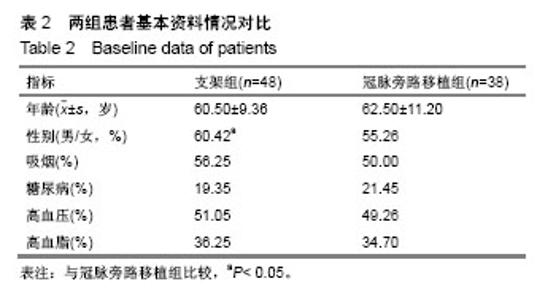
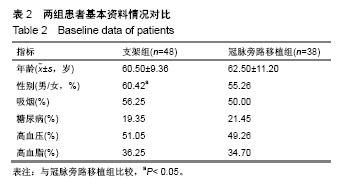
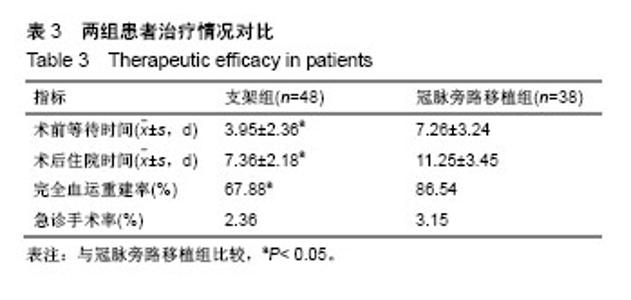
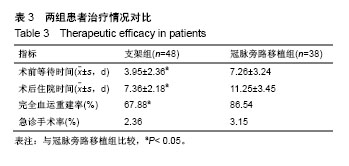
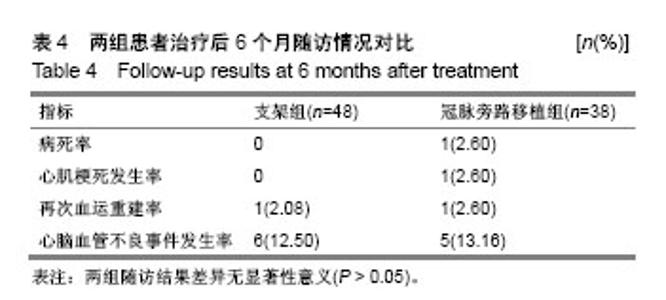
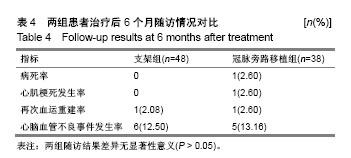
| [1] Wang L, Li HC, Zhou J, et al. Correlation between the GP78 Gene Polymorphism and Coronary Atherosclerotic Heart Disease. Hellenic J Cardiol. 2018;59(1):8-13. [2] Shi JR, Tian CJ, Zeng Q, et al. Expressions of Mast Cell Tryptase and Brain Natriuretic Peptide in Myocardium of Sudden Death due to Hypersensitivity and Coronary Atherosclerotic Heart Disease. Fa Yi Xue Zazhi. 2016;32(3):161-164. [3] Yang RH, Liu YF, Wang XJ, et al. Correlation between high density lipoprotein and monocyte subpopulations among stable coronary atherosclerotic heart disease patients. Int J ClinExp Med. 2015;8(9):16969-16977. [4] Wong MC, Zhang DX, Wang HH. Rapid emergence of atherosclerosis in Asia: a systematic review of coronary atherosclerotic heart disease epidemiology and implications for prevention and control strategies. Curr Opin Lipidol. 2015;26(4): 257-269.[5] 宋昌鹏,王德昭,胡宏宇,等.3.0T高分辨率磁共振评价冠状动脉粥样硬化患者的颈动脉斑块特征[J].中华心血管病杂志,2016,44(1): 38-42.[6] Ellis SG, Tamai H, Nobuyoshi M, et al. Contemporary percutaneous treatment of unprotected left main coronary stenoses: initial results from a multicenter registry analysis 1994-1996. Circulation. 1997;96(11):3867-3872.[7] Kelley MP, Klugherz BD, Hashemi SM, et al. One-year clinical outcomes of protected and unprotected left main coronary artery stenting. Eur Heart J. 2003;24(17):1554-1559.[8] Caracciolo EA, Davis KB, Sopko G, et al. Comparison of surgical and medical group survival in patients with left main equivalent coronary artery disease. Long-term CASS experience. Circulation. 1995;91(9):2335-2344.[9] Park SJ, Kim YH, Lee BK, et al. Sirolimus-eluting stent implantation for unprotected left main coronary artery stenosis: comparison with bare metal stent implantation. J Am Coll Cardiol. 2005;45(3):351-356.[10] Tan Q, Wang Q, Liu D, et al. Intravascular ultrasound-guided unprotected left main coronary artery stenting in the elderly. Saudi Med J. 2015;36(5):549-553. [11] Cassese S, Kufner S, Xhepa E, et al. Three-year efficacy and safety of new- versus early-generation drug-eluting stents for unprotected left main coronary artery disease insights from the ISAR-LEFT MAIN and ISAR-LEFT MAIN 2 trials. Clin Res Cardiol. 2016;105(7):575-584. [12] Ray S, Mazumder A, Kumar S, et al. Angioplasty of unprotected left main coronary stenosis: real world experience of a single-operator group from eastern India. Indian Heart J. 2016;68(1):28-35. [13] 周玉杰,贾德安.批阅三载增删十次-《冠状动脉粥样硬化性心脏病诊断标准》诞生记[J].中国卫生标准管理,2010,1(4):20-21.[14] 杨菲菲,王禹,荆晶,等.无保护左主干病变支架介入治疗后冠状动脉造影随访结果分析[J].中华老年心脑血管病杂志,2010,12(9): 795-798.[15] 高好考,王琼,李寰,等.药物涂层球囊在冠状动脉前降支支架内再狭窄复杂病变的安全性观察[J].岭南心血管病杂志,2017,23(6):649-652.[16] 蔡俊锋,邬祎程,孙延军,等.不同手术方式重建左前降支血运的效果[J].中华胸心血管外科杂志,2013,29(4):209-211.[17] Brener SJ, Galla JM, Bryant R 3rd, et al. Comparison of percutaneous versus surgical revascularization of severe unprotected left main coronary stenosis in matched patients. Am J Cardiol. 2008;101(2):169-172. |