Chinese Journal of Tissue Engineering Research
Previous Articles Next Articles
Safety and clinical application of induced pluripotent stem cells
Zhang Yang, Li Xiu-lan
- Cell Engineering Laboratory of Tianjin Orthopaedic Hospital, Tianjin 300211, China
-
Received:2013-03-21Revised:2013-05-04Online:2013-07-02Published:2013-07-02 -
Contact:Li Xiu-lan, Master, Investigator, Doctoral supervisor, Cell Engineering Laboratory of Tianjin Orthopaedic Hospital, Tianjin 300211, China lixiulan1954@sina -
About author:comZhang Yang★, Master, Associate investigator, Cell Engineering Laboratory of Tianjin Orthopaedic Hospital, Tianjin 300211, China -
Supported by:Tianjin Applied Basis and Cutting-Edge Technology Research Plan, No. 11JCYBJC10200*
CLC Number:
Cite this article
Zhang Yang, Li Xiu-lan. Safety and clinical application of induced pluripotent stem cells[J]. Chinese Journal of Tissue Engineering Research, doi: 10.3969/j.issn.2095-4344.2013.27.021.
share this article
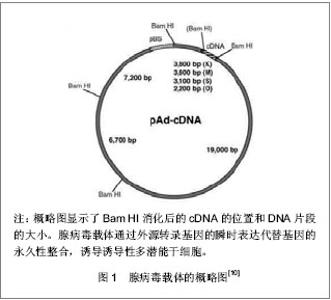
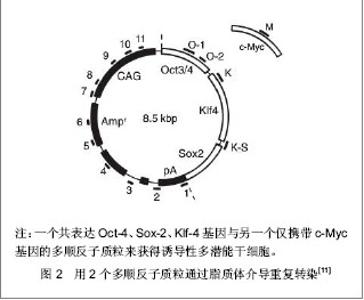
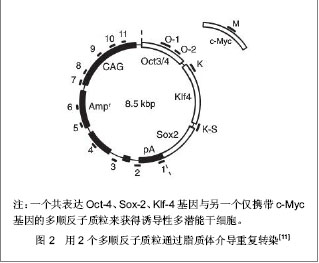
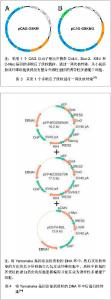
2.1 提高诱导性多潜能干细胞安全性的措施 2.1.1 避免c-Myc基因的使用 在Yamanaka经典方法中,c-Myc基因是4个主要的转录因子之一,同时也是公认的癌基因,外源c-Myc基因的导入具有引发癌变的可能。 Aasen等[5]、Wernig等[6]和Nakagawa等[7]均报道了在不使用c-Myc基因的情况下,仅采用Oct-4、Sox2和Klf4这3个转录因子,也能高质量的诱导小鼠胚胎成纤维细胞和人皮肤成纤维细胞重编程为诱导性多潜能干细胞。Feng等[8]不使用c-Myc和Klf4这两个因子,用Essrb与Oct4和Sox2共同作用把胎鼠成纤维细胞重编程为诱导性多潜能干细胞。Thomson研究小组[9]不使用c-Myc和Klf4,用Oct-4、Sox2、Nanog和Lin28可以诱导人的成纤维细胞为诱导性多潜能干细胞,避开致癌性。去除c-Myc基因后的诱导效率明显低于4个转录因子共同作用,同时会延迟诱导性多潜能干细胞克隆出现的时间,提示c-Myc基因并不是体细胞重编程为诱导性多潜能干细胞所必需的,但细胞重编程中c-Myc基因的参与有助于提高诱导效率。 2.1.2 以其它介导方式代替反转录病毒的使用 诱导性多潜能干细胞前期研究中普遍使用反转录病毒载体,只能感染分裂期细胞而不能感染静止期细胞,目前建立诱导性多潜能干细胞系的传统方法所使用的慢病毒载体属于反转录病毒的一种,可以高效感染分裂细胞和非分裂细胞。不管是采用慢病毒还是反转录病毒基因载体均可能导致载体序列及外源因子序列永久地整合入细胞的基因组DNA中,引起插入突变,影响诱导性多潜能干细胞功能及分化,甚至导致肿瘤发生,限制了诱导性多潜能干细胞在基础研究和临床研究中的应用。 腺病毒介导:由反转录病毒载体介导的基因组插入突变并不是诱导诱导性多潜能干细胞所必需的,利用瞬时转染的方式亦可能获得诱导性多潜能干细胞。Stadtfeld等[10]采用腺病毒载体代替反转录病毒载体,通过外源转录基因的瞬时表达代替基因的永久性整合,诱导诱导性多潜能干细胞均获成功,见图1。该方法一般不会把病毒载体整合进宿主基因组中,但诱导效率低,且仍有外源基因整合进宿主基因组的可能。"
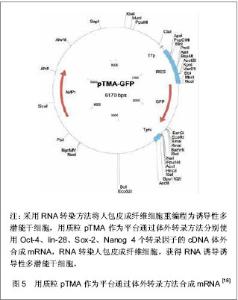
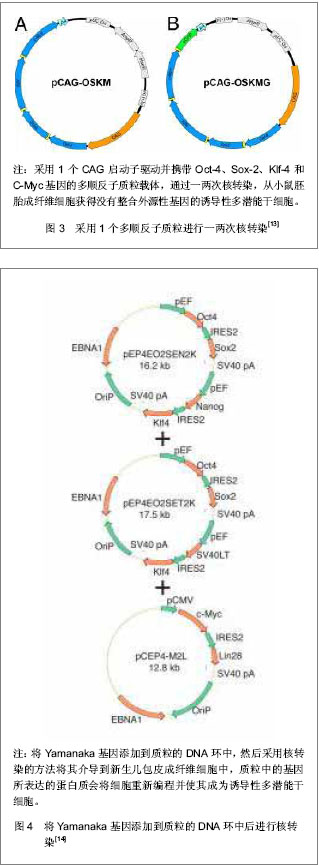
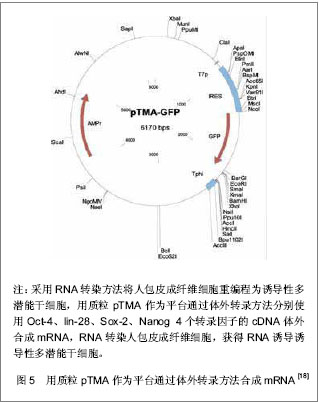
Jia等[15]建立了一个携带POU5Fl、Sox-2、Lin28、Nanog四个重组因子及2A肽段和IRES的质粒,可以诱导人脂肪干细胞重编程为诱导性多潜能干细胞,同时进一步利用PhiC31分子内重组系统使质粒骨架在细菌里被清除和被降解。微环状载体外源沉默机制的低活化作用使其具有更长的异位表达,因此具有较高转染效率,重编程效率低于病毒载体[16-17],高于其他质粒转染重编程方法[11, 14]。该载体仅有1个质粒,无需随后的药物筛选或载体清除,也不包含癌基因。采用质粒转染方法获得的这些诱导性多潜能干细胞没有外源性DNA,从而避免了癌变问题,更适合未来的临床应用。 RNA转染:Yakubov等[18]尝试采用RNA转染方法将人包皮成纤维细胞重编程为诱导性多潜能干细胞,用质粒pTMA作为平台通过体外转录方法分别使用Oct-4、lin-28、Sox-2、Nanog 4个转录因子的cDNA体外合成mRNA,RNA转染人包皮成纤维细胞,成功获得了RNA诱导诱导性多潜能干细胞,见图5。这种方法避免了破坏DNA整合和相关基因组,有可能代替DNA载体诱导诱导性多潜能干细胞。"
| [1] Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663-676.[2] Takahashi K, Tanabe K, Ohnuki M, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;13l(5);861-872.[3] Nishikawa S, Goldstein RA, Nierras CR. The promise of human induced pluripotent stem cells for research and therapy. Nat Rev Mol Cell Biol. 2008;9(9):725-729. [4] Park IH, Arora N, Huo H, et al. Disease-specific induced pluripotent stem cells. Cell. 2008;134(5):877-886. [5] Aasen T, Raya A, Barrero MJ, et al. Efficient and rapid generation of induced pluripotent stem cells from human keratinocytes. Nat Biotechnol. 2008;26(11):1276-1284. [6] Wernig M, Meissner A, Cassady JP, et al. c-Myc is dispensable for direct reprogramming of mouse fibroblasts. Cell Stem Cell. 2008;2(1):10-12. [7] Nakagawa M, Koyanagi M, Tanabe K, et al. Generation of induced pluripotent stem cells without Myc from mouse and human fibroblasts. Nat Biotechnol. 2008;26(1):101-106. [8] Feng B, Jiang J, Kraus P, et al. Reprogramming of fibroblasts into induced pluripotent stem cells with orphan nuclear receptor Esrrb. Nat Cell Biol. 2009;11(2):197-203. [9] Yu J, Vodyanik MA, Smuga-Otto K, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318(5858):1917-1920. [10] Stadtfeld M, Nagaya M, Utikal J, et al. Induced pluripotent stem cells generated without viral integration. Science. 2008;322(5903):945-949. [11] Okita K, Nakagawa M, Hyenjong H, et al. Generation of mouse induced pluripotent stem cells without viral vectors. Science. 2008;322(5903):949-953. [12] Brambrink T, Foreman R, Welstead GG, et al. Sequential expression of pluripotency markers during direct reprogramming of mouse somatic cells. Cell Stem Cell. 2008;2(2):151-159. [13] Gonzalez F, Barragan Monasterio M, Tiscornia G, et al. Generation of mouse-induced pluripotent stem cells by transient expression of a single nonviral polycistronic vector. Proc Natl Acad Sci U S A. 2009;106(22):8918-8922. [14] Yu J, Hu K, Smuga-Otto K, et al. Human induced pluripotent stem cells free of vector and transgene sequences. Science. 2009;324(5928):797-801. [15] Jia F, Wilson KD, Sun N, et al. A nonviral minicircle vector for deriving human iPS cells. Nat Methods. 2010;7(3):197- 199. [16] Okita K, Ichisaka T, Yamanaka S. Generation of germline-competent induced pluripotent stem cells. Nature. 2007;448(7151):313-317. [17] Wernig M, Meissner A, Foreman R, et al. In vitro reprogramming of fibroblasts into a pluripotent ES-cell-like state. Nature. 2007;448(7151):318-324. [18] Yakubov E, Rechavi G, Rozenblatt S, et al. Reprogramming of human fibroblasts to pluripotent stem cells using mRNA of four transcription factors. Biochem Biophys Res Commun. 2010;394(1):189-193. [19] Lin SL, Chang DC, Chang-Lin S, et al. Mir-302 reprograms human skin cancer cells into a pluripotent ES-cell-like state. RNA . 2008;14(10):2115-2124. [20] Judson RL, Babiarz JE, Venere M, et al. Embryonic stem cell-specific microRNAs promote induced pluripotency. Nat Biotechnol. 2009;27(5):459-461. [21] Zhou H, Wu S, Joo JY, et al. Generation of induced pluripotent stem cells using recombinant proteins. Cell Stem Cell. 2009;4(5):381-384. [22] Kim D, Kim CH, Moon JI, et al. Generation of human induced pluripotent stem cells by direct delivery of reprogramming proteins. Cell Stem Cell. 2009;4(6):472-476. [23] Aoi T, Yae K, Nakagawa M, et al. Generation of pluripotent stem cells from adult mouse liver and stomach cells. Science. 2008;321(5889):699-702. [24] Miura K, Okada Y, Aoi T, et al. Variation in the safety of induced pluripotent stem cell lines. Nat Biotechnol. 2009; 27(8):743-745. [25] Eminli S, Utikal J, Arnold K, et al. Reprogramming of neural progenitor cells into induced pluripotent stem cells in the absence of exogenous Sox2 expression. Stem Cells. 2008; 26(10):2467-2474. [26] Kim JB, Zaehres H, Wu G, et al. Pluripotent stem cells induced from adult neural stem cells by reprogramming with two factors. Nature. 2008;454(7204):646-650. [27] Shi Y, Do JT, Desponts C, et al. A combined chemical and genetic approach for the generation of induced pluripotent stem cells. Cell Stem Cell. 2008;2(6):525-528. [28] Huangfu D, Osafune K, Maehr R,et al. Induction of pluripotent stem cells from primary human fibroblasts with only Oct4 and Sox2. Nat Biotechnol. 2008;26(11):1269-1275. [29] Rajasingh J, Lambers E, Hamada H, et al. Cell-free embryonic stem cell extract-mediated derivation of multipotent stem cells from NIH3T3 fibroblasts for functional and anatomical ischemic tissue repair. Circ Res. 2008; 102(11): e107-117. [30] Fu X, Sun X, Li X, et al. Dedifferentiation of epidermal cells to stem cells in vivo. Lancet. 2001;358(9287):1067-1068. [31] Sun X, Fu X, Han W, et al. Dedifferentiation of human terminally differentiating keratinocytes into their precursor cells induced by basic fibroblast growth factor. Biol Pharm Bull. 2011;34(7):1037-1045. [32] Li H, Fu X, Zhang L, et al. In vivo dedifferentiation of human epidermal cells. Cell Biol Int. 2007;31(11):1436-1441. [33] Zhang C, Fu X, Chen P, et al. Dedifferentiation derived cells exhibit phenotypic and functional characteristics of epidermal stem cells. J Cell Mol Med. 2010;14(5):1135-1145. [34] Huang S, Xu Y, Wu C, et al. In vitro constitution and in vivo implantation of engineered skin constructs with sweat glands. Biomaterials. 2010;31(21):5520-5525. [35] Hanna J, Wernig M, Markoulaki S, et al. Treatment of sickle cell anemia mouse model with iPS cells generated from autologous skin. Science. 2007;318(5858):1920-1923. [36] Xu D, Alipio Z, Fink LM, et al. Phenotypic correction of murine hemophilia A using an iPS cell-based therapy. Proc Natl Acad Sci U S A. 2009;106(3):808-813. [37] Ye L, Chang JC, Lin C, et al. Induced pluripotent stem cells offer new approach to therapy in thalassemia and sickle cell anemia and option in prenatal diagnosis in genetic diseases. Proc Natl Acad Sci U S A. 2009;106(24):9826-9830. [38] Wernig M, Zhao JP, Pruszak J, et al. Neurons derived from reprogrammed fibroblasts functionally integrate into the fetal brain and improve symptoms of rats with Parkinson's disease. Proc Natl Acad Sci U S A. 2008;105(15):5856-5861. [39] Soldner F, Hockemeyer D, Beard C, et al. Parkinson's disease patient-derived induced pluripotent stem cells free of viral reprogramming factors. Cell. 2009;136(5):964-977. [40] Nelson TJ, Martinez-Fernandez A, Yamada S, et al. Repair of acute myocardial infarction by human stemness factors induced pluripotent stem cells. Circulation. 2009;120(5): 408-416. [41] Li DS, Warnock GL, Tu HJ, et al. Do immunotherapy and beta cell replacement play a synergistic role in the treatment of type 1 diabetes? Life Sci. 2009;85(15-16):549-556. [42] Dimos JT, Rodolfa KT, Niakan KK, et al. Induced pluripotent stem cells generated from patients with ALS can be differentiated into motor neurons. Science. 2008;321(5893): 1218-1221. [43] Ebert AD, Yu J, Rose FF Jr, et al. Induced pluripotent stem cells from a spinal muscular atrophy patient. Nature. 2009;457(7227):277-280. [44] Tanaka T, Tohyama S, Murata M, et al. In vitro pharmacologic testing using human induced pluripotent stem cell-derived cardiomyocytes. Biochem Biophys Res Commun. 2009; 385(4):497-502. [45] Park IH, Zhao R, West JA, et al. Reprogramming of human somatic cells to pluripotency with defined factors. Nature. 2008;451(7175):141-146.[46] Nakao Y, Narazaki G, Hoshino T, et al. Evaluation of antiangiogenic activity of azumamides by the in vitro vascular organization model using mouse induced pluripotent stem (iPS) cells. Bioorg Med Chem Lett. 2008;18(9):2982-2984. [47] Loh YH, Agarwal S, Park IH, et al. Generation of induced pluripotent stem cells from human blood. Blood. 2009;113(22): 5476-5479. |
| [1] | Pu Rui, Chen Ziyang, Yuan Lingyan. Characteristics and effects of exosomes from different cell sources in cardioprotection [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(在线): 1-. |
| [2] | Wang Haiying, Lü Bing, Li Hui, Wang Shunyi. Posterior lumbar interbody fusion for degenerative lumbar spondylolisthesis: prediction of functional prognosis of patients based on spinopelvic parameters [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1393-1397. |
| [3] | Gu Xia, Zhao Min, Wang Pingyi, Li Yimei, Li Wenhua. Relationship between hypoxia inducible factor 1 alpha and hypoxia signaling pathway [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(8): 1284-1289. |
| [4] | Ji Zhixiang, Lan Changgong. Polymorphism of urate transporter in gout and its correlation with gout treatment [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(8): 1290-1298. |
| [5] | Li Jiacheng, Liang Xuezhen, Liu Jinbao, Xu Bo, Li Gang. Differential mRNA expression profile and competitive endogenous RNA regulatory network in osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(8): 1212-1217. |
| [6] | Tan Jingyu, Liu Haiwen. Genome-wide identification, classification and phylogenetic analysis of Fasciclin gene family for osteoblast specific factor 2 [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(8): 1243-1248. |
| [7] | Zhang Xiumei, Zhai Yunkai, Zhao Jie, Zhao Meng. Research hotspots of organoid models in recent 10 years: a search in domestic and foreign databases [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(8): 1249-1255. |
| [8] | Wang Zhengdong, Huang Na, Chen Jingxian, Zheng Zuobing, Hu Xinyu, Li Mei, Su Xiao, Su Xuesen, Yan Nan. Inhibitory effects of sodium butyrate on microglial activation and expression of inflammatory factors induced by fluorosis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1075-1080. |
| [9] | Wang Xianyao, Guan Yalin, Liu Zhongshan. Strategies for improving the therapeutic efficacy of mesenchymal stem cells in the treatment of nonhealing wounds [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1081-1087. |
| [10] | Wan Ran, Shi Xu, Liu Jingsong, Wang Yansong. Research progress in the treatment of spinal cord injury with mesenchymal stem cell secretome [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1088-1095. |
| [11] | Liao Chengcheng, An Jiaxing, Tan Zhangxue, Wang Qian, Liu Jianguo. Therapeutic target and application prospects of oral squamous cell carcinoma stem cells [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1096-1103. |
| [12] | Zhao Min, Feng Liuxiang, Chen Yao, Gu Xia, Wang Pingyi, Li Yimei, Li Wenhua. Exosomes as a disease marker under hypoxic conditions [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1104-1108. |
| [13] | Xie Wenjia, Xia Tianjiao, Zhou Qingyun, Liu Yujia, Gu Xiaoping. Role of microglia-mediated neuronal injury in neurodegenerative diseases [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1109-1115. |
| [14] | Li Shanshan, Guo Xiaoxiao, You Ran, Yang Xiufen, Zhao Lu, Chen Xi, Wang Yanling. Photoreceptor cell replacement therapy for retinal degeneration diseases [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1116-1121. |
| [15] | Jiao Hui, Zhang Yining, Song Yuqing, Lin Yu, Wang Xiuli. Advances in research and application of breast cancer organoids [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1122-1128. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||