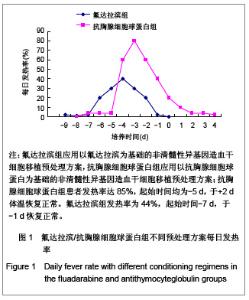
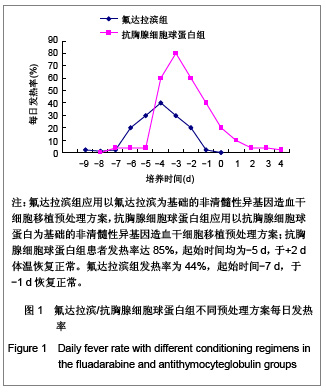
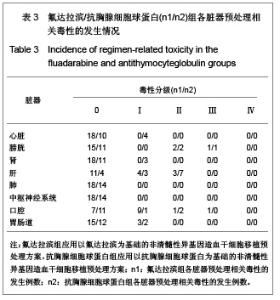
| [1] Baron F,Storb R.Current roles for allogeneic hematopoietic cell transplantation following nonmyeloablative or reduced-intensity conditioning. Clin Adv Hematol Oncol. 2005;3(10):799-819.[2] Weiden PL,Sullivan KM,Flournoy N,et al.Antileukemic effect of chronic graft-versus-host disease:contribution to improved survival after allogeneic marrow transplantation.N Engl J Med.1981;304(25):1529-1533.[3] Li JM,Giver CR,Waller EK.Graft engineering using ex vivo methods to limit GVHD:fludarabine treatment generates superior GVL effects in allogeneic BMT.Exp Hematol.2006; 34(7):895-904.[4] Li QS,Mao P,Wang SQ,et al.Zhonghua Qiguang Yizhi Zazhi. 2004;25(5):308-311. 李庆山,毛平,王顺清,等.供者淋巴细胞输注在恶性血液病非清髓性骨髓移植中的应用[J].中华器官移植杂志,2004,25(5): 308-311.[5] Miehallet M,Le QH,Mohty M,et al.Predictive factors for outcomes after reduced intensity conditioning hematopoietic stem cell transplantation for hematological malignancies:a 10-year retrospective analysis from the Société Francaise de Greffe de Moelle et de Thérapie Cellulaire.Exp Hematol.2008; 36(5):535-544.[6] Zhang ZN.Shen T Beijing:Science Press.2007. 张之南,沈悌.血液病诊断及治疗标准[M].3版.北京:科学出版社, 2007.[7] Deng TF,Li QS,Zhou M.Hainan Yixue. 2011,22(12):26-29. 邓婷芬,李庆山,周铭.单个核细胞绝对计数对恶性血液病患者自体外周血干细胞采集结果及时机选择的意义[J].海南医学,2011, 22(12):26-29.[8] Li QS,Mao P,Wang SQ,et al.Linchuang Huicui. 2008; 21(21): 1563-1565. 李庆山,毛平,王顺清,等.非清髓性造血干细胞移植在血液病治疗中应用抗胸腺细胞球蛋白或抗淋巴细胞球蛋白安全性观察[J].临床荟萃,2008,21(21):1563-1565.[9] Li QS,Mao P,Wang SQ,et al.Zhonghua Qiguang Yizhi Zazhi. 2008;29(11):689-692. 李庆山,毛平,王顺清,等.减低强度预处理造血干细胞移植联合低剂量环孢素A治疗恶性血液病[J].中华器官移植杂志,2008, 29(11): 689-692.[10] Bearman SI,Appelbaum FR,Buckner CD,et al. Regimen-related toxicity in patients undergoing bone marrow transplantation.J Clin Oncol.1988;6(10):1562-1568.[11] Pihuseh R,Holler E,Mühlbayer D,et al.The impact of antithymocyte globulin on short-term toxicity after allogeneic stem cell transplantation.Bone Marrow Transplant.2002; 30(6): 347-354.[12] Gupta V,Gordon-Smith EC,Cook G,et al.A third course of antithymocyte globulin in aplastic anaemia is only beneficial in previous responders.Br J Haemat0l.2005;129(1):110-117.[13] Besien K, Devine S, Wickrema A,et al.Regimen-related toxicity after ?udarabine–melphalan conditioning:a prospective study of 31 patients with hematologic malignancies.Bone Marrow. Transplantation.2003; 32(5):471-476.[14] KrÖger N,Schetelig J,Zabelina T,et al.A ?udarabine-based dose-reduced conditioning regimen followed by allogeneic stem cell transplantation from related or unrelated donors in patients with myelodysplastic syndrome. Bone Marrow Transplantation.2001;28(7): 643-647.[15] Gluckman E,Jolivet I,Scrobohaci ML,et al.Use of prostaglandinE1 for prevention of liver veno-occlusive disease in leukaemic patients treated by allogeneic bone marrow transplantation.Br J Heamatol.1990;74(3):277-281. |