Chinese Journal of Tissue Engineering Research ›› 2018, Vol. 22 ›› Issue (14): 2190-2194.doi: 10.3969/j.issn.2095-4344.0748
Previous Articles Next Articles
Preparation and physicochemical characterization of levofloxacin-loaded poly(lactic-co-glycolic acid) nanoparticles
Peng Xu-feng1, 2, Luo Wen-qiang1, 2, Zhang Xin-ru1, 2, Wang Ji-hong1, 2
- 1Department of Urology, Shanghai Sixth People’s Hospital of Shanghai Jiao Tong University, 2Shanghai Eastern Institute for Urologic Repair and Reconstruction, Shanghai 200233, China
-
Received:2017-12-09Online:2018-05-18Published:2018-05-18 -
Contact:Wang Ji-hong, M.D., Department of Urology, Shanghai Sixth People’s Hospital of Shanghai Jiao Tong University, Shanghai 200233, China -
About author:Peng Xu-feng, Master candidate, Department of Urology, Shanghai Sixth People’s Hospital of Shanghai Jiao Tong University, Shanghai 200233, China
CLC Number:
Cite this article
Peng Xu-feng, Luo Wen-qiang, Zhang Xin-ru, Wang Ji-hong. Preparation and physicochemical characterization of levofloxacin-loaded poly(lactic-co-glycolic acid) nanoparticles[J]. Chinese Journal of Tissue Engineering Research, 2018, 22(14): 2190-2194.
share this article
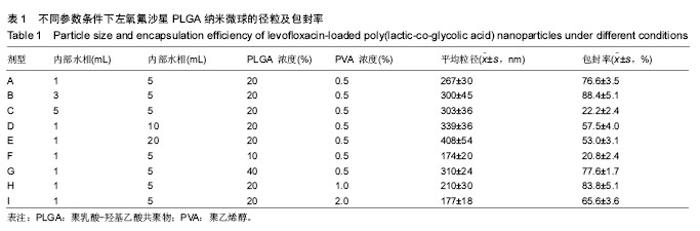
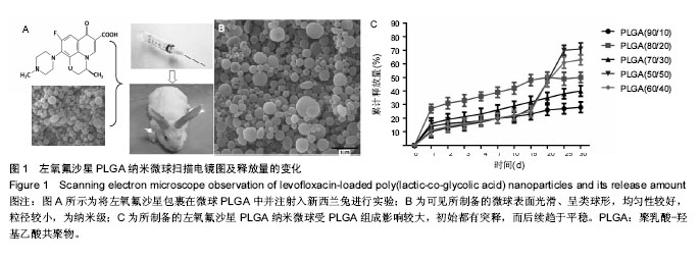
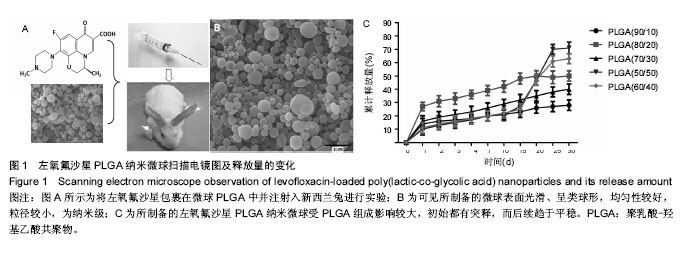
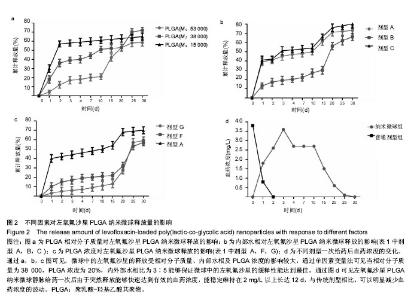
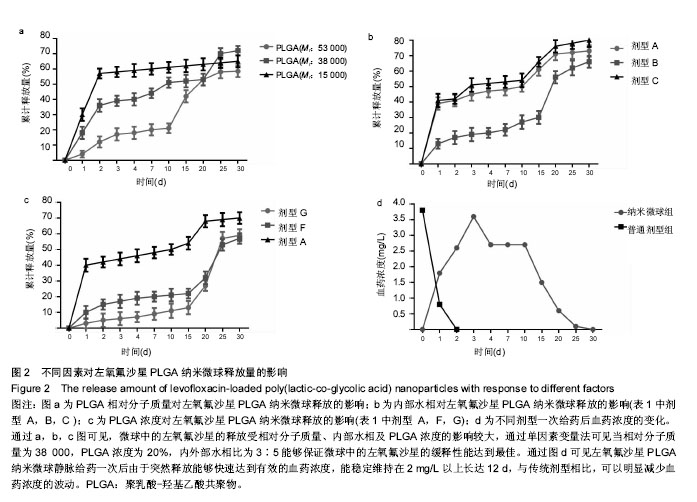
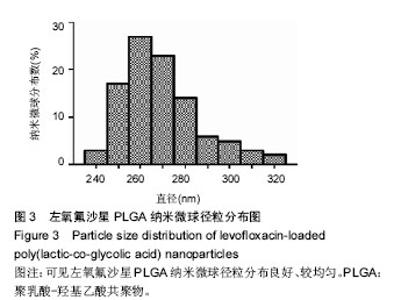
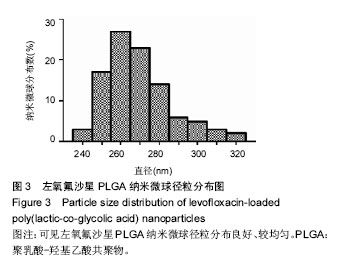
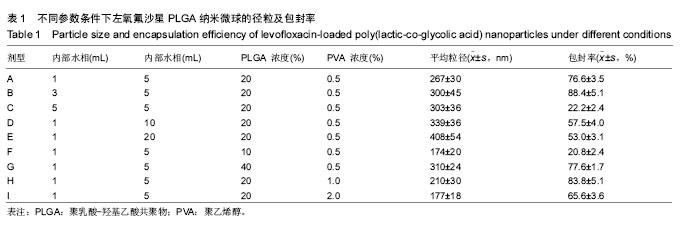
2.1 实验动物数量分析 共纳入新西兰大白兔69只,有1只因感染而死于败血症未补充,未纳入结果分析。 2.2 左氧氟沙星PLGA纳米微球的鉴定 采用乳化溶剂挥发法,以二氯甲烷为溶剂相,PLGA为载体相,左氧氟沙星为包埋对象,聚乙烯醇为乳化剂成功制备了基于PLGA不同批次的左氧氟沙星纳米微球。在制作过程中采用单因素变量法探讨多种因素对纳米微球直径大小、载药量、包封率的影响(表1,图1,2),包括PLGA的组成、PLGA相对分子质量的大小、内外部水相比PLGA的浓度。从图1C可见当乳酸/羟基乙酸比为60/40,50/50时累计释放曲线呈现典型的三段式,当组成比为70/30,80/20,90/10时,PLGA纳米微球有早期的突然释放和之后趋于平稳的缓释,考虑到初始用药需尽早达到药物的有效抑菌溶度和后期的维持时间,当比例为80/20时是最为理想的组成。从图2a,b,c结合表1可见,当PLGA的相对分子质量为38 000,浓度为20%,内外部水相比为3∶5能够保证PLGA纳米微球中的左氧氟沙星载药量达到较高的同时包封率较高且缓释性能达到最佳,故以此条件下的PLGA纳米微球进行后续实验。"
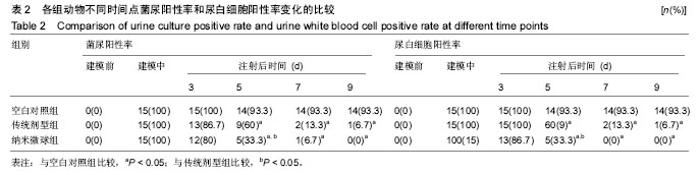
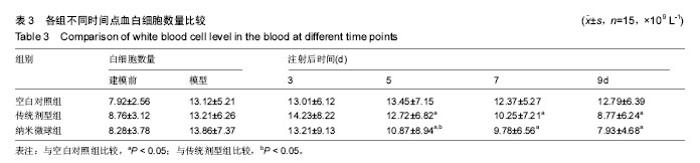
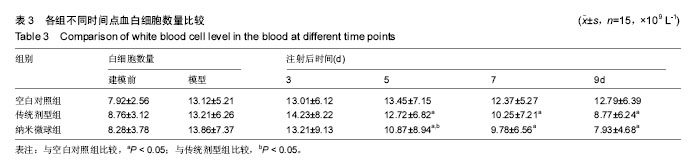
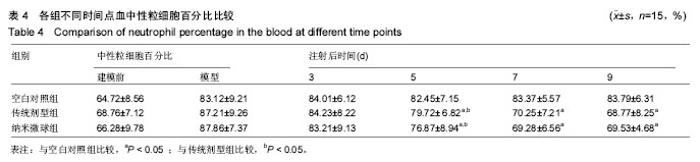
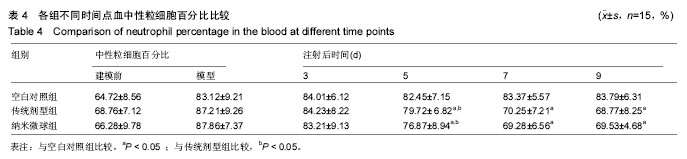
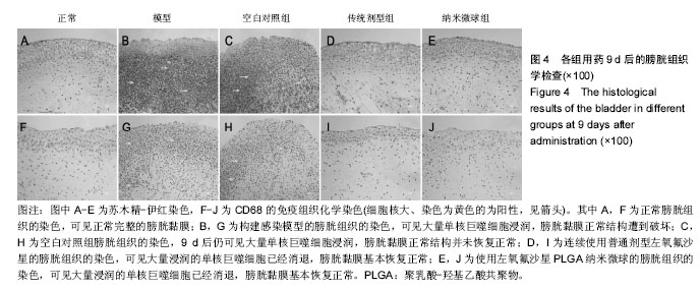
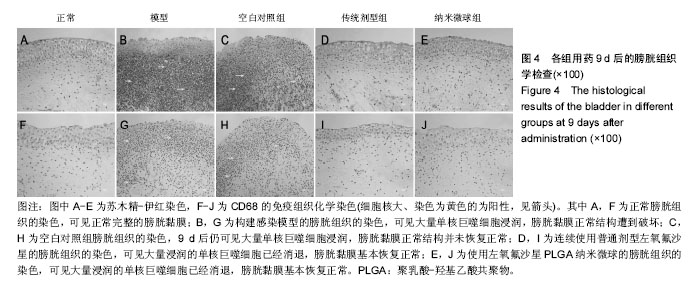
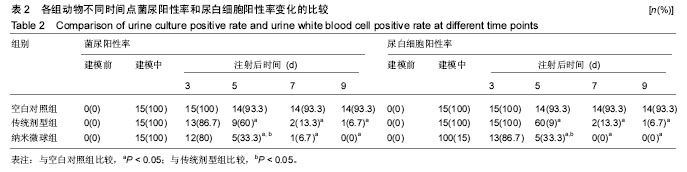
2.4 左氧氟沙星PLGA纳米微球的体内药代动力学特性 通过高效液相色谱法分析发现,最优条件下的左氧氟沙星PLGA纳米微球其平均载药量为(13.2±0.6)%,包封率为88.9%。体内药代动力学研究表明,传统剂型的左氧氟沙星在静脉应用后能迅速达到血药浓度的峰值为(3.8± 1.2) mg/L,其消除相半衰期约为13 h,约2 d基本已完全从 体内代谢消失。而左氧氟沙星PLGA纳米微球在应用后血药浓度逐步升高,并达到峰值(3.6±1.3)mg/L,之后逐步稳定能维持在质量浓度2 mg/L以上约12 d,最终逐步下降,30 d后从体内基本无法检出(图2d)。 2.5 左氧氟沙星PLGA纳米微球的抗菌性能评估 不同时间点尿中大肠杆菌阳性率的比较:见表2。制模前各组动物菌尿阳性率均为阴性,造模后菌尿阳性率达100%,说明建模成功。开始用药后,传统剂型组和纳米微球组抑菌作用逐步显现,到用药的第5天,两组与空白对照组差异有显著性意义,且一直延续到第9天,其中在第5天时传统剂型组与纳米微球组之间差异有显著性意义(P < 0.05)。 不同时间点尿中白细胞阳性率的比较:见表2。制模前各组动物尿白细胞阳性率均为阴性,造模后菌尿阳性率达100%。用药后第3天纳米微球组阳性率开始下降,传统剂型组阳性率第5天开始下降,一直持续到第9天。"
| [1] McLellan LK, Hunstad DA. Urinary Tract Infection: Pathogenesis and Outlook. Trends Mol Med. 2016;22(11):946-957. [2] Anderson VR, Perry CM. Levofloxacin: a review of its use as a high-dose, short-course treatment for bacterial infection. Drugs. 2008; 8(4):535-565.[3] Derevianko II, Lavrinova LN, Kudriashova EE. Effectiveness of levofloxacin (Tavanik, "Aventis Pharma") in the treatment of complicated infections of the urogenital organs. Urologiia. 2003;(1): 31-34.[4] Guan J, Cheng P, Huang SJ, et al. Optimized Preparation of Levofloxacin-loaded Chitosan Nanoparticles by Ionotropic Gelation. 2011 International Conference on Physics Science and Technology (Icpst). 2011;22(11):163-169.[5] Bernkop-Schnurch A, Dunnhaupt S. Chitosan-based drug delivery systems. Eur J Pharm Biopharm. 2012;81(3):463-469. [6] Khan FI, Rahman S, Queen A, et al. Implications of molecular diversity of chitin and its derivatives. Appl Microbiol Biotechnol. 2017;101(9): 3513-3536.[7] Das S, Khuda-Bukhsh AR. PLGA-loaded nanomedicines in melanoma treatment: future prospect for efficient drug delivery. Indian J Med Res. 2016;144(2):181-193. [8] Kapoor DN, Bhatia A, Kaur R, et al. PLGA: a unique polymer for drug delivery. Ther Deliv. 2015;6(1):41-58. [9] Sah H, Thoma LA, Desu HR, et al. Concepts and practices used to develop functional PLGA-based nanoparticulate systems. Int J Nanomed. 2013;8:747-765.[10] Liu Y, Bharadwaj S, Lee SJ, et al. Optimization of a natural collagen scaffold to aid cell-matrix penetration for urologic tissue engineering. Biomaterials. 2009;30(23-24):3865-3873.[11] Blum F. High performance liquid chromatography. Br J Hosp Med (Lond). 2014;75(2):C18-C21.[12] Cadieux PA, Chew BH, Knudsen BE, et al. Triclosan loaded ureteral stents decrease proteus mirabilis 296 infection in a rabbit urinary tract infection model. J Urol. 2006;175(6):2331-2335.[13] Xie M, Xu Y, Song L, et al. Tissue-engineered buccal mucosa using silk fibroin matrices for urethral reconstruction in a canine model. J Surg Res. 2014;188(1):1-7.[14] Sa Y, Li C, Li H, et al. TIMP-1 Induces alpha-Smooth Muscle Actin in Fibroblasts to Promote Urethral Scar Formation. Cell Physiol Biochem. 2015;35(6):2233-2243.[15] Sheerin NS. Urinary tract infection. Medicine. 2013;29(3):699-715. [16] Jin L, Ding Y, Feng M, et al. Preparation oral levofloxacin colon-specific microspheres delivery: in vitro and in vivo studies. Drug Deliv. 2016;23(3):992-998. [17] 张心如,鲁文龙,冯超,等.血管内皮生长因子纳米微球-细菌纤维素复合支架与多种细胞构建组织工程膀胱[J].中国组织工程研究, 2016,20(21): 3088-3096.[18] Wolfram J, Zhu M, Yang Y, et al. Safety of Nanoparticles in Medicine. Curr Drug Targets. 2015;16(14):1671-1681.[19] Johannesson G, Stefansson E, Loftsson T. Microspheres and Nanotechnology for Drug Delivery. Dev Ophthalmol. 2016;55:93-103. [20] van Rijt S, Habibovic P. Enhancing regenerative approaches with nanoparticles. J R Soc Interface. 2017;14(129):20170093.[21] Prajapati VD, Jani GK, Kapadia JR. Current knowledge on biodegradable microspheres in drug delivery. Expert Opin Drug Deliv. 2015;12(8):1283-1299. [22] Gaspar MC, Sousa JJ, Pais AA, et al. Optimization of levofloxacin-loaded crosslinked chitosan microspheres for inhaled aerosol therapy. Eur J Pharm Biopharm. 2015;96:65-75.[23] Zhang K, Fu Q, Yoo J, et al. 3D bioprinting of urethra with PCL/PLCL blend and dual autologous cells in fibrin hydrogel: an in vitro evaluation of biomimetic mechanical property and cell growth environment. Acta Biomater. 2017;50:154-164. [24] Gilert A, Baruch L, Bronshtein T, et al. PLGA-Listeriolysin O microspheres: Opening the gate for cytosolic delivery of cancer antigens. Biomed Microdevices. 2016;18(2):23.[25] Crawford ED, Moul JW, Sartor O, et al. Extended release, 6-month formulations of leuprolide acetate for the treatment of advanced prostate cancer: achieving testosterone levels below 20 ng/dl. Expert Opin Drug Metab Toxicol. 2015;11(9):1465-1474. [26] Shirangi M, Najafi M, Rijkers DT, et al. Inhibition of Octreotide Acylation Inside PLGA Microspheres by Derivatization of the Amines of the Peptide with a Self-Immolative Protecting Group. Bioconjug Chem. 2016;27(3):576-585. [27] Xu Y, Kim CS, Saylor DM, et al. Polymer degradation and drug delivery in PLGA-based drug-polymer applications: a review of experiments and theories. J Biomed Mater Res B Appl Biomater. 2017;105(6): 1692-1716.[28] Pal S, Tak YK, Song JM. Does the antibacterial activity of silver nanoparticles depend on the shape of the nanoparticle? A study of the Gram-negative bacterium Escherichia coli. Appl Environ Microbiol. 2007;73(6):1712-1720. [29] Feng C, Xu YM, Fu Q, et al. Reconstruction of three-dimensional neourethra using lingual keratinocytes and corporal smooth muscle cells seeded acellular corporal spongiosum. Tissue Eng Part A. 2011; 17(23-24):3011-309.[30] Lv X, Li Z, Chen S, et al. Structural and functional evaluation of oxygenating keratin/silk fibroin scaffold and initial assessment of their potential for urethral tissue engineering. Biomaterials. 2016;84:99-110. |
| [1] | Zhang Tongtong, Wang Zhonghua, Wen Jie, Song Yuxin, Liu Lin. Application of three-dimensional printing model in surgical resection and reconstruction of cervical tumor [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1335-1339. |
| [2] | Zeng Yanhua, Hao Yanlei. In vitro culture and purification of Schwann cells: a systematic review [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1135-1141. |
| [3] | Xu Dongzi, Zhang Ting, Ouyang Zhaolian. The global competitive situation of cardiac tissue engineering based on patent analysis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(5): 807-812. |
| [4] | Wu Zijian, Hu Zhaoduan, Xie Youqiong, Wang Feng, Li Jia, Li Bocun, Cai Guowei, Peng Rui. Three-dimensional printing technology and bone tissue engineering research: literature metrology and visual analysis of research hotspots [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 564-569. |
| [5] | Chang Wenliao, Zhao Jie, Sun Xiaoliang, Wang Kun, Wu Guofeng, Zhou Jian, Li Shuxiang, Sun Han. Material selection, theoretical design and biomimetic function of artificial periosteum [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 600-606. |
| [6] | Liu Fei, Cui Yutao, Liu He. Advantages and problems of local antibiotic delivery system in the treatment of osteomyelitis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 614-620. |
| [7] | Li Xiaozhuang, Duan Hao, Wang Weizhou, Tang Zhihong, Wang Yanghao, He Fei. Application of bone tissue engineering materials in the treatment of bone defect diseases in vivo [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 626-631. |
| [8] | Zhang Zhenkun, Li Zhe, Li Ya, Wang Yingying, Wang Yaping, Zhou Xinkui, Ma Shanshan, Guan Fangxia. Application of alginate based hydrogels/dressings in wound healing: sustained, dynamic and sequential release [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 638-643. |
| [9] | Chen Jiana, Qiu Yanling, Nie Minhai, Liu Xuqian. Tissue engineering scaffolds in repairing oral and maxillofacial soft tissue defects [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 644-650. |
| [10] | Xing Hao, Zhang Yonghong, Wang Dong. Advantages and disadvantages of repairing large-segment bone defect [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(3): 426-430. |
| [11] | Chen Siqi, Xian Debin, Xu Rongsheng, Qin Zhongjie, Zhang Lei, Xia Delin. Effects of bone marrow mesenchymal stem cells and human umbilical vein endothelial cells combined with hydroxyapatite-tricalcium phosphate scaffolds on early angiogenesis in skull defect repair in rats [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3458-3465. |
| [12] | Wang Hao, Chen Mingxue, Li Junkang, Luo Xujiang, Peng Liqing, Li Huo, Huang Bo, Tian Guangzhao, Liu Shuyun, Sui Xiang, Huang Jingxiang, Guo Quanyi, Lu Xiaobo. Decellularized porcine skin matrix for tissue-engineered meniscus scaffold [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3473-3478. |
| [13] | Mo Jianling, He Shaoru, Feng Bowen, Jian Minqiao, Zhang Xiaohui, Liu Caisheng, Liang Yijing, Liu Yumei, Chen Liang, Zhou Haiyu, Liu Yanhui. Forming prevascularized cell sheets and the expression of angiogenesis-related factors [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3479-3486. |
| [14] | Liu Chang, Li Datong, Liu Yuan, Kong Lingbo, Guo Rui, Yang Lixue, Hao Dingjun, He Baorong. Poor efficacy after vertebral augmentation surgery of acute symptomatic thoracolumbar osteoporotic compression fracture: relationship with bone cement, bone mineral density, and adjacent fractures [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3510-3516. |
| [15] | Liu Liyong, Zhou Lei. Research and development status and development trend of hydrogel in tissue engineering based on patent information [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3527-3533. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||