Chinese Journal of Tissue Engineering Research ›› 2018, Vol. 22 ›› Issue (1): 126-132.doi: 10.3969/j.issn.2095-4344.0422
Previous Articles Next Articles
Adipose-derived stem cells: current progress and future perspectives regarding the myogenic differentiation and use in the repair of skeletal muscle defects
Chen You-bai1, 2, Zhang Wei3, Li Li1, Zhang Qi-xu2, Han Yan1
- 1Department of Plastic and Reconstructive Surgery, 3Department of Orthopedics, Chinese PLA General Hospital, Beijing 100853, China; 2Department of Plastic Surgery, University of Texas MD Anderson Cancer Center, Texas, USA)
-
Revised:2017-08-13Online:2018-01-08Published:2018-01-08 -
Contact:Han Yan, Chief physician, Professor, Doctoral supervisor, Department of Plastic and Reconstructive Surgery, Chinese PLA General Hospital, Beijing 100853, China;Zhang Qi-xu, Associate professor, Department of Plastic Surgery, University of Texas MD Anderson Cancer Center, Texas, USA -
About author:Chen You-bai, M.D., Attending physician, Department of Plastic and Reconstructive Surgery, Chinese PLA General Hospital, Beijing 100853, China; Department of Plastic Surgery, University of Texas MD Anderson Cancer Center, Texas, USA
CLC Number:
Cite this article
Chen You-bai, Zhang Wei, Li Li, Zhang Qi-xu, Han Yan. Adipose-derived stem cells: current progress and future perspectives regarding the myogenic differentiation and use in the repair of skeletal muscle defects[J]. Chinese Journal of Tissue Engineering Research, 2018, 22(1): 126-132.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks

2.1 脂肪干细胞向骨骼肌成肌细胞分化的过程 脂肪干细胞在定向诱导下向骨骼肌成肌细胞分化是多种基因、蛋白和信号通路交互作用的复杂过程。诱导1 d后脂肪干细胞开始表达MyoG;3 d后细胞体积增大,胞浆内富含嗜碱性颗粒和腔状结构,肌浆网扩张,由短梭形变为长梭形;4-6 d时表达骨骼肌成肌细胞特异性标记物MyoD1和肌球蛋白重链(myosin heavy chain,MHC);10 d时表达肌间线蛋白(desmin);14 d后细胞融合,平行排列形成多核的肌管样结构,并表达myf5、myf6、肌细胞生成素(myogenin)、PAX7和肌动蛋白[1]。 2.2 影响脂肪干细胞成肌分化的因素及机制 见表1。"

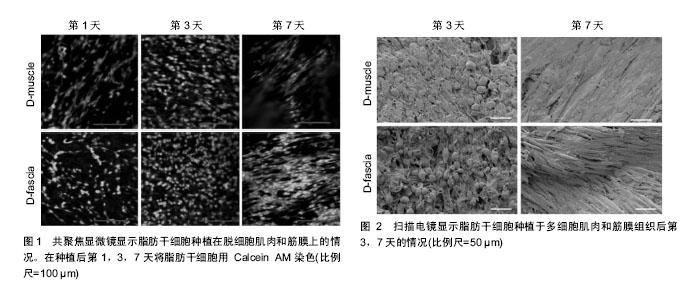
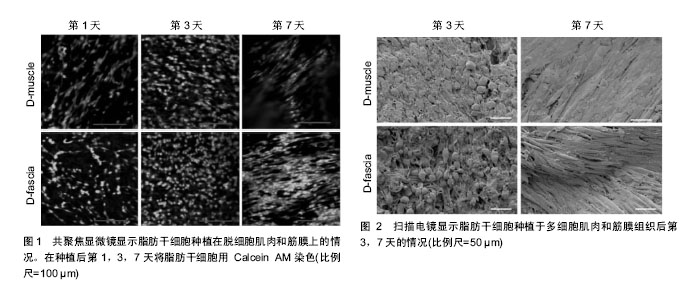
2.2.1 化学药物 ①5-氮杂胞苷:是体外诱导脂肪干细胞成肌分化的主要化学因子,通过阻止新复制DNA的胞嘧啶甲基化,激活原本转录失活的成肌相关基因。Mizuno等[1]最早利用添加了5-氮杂胞苷、地塞米松、氢化可的松和血清的基础培养液诱导脂肪干细胞向骨骼肌成肌细胞分化。但5-氮杂胞苷的诱导分化效率较低,且其细胞毒性与浓度呈正比,20 μmol/L的5-氮杂胞苷可致30%的细胞死亡。研究显示10 μmol/L的5-氮杂胞苷可获得较高的成肌诱导率,同时将其细胞毒性控制在较低水平[2];②硼:Apdik等[3]研究显示利用81.9 μmol/L和819 μmol/L的硼酸处理脂肪干细胞4 d后,MHC、MyoD、myogenin和desmin等表达增高,证实硼元素可促进脂肪干细胞的成肌分化。 2.2.2 生物力学 Bayati等[4]研究显示每天1 h的10%振幅0.5 Hz频率的循环单轴应力可显著刺激脂肪干细胞向骨骼肌细胞分化。 2.2.3 微环境 利用条件培养基或与肌细胞共培养,也可促使脂肪干细胞分化为骨骼肌成肌细胞。Stern-Straeter等[5]对比了间充质干细胞培养基、间充质干细胞培养基+5-氮杂胞苷、骨骼肌成肌细胞培养基、以及5%,30%和50%的骨骼肌卫星细胞条件培养基对脂肪干细胞成肌分化的诱导作用,结果发现30%卫星细胞条件培养基培养的脂肪干细胞成肌分化的比例最高。此外,将脂肪干细胞与鼠骨骼肌细胞共培养,或将脂肪干细胞移植到假肥大型肌营养不良小鼠体内,均可见脂肪干细胞分化为骨骼肌细胞[6]。但Vieira等[7]发现,将脂肪干细胞与假肥大型肌营养不良患者的骨骼肌细胞共培养后,脂肪干细胞并非直接分化为肌细胞,而是与病变肌细胞融合,促进细胞再生和增殖。微环境对脂肪干细胞分化的诱导机制尚不清楚,可能与条件培养基中或周围肌细胞分泌的各种细胞因子相关[8]。 2.2.4 成肌调节因子 主要包括MyoD、myf5、myogenin与肌调节因子4(myogenic regulatory factors,MRF4,或称myf6),上述转录因子激活MHC,肌动蛋白和肌钙蛋白的转录[9]。其中MyoD与Myf5主要在脂肪干细胞分化为成肌细胞中起作用,而myogenin与MRF4位于MyoD的下游,在成肌细胞分化为成熟肌纤维和肌管中发挥功能。MyoD是骨骼肌细胞分化的开关基因,通过与靶基因的启动子区或增强子区的一致性保守序列E-box结合,激活肌肉特异性基因的转录,促进成肌分化[10]。敲除MyoD基因的小鼠没有肌肉形成,而敲除myogenin基因可产生正常数目的成肌细胞,但不能向肌细胞分化。此外,研究显示通过基因转染在鸡、鼠、人的成纤维细胞和脂肪细胞中高表达MyoD,可促使上述细胞转化为骨骼肌成肌细胞,并形成肌管[11]。 2.2.5 细胞因子 ①血管内皮生长因子(vascular endothelial growth factor, VEGF):阻断脂肪干细胞中血管内皮生长因子的表达,脂肪干细胞无法分化为肌细胞[12],其机制可能由于血管内皮生长因子通过促进血管新生进而促进肌肉再生[13];②肝细胞生长因子(hepatocyte growth factor, HGF):肝细胞生长因子能激活静止的卫星细胞,促进成肌细胞增殖和迁移,从而促进骨骼肌再生。肝细胞生长因子仅在骨骼肌卫星细胞及再生的肌纤维中表达,其表达量与肌肉的损伤程度成正比[14];③胰岛素样生长因子1(insulin-like growth factor, IGF-1):胰岛素样生长因子1可激活骨骼肌卫星细胞,促进成肌细胞增殖与分化,通过增加PI3K水平,抑制细胞凋亡和炎症反应,减少骨骼肌纤维化,促进损伤骨骼肌再生[14-15];④转化生长因子β(transforming growth factor, TGF-β):转化生长因子β通过重新编码肌肉细胞基因,抑制MyoD、myf5、myogenin等成肌调节因子表达[16],促进骨骼肌纤维化。脂肪干细胞能调控转化生长因子β表达,抑制骨骼肌纤维化;⑤成纤维细胞生长因子(fibroblast growth factor, FGF):成纤维细胞生长因子可降低肌肉纤维化程度。添加了成纤维细胞生长因子的培养液可促进胚胎干细胞分化为心肌细胞[17],但目前尚无研究证实成纤维细胞生长因子对脂肪干细胞向骨骼肌成肌细胞分化的影响;⑥表皮生长因子(epidermal growth factor,EGF):表皮生长因子可促进脂肪干细胞的增殖和分化,且与浓度和作用时间成正比;⑦骨形态发生蛋白(bone morphogenetic protein,BMP):骨形态发生蛋白2和骨形态发生蛋白4均可抑制脂肪干细胞的成肌分化,并促进其成骨分化。Shi等[18]发现抑制骨形态发生蛋白信号通路可促进肌纤维再生和修复;⑧白血病抑制因子(leukemia inhibitory factor,LIF):白血病抑制因子通过JAK2和STAT3信号通路调节卫星细胞和成肌细胞增殖,通过PI3K信号通路抑制成肌细胞凋亡,促进骨骼肌损伤修复[19]。 2.2.6 MicroRNA ①miR-23a:下调MHC的表达,抑制成肌分化;②miR-26a:肌肉损伤后miR-26a的表达升高,敲除miR-26a后会导致肌细胞修复延迟,提示miR-26a对成肌分化有促进作用;③miR-29:通过抑制Smad3的表达,调节组蛋白去乙酰化酶,抑制转化生长因子β,进而抑制骨骼肌纤维化[20];④miR-125b:肌肉损伤后miR-125b的表达下调,其下游靶基因IGF的表达上调,促进骨骼肌再生;⑤miR-128a:通过调节胰岛素受体底物抑制成肌细胞的增殖,抑制miR-128a的表达会促进肌管成熟;⑥miR-133:抑制血清反应因子表达,促进肌动蛋白的重构,促进卫星细胞增殖和成肌分化,同时抑制卫星细胞向脂肪细胞分化;⑦miR-148a:通过调节ROCK1基因,促进成肌分化[21];⑧miR-1和miR-206:miR-1和miR-206在成肌细胞分化过程中表达上调,通过notch信号通路,下调pax7、notch3和胰岛素样生长因子结合蛋白5的表达,同时在肌细胞融合过程中下调间隙连接蛋白的蛋白水平,促进卫星细胞的分化和融合[22]。Nakasa等[23]向刀片割伤的胫骨前肌中注射miR-1、miR-206和miR-133混合物,1周后MyoD和Myogenin的表达增加,血管生成增加,肌肉纤维化降低;⑨miR-214:通过下调原癌基因N-ras,使细胞退出有丝分裂,促进成肌分化[24];⑩miR-351:抑制细胞周期抑制子E2f3的表达,使细胞进入细胞周期从而促进细胞的增殖,抑制miR-351会导致成肌细胞增殖能力下降;?miR-486:在成肌分化过程中miR-486表达上调,通过下调靶基因pax7的表达,促进成肌分化。 2.2.7 长链非编码RNA Wang等[25]研究显示长链非编码RNA——Dum在骨骼肌成肌细胞的肌肉生成中被MyoD转录,通过招募Dnmt1、Dnmt3a和Dnmt3b,沉默Dppa2基因,诱导骨骼肌成肌细胞分化和受损肌细胞的再生。 2.3 利用脂肪干细胞修复骨骼肌损伤的研究进展 脂肪干细胞移植治疗急性心肌梗死和慢性心脏病的动物及临床试验开展较早,目前取得了较大的进展。Miyahara等[26]将脂肪干细胞制成细胞单层薄片移植到心肌梗死区域,部分脂肪干细胞分化为心肌细胞,改善了心脏功能。Zhang等[27]将脂肪干细胞与纤维蛋白胶结合后注射到心肌梗死大鼠的左心室壁,发现心脏功能有明显提高。临床试验也显示脂肪干细胞可有效治疗急性心肌梗死[28],增加心脏泵血能力,减少瘢痕面积。同样,脂肪干细胞可通过直接分化为骨骼肌细胞,或旁分泌各种细胞因子[29],调控炎症反应,抑制细胞凋亡[30],保护受损骨骼肌细胞[31],促进血管形成,招募内源性干细胞,增加骨骼肌质量和再生肌纤维面积,提高肌纤维收缩强度,减少骨骼肌纤维化,促进骨骼肌功能和形态的恢复。Liu等[32]发现移植至假肥大型肌营养不良小鼠骨骼肌的脂肪干细胞可促进抗肌萎缩蛋白的表达,改善肌肉萎缩状况。Pinheiro等[33]和Winkler等[34]研究显示脂肪干细胞移植可显著增加损伤骨骼肌的收缩力量、抗疲劳能力、肌纤维横断面积、肌纤维数量和肌细胞生成素的含量,促进萎缩骨骼肌再生。 单纯的细胞疗法不能完全修复较大的结构性肌肉缺损,因此将脂肪干细胞与左旋聚乳酸、聚乙二醇、聚羟基乙酸、乙醇酸乙酯、聚己内酯等合成的共聚物支架或脱细胞的肌肉组织支架结合,构建组织工程肌肉是目前的研究热点。尤其是由胶原蛋白、层粘连蛋白、黏多糖及各种生长因子组成的脱细胞肌肉组织支架,保留了肌肉组织原有的三维结构,具有较强的力学性能,为脂肪干细胞的附着、增殖和分化提供了有力的条件(图1,2)[35]。课题组曾对鼠、狗、猪、人等肌肉组织进行脱细胞处理,现将步骤概括如下:将肌肉标本冷冻于-80 ℃后室温融化,重复3次;超纯水漂洗2 d,0.5 mol/L和1 mol/L盐水各漂洗4 h,超纯水过夜漂洗,重复1次。37 ℃下用0.25%胰蛋白酶+EDTA处理2 h,去离子水漂洗1 h,用1%的Triton X-100浸泡5 d,每天换液,然后用DNA酶处理3 h,再用超纯水漂洗2 d,PBS漂洗1 d,4 ℃下储存待用。组织工程的应用局限性在于复合物的早期血管化程度,如何在移植早期建立血运,成为限制组织工程临床应用的瓶颈[36]。"
| [1] Mizuno H, Zuk PA, Zhu M, et al. Myogenic differentiation by human processed lipoaspirate cells. Plast Reconstr Surg. 2002;109(1):199-209.[2] Antonitsis P, Ioannidou-Papagiannaki E, Kaidoglou A, et al. In vitro cardiomyogenic differentiation of adult human bone marrow mesenchymal stem cells. The role of 5-azacytidine. Interact Cardiovasc Thorac Surg. 2007;6(5):593-597.[3] Apdik H, Do?an A, Demirci S, et al. Dose-dependent Effect of Boric Acid on Myogenic Differentiation of Human Adipose-derived Stem Cells (hADSCs). Biol Trace Elem Res. 2015;165(2):123-130.[4] Bayati V, Sadeghi Y, Shokrgozar MA, et al. The evaluation of cyclic uniaxial strain on myogenic differentiation of adipose-derived stem cells. Tissue Cell. 2011;43(6):359-366.[5] Stern-Straeter J, Bonaterra GA, Juritz S, et al. Evaluation of the effects of different culture media on the myogenic differentiation potential of adipose tissue- or bone marrow-derived human mesenchymal stem cells. Int J Mol Med. 2014;33(1):160-170.[6] Lee EM, Kim AY, Lee EJ, et al. Therapeutic effects of mouse adipose-derived stem cells and losartan in the skeletal muscle of injured mdx mice. Cell Transplant. 2015;24(5):939-53.[7] Vieira NM, Brandalise V, Zucconi E, et al. Human multipotent adipose-derived stem cells restore dystrophin expression of Duchenne skeletal-muscle cells in vitro. Biol Cell. 2008; 100(4):231-241.[8] 陈犹白,陈聪慧,Qixu Zhang,等.脂肪干细胞表型和标记物的研究进展[J].中国美容医学,2016,25(3):91-100.[9] Montarras D, Chelly J, Bober E, et al. Developmental patterns in the expression of Myf5, MyoD, myogenin, and MRF4 during myogenesis. New Biol. 1991;3(6):592-600.[10] Sung MS, Mun JY, Kwon O, et al. Efficient myogenic differentiation of human adipose-derived stem cells by the transduction of engineered MyoD protein. Biochem Biophys Res Commun. 2013;437(1):156-161.[11] Schenborn ET, Berg LD. Enhanced expression of transfected genes by MyoD. Molecular Biology of the Cell. 2000; 11:14A-15A.[12] Kim MH, Hong HN, Hong JP, et al. The effect of VEGF on the myogenic differentiation of adipose tissue derived stem cells within thermosensitive hydrogel matrices. Biomaterials. 2010; 31(6):1213-1218.[13] 陈犹白,张启旭, Butler CE,等.DOTAP脂质体介导VEGF基因转染人脂肪干细胞及目的基因的表达[J].临床耳鼻咽喉头颈外科, 2016,30(12):966-971.[14] Sumino Y, Hanada M, Hirata Y, et al. The effects of hepatocyte growth factor and insulin-like growth factor-1 on the myogenic differentiation of satellite cells in human urethral rhabdosphincter. Neurourol Urodyn. 2010; 29(3): 470-475.[15] Prelle K, Wobus AM, Krebs O, et al. Overexpression of insulin-like growth factor-II in mouse embryonic stem cells promotes myogenic differentiation. Biochem Biophys Res Commun. 2000;277(3):631-638.[16] Bourlier V, Sengenès C, Zakaroff-Girard A, et al. TGFbeta family members are key mediators in the induction of myofibroblast phenotype of human adipose tissue progenitor cells by macrophages. PLoS One. 2012;7(2): e31274.[17] Kawai T, Takahashi T, Esaki M, et al. Efficient cardiomyogenic differentiation of embryonic stem cell by fibroblast growth factor 2 and bone morphogenetic protein 2. Circ J. 2004;68(7):691-702.[18] Shi S, Hoogaars WM, de Gorter DJ, et al. BMP antagonists enhance myogenic differentiation and ameliorate the dystrophic phenotype in a DMD mouse model. Neurobiol Dis. 2011;41(2):353-360.[19] Diao Y, Wang X, Wu Z. SOCS1, SOCS3, and PIAS1 promote myogenic differentiation by inhibiting the leukemia inhibitory factor-induced JAK1/STAT1/STAT3 pathway. Mol Cell Biol. 2009;29(18):5084-5093.[20] Winbanks CE, Wang B, Beyer C, et al. TGF-beta regulates miR-206 and miR-29 to control myogenic differentiation through regulation of HDAC4. J Biol Chem. 2011;286(16): 13805-13814. [21] Zhang J, Ying ZZ, Tang ZL, et al. MicroRNA-148a promotes myogenic differentiation by targeting the ROCK1 gene. J Biol Chem. 2012;287(25):21093-21101.[22] Nakajima N, Takahashi T, Kitamura R, et al. MicroRNA-1 facilitates skeletal myogenic differentiation without affecting osteoblastic and adipogenic differentiation. Biochem Biophys Res Commun. 2006;350(4):1006-1012.[23] Nakasa T, Ishikawa M, Shi M, et al. Acceleration of muscle regeneration by local injection of muscle-specific microRNAs in rat skeletal muscle injury model. J Cell Mol Med. 2010; 14(10):2495-2505.[24] Liu J, Luo XJ, Xiong AW, et al. MicroRNA-214 promotes myogenic differentiation by facilitating exit from mitosis via down-regulation of proto-oncogene N-ras. J Biol Chem. 2010;285(34):26599-26607.[25] Wang L, Zhao Y, Bao X, et al. LncRNA Dum interacts with Dnmts to regulate Dppa2 expression during myogenic differentiation and muscle regeneration. Cell Res. 2015; 25(3):335-350.[26] Miyahara Y, Nagaya N, Kataoka M, et al. Monolayered mesenchymal stem cells repair scarred myocardium after myocardial infarction. Nat Med. 2006;12(4):459-465.[27] Zhang X, Wang H, Ma X, et al. Preservation of the cardiac function in infarcted rat hearts by the transplantation of adipose-derived stem cells with injectable fibrin scaffolds. Exp Biol Med (Maywood). 2010;235(12):1505-1515.[28] Beitnes JO, Lunde K, Brinchmann JE, et al. Stem cells for cardiac repair in acute myocardial infarction. Expert Rev Cardiovasc Ther. 2011;9(8):1015-1025.[29] 陈犹白,陈聪慧,Qixu Zhang,等.脂肪干细胞成脂分化的研究进展[J].中国美容医学,2016,25(4):86-93.[30] 陈犹白,陈聪慧,Qixu Zhang,等.脂肪干细胞成骨分化的研究进展[J/CD].中华损伤与修复杂志:电子版,2016,11(2):126-134.[31] 陈犹白,郝永红,王岚,等.脂肪干细胞成脂分化的分子机制和信号通路[J].中国组织工程研究,2017,21(1):154-158.[32] Liu Y, Yan X, Sun Z, et al. Flk-1+ adipose-derived mesenchymal stem cells differentiate into skeletal muscle satellite cells and ameliorate muscular dystrophy in mdx mice. Stem Cells Dev. 2007;16(5):695-706.[33] Pinheiro CH, de Queiroz JC, Guimarães-Ferreira L, et al. Local injections of adipose-derived mesenchymal stem cells modulate inflammation and increase angiogenesis ameliorating the dystrophic phenotype in dystrophin-deficient skeletal muscle. Stem Cell Rev. 2012;8(2):363-374.[34] Winkler T, von Roth P, Radojewski P, et al. Immediate and delayed transplantation of mesenchymal stem cells improve muscle force after skeletal muscle injury in rats. J Tissue Eng Regen Med. 2012;6 Suppl 3:s60-67.[35] Wang L, Johnson JA, Chang DW, et al. Decellularized musculofascial extracellular matrix for tissue engineering. Biomaterials. 2013;34(11):2641-2654.[36] Zhang Q, Johnson JA, Dunne LW, et al. Decellularized skin/adipose tissue flap matrix for engineering vascularized composite soft tissue flaps. Acta Biomater. 2016;35:166-184.[37] 陈犹白,陈聪慧,Qixu Zhang,等.脂肪干细胞的分离、纯化和保存:研究进展和发展方向[J].中国组织工程研究,2016, 20(10): 1508-1520.[38] Muehlberg FL, Song YH, Krohn A, et al. Tissue-resident stem cells promote breast cancer growth and metastasis. Carcinogenesis. 2009;30(4):589-597.[39] Yu JM, Jun ES, Bae YC, et al. Mesenchymal stem cells derived from human adipose tissues favor tumor cell growth in vivo. Stem Cells Dev. 2008;17(3):463-473.[40] Galiè M, Konstantinidou G, Peroni D, et al. Mesenchymal stem cells share molecular signature with mesenchymal tumor cells and favor early tumor growth in syngeneic mice. Oncogene. 2008;27(18):2542-2551.[41] Kucerova L, Matuskova M, Hlubinova K, et al. Tumor cell behaviour modulation by mesenchymal stromal cells. Mol Cancer. 2010;9:129.[42] Prantl L, Muehlberg F, Navone NM, et al. Adipose tissue-derived stem cells promote prostate tumor growth. Prostate. 2010;70(15):1709-1715.[43] Eterno V, Zambelli A, Pavesi L, et al. Adipose-derived Mesenchymal Stem Cells (ASCs) may favour breast cancer recurrence via HGF/c-Met signaling. Oncotarget. 2014; 5(3):613-633.[44] Zhu Y, Sun Z, Han Q, et al. Human mesenchymal stem cells inhibit cancer cell proliferation by secreting DKK-1. Leukemia. 2009;23(5):925-933.[45] Cousin B, Ravet E, Poglio S, et al. Adult stromal cells derived from human adipose tissue provoke pancreatic cancer cell death both in vitro and in vivo. PLoS One. 2009;4(7):e6278.[46] Donahue HJ, Saunders MM, Li Z, et al. A potential role for gap junctions in breast cancer metastasis to bone. J Musculoskelet Neuronal Interact. 2003;3(2):156-161.[47] Bielli A, Scioli MG, Gentile P, et al. Adult adipose-derived stem cells and breast cancer: a controversial relationship. Springerplus. 2014;3:345.[48] Delay E, Garson S, Tousson G, et al. Fat injection to the breast: technique, results, and indications based on 880 procedures over 10 years. Aesthet Surg J. 2009;29(5): 360-376.[49] Petit JY, Botteri E, Lohsiriwat V, et al. Locoregional recurrence risk after lipofilling in breast cancer patients. Ann Oncol. 2012;23(3):582-588.[50] Rietjens M, De Lorenzi F, Rossetto F, et al. Safety of fat grafting in secondary breast reconstruction after cancer. J Plast Reconstr Aesthet Surg. 2011;64(4):477-483. |
| [1] | Pu Rui, Chen Ziyang, Yuan Lingyan. Characteristics and effects of exosomes from different cell sources in cardioprotection [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(在线): 1-. |
| [2] | Lin Qingfan, Xie Yixin, Chen Wanqing, Ye Zhenzhong, Chen Youfang. Human placenta-derived mesenchymal stem cell conditioned medium can upregulate BeWo cell viability and zonula occludens expression under hypoxia [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(在线): 4970-4975. |
| [3] | Zhang Tongtong, Wang Zhonghua, Wen Jie, Song Yuxin, Liu Lin. Application of three-dimensional printing model in surgical resection and reconstruction of cervical tumor [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1335-1339. |
| [4] | Zhang Xiumei, Zhai Yunkai, Zhao Jie, Zhao Meng. Research hotspots of organoid models in recent 10 years: a search in domestic and foreign databases [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(8): 1249-1255. |
| [5] | Hou Jingying, Yu Menglei, Guo Tianzhu, Long Huibao, Wu Hao. Hypoxia preconditioning promotes bone marrow mesenchymal stem cells survival and vascularization through the activation of HIF-1α/MALAT1/VEGFA pathway [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 985-990. |
| [6] | Shi Yangyang, Qin Yingfei, Wu Fuling, He Xiao, Zhang Xuejing. Pretreatment of placental mesenchymal stem cells to prevent bronchiolitis in mice [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 991-995. |
| [7] | Liang Xueqi, Guo Lijiao, Chen Hejie, Wu Jie, Sun Yaqi, Xing Zhikun, Zou Hailiang, Chen Xueling, Wu Xiangwei. Alveolar echinococcosis protoscolices inhibits the differentiation of bone marrow mesenchymal stem cells into fibroblasts [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 996-1001. |
| [8] | Fan Quanbao, Luo Huina, Wang Bingyun, Chen Shengfeng, Cui Lianxu, Jiang Wenkang, Zhao Mingming, Wang Jingjing, Luo Dongzhang, Chen Zhisheng, Bai Yinshan, Liu Canying, Zhang Hui. Biological characteristics of canine adipose-derived mesenchymal stem cells cultured in hypoxia [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1002-1007. |
| [9] | Geng Yao, Yin Zhiliang, Li Xingping, Xiao Dongqin, Hou Weiguang. Role of hsa-miRNA-223-3p in regulating osteogenic differentiation of human bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1008-1013. |
| [10] | Lun Zhigang, Jin Jing, Wang Tianyan, Li Aimin. Effect of peroxiredoxin 6 on proliferation and differentiation of bone marrow mesenchymal stem cells into neural lineage in vitro [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1014-1018. |
| [11] | Zhu Xuefen, Huang Cheng, Ding Jian, Dai Yongping, Liu Yuanbing, Le Lixiang, Wang Liangliang, Yang Jiandong. Mechanism of bone marrow mesenchymal stem cells differentiation into functional neurons induced by glial cell line derived neurotrophic factor [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1019-1025. |
| [12] | Duan Liyun, Cao Xiaocang. Human placenta mesenchymal stem cells-derived extracellular vesicles regulate collagen deposition in intestinal mucosa of mice with colitis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1026-1031. |
| [13] | Pei Lili, Sun Guicai, Wang Di. Salvianolic acid B inhibits oxidative damage of bone marrow mesenchymal stem cells and promotes differentiation into cardiomyocytes [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1032-1036. |
| [14] | Guan Qian, Luan Zuo, Ye Dou, Yang Yinxiang, Wang Zhaoyan, Wang Qian, Yao Ruiqin. Morphological changes in human oligodendrocyte progenitor cells during passage [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1045-1049. |
| [15] | Wang Zhengdong, Huang Na, Chen Jingxian, Zheng Zuobing, Hu Xinyu, Li Mei, Su Xiao, Su Xuesen, Yan Nan. Inhibitory effects of sodium butyrate on microglial activation and expression of inflammatory factors induced by fluorosis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1075-1080. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||