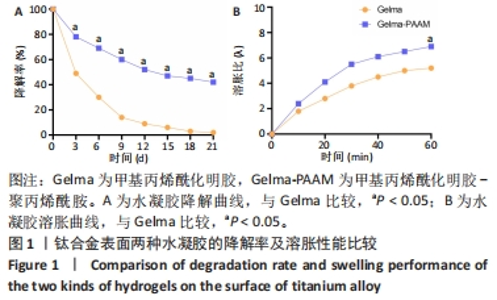
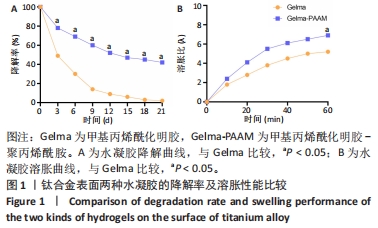
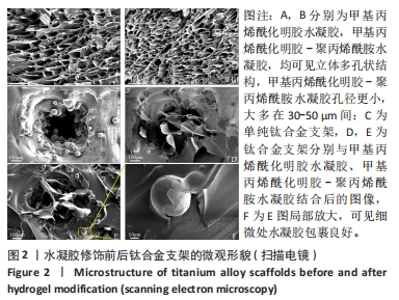
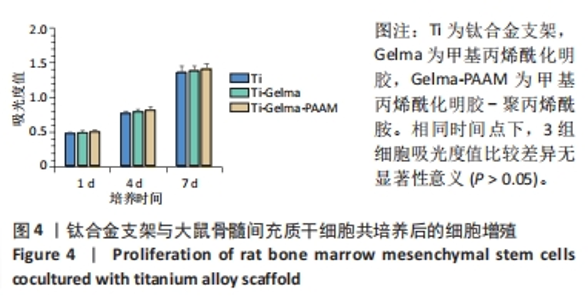
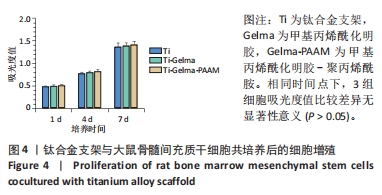
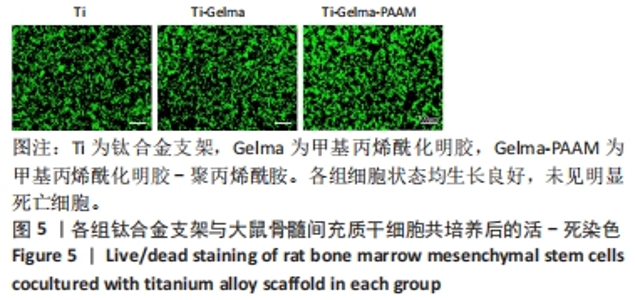
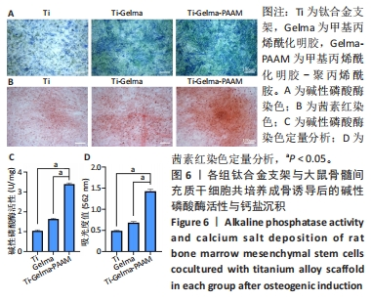
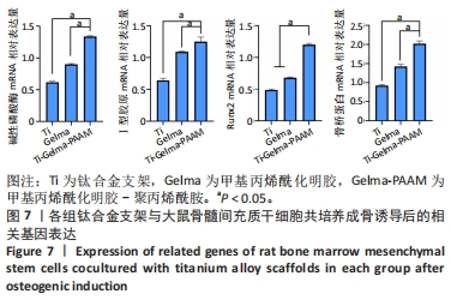
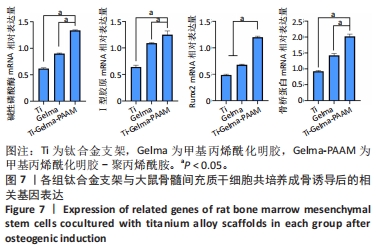
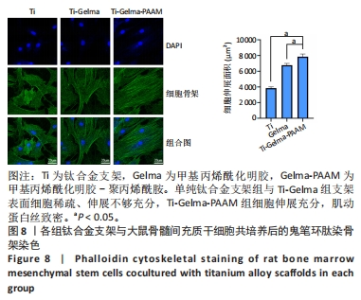
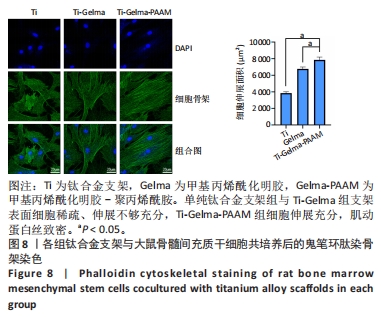
Chinese Journal of Tissue Engineering Research ›› 2024, Vol. 28 ›› Issue (22): 3472-3477.doi: 10.12307/2024.563
Previous Articles Next Articles
Osteogenic properties of polyacrylamide-modified gelatin methacryloyl grafted titanium alloy scaffold
Chu Fuchao1, Wang Zhenxin1, Zhang Dazhen1, Yuan Feng2
- 1Xuzhou Medical University, Xuzhou 221004, Jiangsu Province, China; 2Department of Orthopedics, Affiliated Hospital of Xuzhou Medical University, Key Laboratory of Bone Tissue Regeneration and Digital Medicine, Xuzhou 221006, Jiangsu Province, China
-
Received:2023-10-11Accepted:2023-11-27Online:2024-08-08Published:2024-01-20 -
Contact:Yuan Feng, Chief physician, Professor, Doctoral supervisor, Department of Orthopedics, Affiliated Hospital of Xuzhou Medical University, Key Laboratory of Bone Tissue Regeneration and Digital Medicine, Xuzhou 221006, Jiangsu Province, China -
About author:Chu Fuchao, Master candidate, Xuzhou Medical University, Xuzhou 221004, Jiangsu Province, China -
Supported by:Key Scientific Research Project of Jiangsu Provincial Health Commission, No. ZD2022064 (to YF); Jiangsu Provincial Social Development-Clinical Frontier Technology Project, No. BE2022708 (to YF)
CLC Number:
Cite this article
Chu Fuchao, Wang Zhenxin, Zhang Dazhen, Yuan Feng. Osteogenic properties of polyacrylamide-modified gelatin methacryloyl grafted titanium alloy scaffold[J]. Chinese Journal of Tissue Engineering Research, 2024, 28(22): 3472-3477.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
| [1] ZHANG LC, CHEN LY. A Review on Biomedical Titanium Alloys: Recent Progress and Prospect. Adv Eng Mater. 2019;21(4):1801215. [2] CHEN LY, CUI YW, ZHANG LC. Recent Development in Beta Titanium Alloys for Biomedical Applications. Metals. 2020;10(9):1139. [3] TAKIZAWA T, NAKAYAMA N, HANIU H, et al. Titanium Fiber Plates for Bone Tissue Repair. Adv Mater. 2018;30(4):1703608. [4] LIU X, CHEN S, TSOI JKH, et al. Binary titanium alloys as dental implant materials-a review. Regen Biomater. 2017;4(5):315. [5] TORSTRICK FB, LIN ASP, POTR D, et al. Porous PEEK improves the bontee-implant interface compared to plasma-sprayed titanium coating on PEEK. Biomaterials. 2018;185:106-116. [6] RUBSHTEIN AP, TRAKHTENBERG I, MAKAROVA EB, et al. Porous material based on spongy titanium granules: structure, mechanical properties, and osseointegration. Mater Sci Eng C Mater Biol Appl. 2014;35:363-369. [7] CHEN J, ZHANG X, HUANG C, et al. Osteogenic activity and antibacterial effect of porous titanium modified with metal-organic framework films. Journal of biomedical materials research Part A. 2017;105(3):834-846. [8] KIM J, KIM HN, LIM KT, et al. Synergistic effects of nanotopography and co-culture with endothelial cells on osteogenesis of mesenchymal stem cells. Biomaterials. 2013;34(30):7257-7268. [9] LLOPIS-GRIMALT MA, ARBOS A, GIL-MIR M, et al. Multifunctional Properties of Quercitrin-Coated Porous Ti-6Al-4V Implants for Orthopaedic Applications Assessed In Vitro. J Clin Med. 2020;9(3):855. [10] SMIRNOV IV, DEEV RV, BOZO II, et al. Octacalcium phosphate coating for 3D printed cranioplastic porous titanium implants. Surf Coat Technol. 2020; 383:125192. [11] ZHAO Z, GAO W, BAI H. A mineral layer as an effective binder to achieve strong bonding between a hydrogel and a solid titanium substrate. J Mater Chem B. 2018;6(23):3859-3864. [12] GARCIA GARCIA A, HÉBRAUD A, DUVAL JL, et al. Poly(ε-caprolactone)/Hydroxyapatite 3D Honeycomb Scaffolds for a Cellular Microenvironment Adapted to Maxillofacial Bone Reconstruction. ACS Biomater Sci Eng. 2018; 4(9):3317-3326. [13] SUN Y, ZHANG X, LUO M, et al. Plasma Spray vs. Electrochemical Deposition: Toward a Better Osteogenic Effect of Hydroxyapatite Coatings on 3D-Printed Titanium Scaffolds. Front Bioeng Biotechnol. 2021;9:727386. [14] XIE M, YU K, SUN Y, et al. Protocols of 3D Bioprinting of Gelatin Methacryloyl Hydrogel Based Bioinks. J Vis Exp. 2019;(154). doi: 10.3791/60545. [15] HERRERA-RUIZ A, TOVAR BB, GARCÍA RG, et al. Nanomaterials-Incorporated Chemically Modified Gelatin Methacryloyl-Based Biomedical Composites: A Novel Approach for Bone Tissue Engineering. Pharmaceutics. 2022;14(12): 2645. [16] YUE K, TRUJILLO-DE SANTIAGO G, ALVAREZ MM, et al. Synthesis, properties, and biomedical applications of gelatin methacryloyl (GelMA) hydrogels. Biomaterials. 2015;73:254-271. [17] MAMIDI N, IJADI F, NORAHAN MH. Leveraging the Recent Advancements in GelMA Scaffolds for Bone Tissue Engineering: An Assessment of Challenges and Opportunities. Biomacromolecules. 2023.doi: 10.1021/acs.biomac.3c00279. [18] ZHANG S, GUO Y, DONG Y, et al. A novel nanosilver/nanosilica hydrogel for bone regeneration in infected bone defects. ACS Appl Mater Interfaces. 2016;8(21):13242-13250. [19] TODA H, YAMAMOTO M, UYAMA H, et al. Fabrication of hydrogels with elasticity changed by alkaline phosphatase for stem cell culture. Acta Biomater. 2016;29:215-227. [20] HE M, HOU Y, ZHU C, et al. 3D-Printing Biodegradable PU/PAAM/Gel Hydrogel Scaffold with High Flexibility and Self-Adaptibility to Irregular Defects for Nonload-Bearing Bone Regeneration. Bioconjug Chem. 2021; 32(8):1915-1925. [21] Wang S, Zhao X, Hsu Y, et al. Surface modification of titanium implants with Mg-containing coatings to promote osseointegration. Acta biomaterialia. 2023;169:19-44. [22] SARRAF M, REZVANI GHOMI E, ALIPOUR S, et al. A state-of-the-art review of the fabrication and characteristics of titanium and its alloys for biomedical applications. Biodes Manuf. 2021;5(2):371-395. [23] SHAO H, MA M, WANG Q, et al. Advances in the superhydrophilicity-modified titanium surfaces with antibacterial and pro-osteogenesis properties: A review. Front Bioeng Biotechnol. 2022;10:1000401. [24] MENG F, YIN Z, REN X, et al. Construction of Local Drug Delivery System on Titanium-Based Implants to Improve Osseointegration. Pharmaceutics. 2022;14(5):1069. [25] LENG J, HE Y, YUAN Z, et al. Enzymatically-degradable hydrogel coatings on titanium for bacterial infection inhibition and enhanced soft tissue compatibility via a self-adaptive strategy. Bioactive materials. Bioact Mater. 2021;6(12):4670-4685. [26] ZHAO C, ZHOU L, CHIAO M, et al. Antibacterial hydrogel coating: Strategies in surface chemistry. Adv Colloid Interface Sci. 2020;285:102280. [27] YANG Z, YANG Z, DING L, et al. Self-Adhesive Hydrogel Biomimetic Periosteum to Promote Critical-Size Bone Defect Repair via Synergistic Osteogenesis and Angiogenesis. ACS Appl Mater Interfaces. 2022;14(32): 36395-36410. [28] GENG X, QI Y, LIU X, et al. A multifunctional antibacterial and self-healing hydrogel laden with bone marrow mesenchymal stem cell-derived exosomes for accelerating diabetic wound healing. Biomater Adv. 2022;133:112613. [29] ECHAVE MC, EREZUMA I, GOLAFSHAN N, et al. Bioinspired gelatin/bioceramic composites loaded with bone morphogenetic protein-2 (BMP-2) promote osteoporotic bone repair. Biomaterials advances. 2022;134: 112539. [30] WU X, LIU S, CHEN K, et al. 3D printed chitosan-gelatine hydrogel coating on titanium alloy surface as biological fixation interface of artificial joint prosthesis. Int J Biol Macromol. 2021;182:669-679. [31] LI Y, LIU Y, BAI H, et al. Sustained Release of VEGF to Promote Angiogenesis and Osteointegration of Three-Dimensional Printed Biomimetic Titanium Alloy Implants. Front Bioeng Biotechnol. 2021;9:757767. [32] ZHENG J, WANG Y, WANG Y, et al. Gelatin/Hyaluronic Acid Photocrosslinked Double Network Hydrogel with Nano-Hydroxyapatite Composite for Potential Application in Bone Repair. Gels. 2023;9(9):742. [33] GUO B, LIANG Y, DONG R. Physical dynamic double-network hydrogels as dressings to facilitate tissue repair. Nat Protoc. 2023;18(11):3322-3354. [34] ZHANG Z, HU Y, MA H, et al. MXene/Gelatin/Polyacrylamide Nanocomposite Double Network Hydrogel with Improved Mechanical and Photothermal Properties. Polymers. 2022;14(23):5247. [35] CHEN H, DING Z, YAN D, et al. Double-network composites based on inorganic fillers reinforced dextran-based hydrogel with high strength. Carbohydr Polym. 2022;296:119900. [36] DONG W, MA W, ZHAO S, et al. The surface modification of long carbon fiber reinforced polyether ether ketone with bioactive composite hydrogel for effective osteogenicity. Mater Sci Eng C Mater Biol Appl. 2021;130:112451. [37] HE M, HOU Y, ZHU C, et al. 3D-Printing Biodegradable PU/PAAM/Gel Hydrogel Scaffold with High Flexibility and Self-Adaptibility to Irregular Defects for Nonload-Bearing Bone Regeneration. Bioconjug Chem. 2021; 32(8):1915-1925. [38] XUE X, ZHANG H, LIU H, et al. Rational Design of Multifunctional CuS Nanoparticle‐PEG Composite Soft Hydrogel‐Coated 3D Hard Polycaprolactone Scaffolds for Efficient Bone Regeneration. Adv Funct Mater. 2022;32(33):2202470. [39] ZHANG J, TONG D, SONG H, et al. Osteoimmunity‐Regulating Biomimetically Hierarchical Scaffold for Augmented Bone Regeneration. Adv Mater. 2022; 34(36):e2202044. [40] BERNAR A, GEBETSBERGER JV, BAUER M, et al. Optimization of the Alizarin Red S Assay by Enhancing Mineralization of Osteoblasts. Int J Mol Sci. 2022; 24(1):723. |
| [1] | Liu Zhaohui, Han Xiaoqian, Duan Xin, Guo Pengda, Zhang Yuntao. Salidroside promotes osteogenic differentiation of MC3T3-E1 cells: an in vitro experiment [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(2): 231-237. |
| [2] | Zhou Shijie, Li Muzhe, Yun Li, Zhang Tianchi, Niu Yuanyuan, Zhu Yihua, Zhou Qinfeng, Guo Yang, Ma Yong, Wang Lining. Effect of Wenshen Tongluo Zhitong formula on mouse H-type bone microvascular endothelial cell/bone marrow mesenchymal stem cell co-culture system [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(1): 8-15. |
| [3] | Zheng Qian, Liu Pingping, Gu Yujie, Xie Lei. Effect of ursolic acid on osteogenic differentiation of human periodontal ligament stem cells#br# [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(1): 80-86. |
| [4] | Yang Yufang, Yang Zhishan, Duan Mianmian, Liu Yiheng, Tang Zhenglong, Wang Yu. Application and prospects of erythropoietin in bone tissue engineering [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(9): 1443-1449. |
| [5] | Dai Yuexing, Zheng Liqin, Wu Minhui, Li Zhihong, Li Shaobin, Zheng Desheng, Lin Ziling. Effect of vessel number on computational fluid dynamics in vascular networks [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(8): 1206-1210. |
| [6] | Wang Shanshan, Shu Qing, Tian Jun. Physical factors promote osteogenic differentiation of stem cells [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(7): 1083-1090. |
| [7] | Wang Wu, Fan Xiaolei, Xie Jie, Hu Yihe, Zeng Min. Hydroxyapatite-polyvinyl alcohol/collagen-chitosan-gelatin composite hydrogel for repairing rabbit osteochondral defect [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(5): 682-689. |
| [8] | Zhang Ya, Mu Qiuju, Wang Zilin, Liu Hongjie, Zhu Lili. Hydrogel loaded with platelet-rich plasma promotes wound healing in diabetic rats [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(5): 690-696. |
| [9] | Shen Ziqing, Xia Tian, Shan Yibo, Zhu Ruijun, Wan Haoxin, Ding Hao, Pan Shu, Zhao Jun. Vascularized tracheal substitutes constructed by exosome-load hydrogel-modified 3D printed scaffolds [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(5): 697-705. |
| [10] | Lan Weiwei, Yu Yaodong, Huang Di, Chen Weiyi. In vitro degradation behavior of Mg-Zn-Ca alloys [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(5): 717-723. |
| [11] | Tian Xin, Liu Tao, Yang Huilin, He Fan. In vitro evaluation of sustained release Kartogenin by gelatin methacryloyl microspheres for repairing nucleus pulposus degeneration [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(5): 724-730. |
| [12] | Zhou Xiaowen, Fu Zuchang, Huang Fei, Ai Jianguo, Zhao Feng. Bone defect blocked by bone cement segmental filling in single-plane tibial bone transport [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(5): 736-740. |
| [13] | Zhu Liwei, Wang Jiangyue, Bai Ding. Application value of nanocomposite gelatin methacryloyl hydrogels in different bone defect environments [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(5): 753-758. |
| [14] | Chen Xiaofang, Zheng Guoshuang, Li Maoyuan, Yu Weiting. Preparation and application of injectable sodium alginate hydrogels [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(5): 789-794. |
| [15] | Yang Yuqing, Chen Zhiyu. Role and application of early transient presence of M1 macrophages in bone tissue engineering [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(4): 594-601. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||