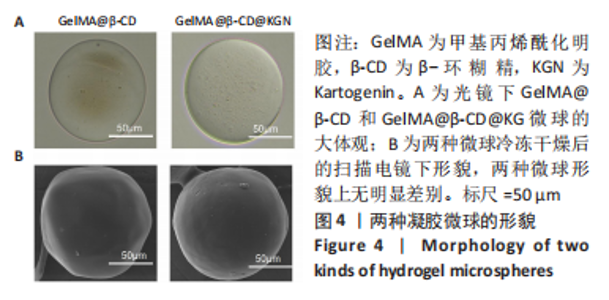
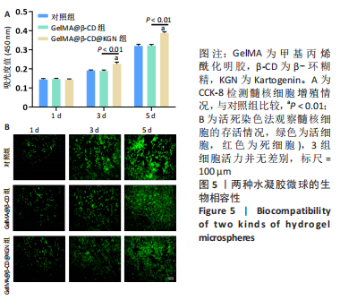
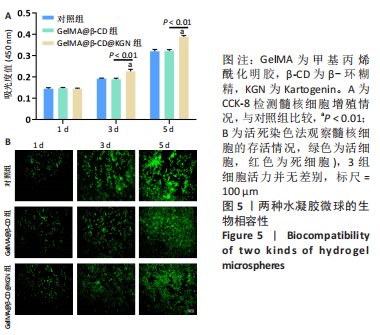
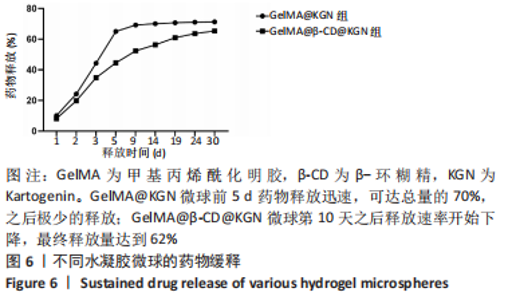
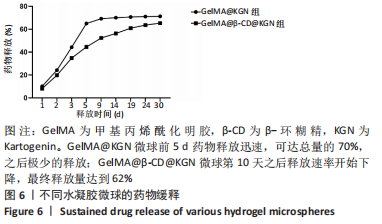
Chinese Journal of Tissue Engineering Research ›› 2024, Vol. 28 ›› Issue (5): 724-730.doi: 10.12307/2024.247
Previous Articles Next Articles
In vitro evaluation of sustained release Kartogenin by gelatin methacryloyl microspheres for repairing nucleus pulposus degeneration
Tian Xin1, 2, Liu Tao1, Yang Huilin1, 2, He Fan1, 2
- 1Department of Orthopedics, First Affiliated Hospital of Soochow University, Suzhou 215006, Jiangsu Province, China; 2Institute of Orthopedics at Soochow University, Suzhou 215007, Jiangsu Province, China
-
Received:2022-12-09Accepted:2023-02-08Online:2024-02-18Published:2023-08-16 -
Contact:He Fan, PhD, Researcher, Doctoral supervisor, Department of Orthopedics, First Affiliated Hospital of Soochow University, Suzhou 215006, Jiangsu Province, China; Institute of Orthopedics at Soochow University, Suzhou 215007, Jiangsu Province, China -
About author:Tian Xin, Master candidate, Department of Orthopedics, First Affiliated Hospital of Soochow University, Suzhou 215006, Jiangsu Province, China; Institute of Orthopedics at Soochow University, Suzhou 215007, Jiangsu Province, China -
Supported by:Natural Science Foundation of Jiangsu Province, No. BK20220046 (to HF)
CLC Number:
Cite this article
Tian Xin, Liu Tao, Yang Huilin, He Fan. In vitro evaluation of sustained release Kartogenin by gelatin methacryloyl microspheres for repairing nucleus pulposus degeneration[J]. Chinese Journal of Tissue Engineering Research, 2024, 28(5): 724-730.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
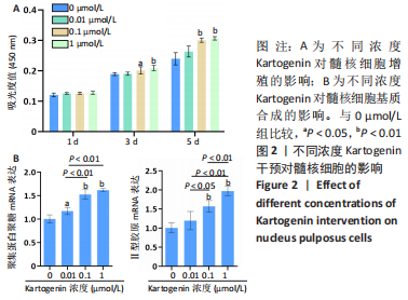
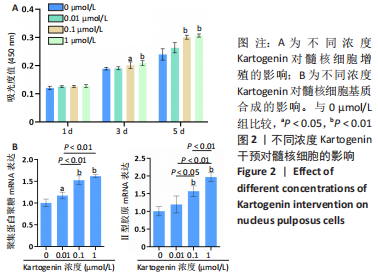
2.2 KGN干预对髓核细胞的影响 CCK-8检测显示,培养第1天,4组细胞增殖吸光度值比较差异无显著性意义(P > 0.05);培养第3天,与0 μmol/L组比较,0.1 μmol/L和1 μmol/L组细胞增殖吸光度值升高(P < 0.05,P < 0.01);培养第5天,与0 μmol/L组比较,0.1 μmol/L和1 μmol/L组细胞增殖吸光度值升高(P < 0.01),见图2A。 qRT-PCR检测显示,与0 μmol/L组比较,0.01 μmol/L组聚集蛋白聚糖的mRNA表达上调17%(P < 0.05),0.1 μmol/L组聚集蛋白聚糖、Ⅱ型胶原的mRNA表达分别上调53%和57%(P < 0.01),1 μmol/L组聚集蛋白聚糖、Ⅱ型胶原的mRNA表达分别上调62%和97%(P < 0.01),见图2B。"
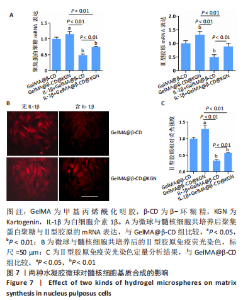
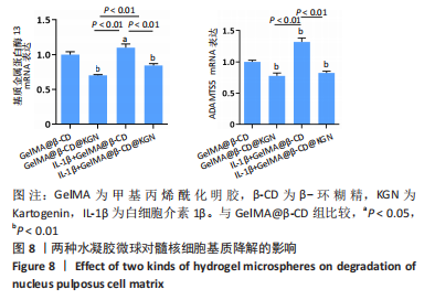
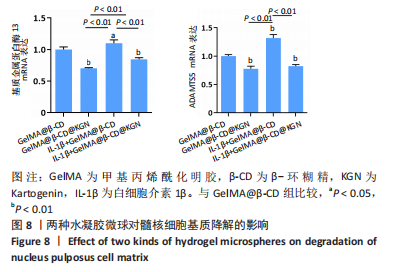
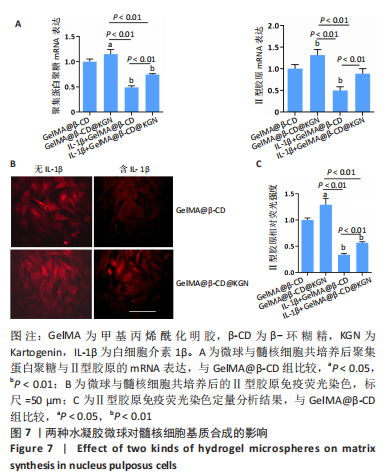
2.7 水凝胶微球对髓核细胞基质合成的影响 qRT-PCR检测显示,与GelMA@β-CD组相比,GelMA@β-CD@KGN组聚集蛋白聚糖与Ⅱ型胶原的mRNA表达分别上调了15%,32%(P < 0.05,P < 0.01),白细胞介素1β+GelMA@β-CD组聚集蛋白聚糖与Ⅱ型胶原的mRNA表达分别下调了51%,50%(P < 0.01),白细胞介素1β+GelMA@β-CD@KGN组聚集蛋白聚糖与Ⅱ型胶原的mRNA表达分别下调了25%,11%(P < 0.01),见图7A。 免疫荧光染色显示,与GelMA@β-CD组相比,GelMA@β-CD@KGN组Ⅱ型胶原荧光信号增强,白细胞介素1β+GelMA@β-CD组、白细胞介素1β+GelMA@β-CD@KGN组Ⅱ型胶原荧光信号减弱,白细胞介素1β+GelMA@β-CD@KGN组Ⅱ型胶原荧光信号强于白细胞介素1β+GelMA@β-CD微球组,见图7B、C。"
| [1] DEYO RA, WEINSTEIN JN. Low back pain. N Engl J Med. 2001;344(5):363-370. [2] HARTVIGSEN J, HANCOCK MJ, KONGSTED A, et al. What low back pain is and why we need to pay attention. Lancet. 2018;391(10137):2356-2367. [3] FRANCISCO V, PINO J, GONZÁLEZ-GAY MÁ, et al. A new immunometabolic perspective of intervertebral disc degeneration. Nat Rev Rheumatol. 2022; 18(1):47-60. [4] ANTONIOU J, STEFFEN T, NELSON F, et al. The human lumbar intervertebral disc: evidence for changes in the biosynthesis and denaturation of the extracellular matrix with growth, maturation, ageing, and degeneration. J Clin Invest. 1996;98(4):996-1003. [5] BINCH ALA, RATCLIFFE LPD, MILANI AH, et al. Site-Directed Differentiation of Human Adipose-Derived Mesenchymal Stem Cells to Nucleus Pulposus Cells Using an Injectable Hydroxyl-Functional Diblock Copolymer Worm Gel. Biomacromolecules. 2021;22(2):837-845. [6] JOHNSON K, ZHU S, TREMBLAY MS, et al. A stem cell-based approach to cartilage repair. Science. 2012;336(6082):717-721. [7] YU C, LI D, WANG C, et al. Injectable kartogenin and apocynin loaded micelle enhances the alleviation of intervertebral disc degeneration by adipose-derived stem cell. Bioact Mater. 2021;6(10):3568-3579. [8] VAN DEN BULCKE AI, BOGDANOV B, DE ROOZE N, et al. Structural and rheological properties of methacrylamide modified gelatin hydrogels. Biomacromolecules. 2000;1(1):31-38. [9] DONG Z, MENG X, YANG W, et al. Progress of gelatin-based microspheres (GMSs) as delivery vehicles of drug and cell. Mater Sci Eng C Mater Biol Appl. 2021;122:111949. [10] XU J, LI J, LIN S, et al. Nanocarrier-mediated codelivery of small molecular drugs and siRNA to enhance chondrogenic differentiation and suppress hypertrophy of human mesenchymal stem cells. Adv Funct Mater. 2016;26: 2463-2472. [11] MURA P. Advantages of the combined use of cyclodextrins and nanocarriers in drug delivery: A review. Int J Pharm. 2020;579:119181. [12] XU J, FENG Q, LIN S, et al. Injectable stem cell-laden supramolecular hydrogels enhance in situ osteochondral regeneration via the sustained co-delivery of hydrophilic and hydrophobic chondrogenic molecules. Biomaterials. 2019;210:51-61. [13] YUE K, TRUJILLO-DE SANTIAGO G, ALVAREZ MM, et al. Synthesis, properties, and biomedical applications of gelatin methacryloyl (GelMA) hydrogels. Biomaterials. 2015;73:254-271. [14] XU X, LIANG Y, LI X, et al. Exosome-mediated delivery of kartogenin for chondrogenesis of synovial fluid-derived mesenchymal stem cells and cartilage regeneration. Biomaterials. 2021;269:120539. [15] FRAPIN L, CLOUET J, CHÉDEVILLE C, et al. Controlled release of biological factors for endogenous progenitor cell migration and intervertebral disc extracellular matrix remodelling. Biomaterials. 2020;253:120107. [16] ZHOU W, DUAN Z, ZHAO J, et al. Glucose and MMP-9 dual-responsive hydrogel with temperature sensitive self-adaptive shape and controlled drug release accelerates diabetic wound healing. Bioact Mater. 2022;17:1-17. [17] RIBEIRO JS, BORDINI EAF, FERREIRA JA, et al. Injectable MMP-Responsive Nanotube-Modified Gelatin Hydrogel for Dental Infection Ablation. ACS Appl Mater Interfaces. 2020;12(14):16006-16017. [18] CAI C, ZHANG X, LI Y, et al. Self-Healing Hydrogel Embodied with Macrophage-Regulation and Responsive-Gene-Silencing Properties for Synergistic Prevention of Peritendinous Adhesion. Adv Mater. 2022;34(5): e2106564. [19] CHANG H, CAI F, ZHANG Y, et al. Silencing Gene-Engineered Injectable Hydrogel Microsphere for Regulation of Extracellular Matrix Metabolism Balance. Small Methods. 2022;6(4):e2101201. [20] LI L, DU Y, XIONG Y, et al. Injectable negatively charged gelatin microsphere-based gels as hemostatic agents for intracavitary and deep wound bleeding in surgery. J Biomater Appl. 2018;33(5):647-661. [21] BIAN J, CAI F, CHEN H, et al. Modulation of Local Overactive Inflammation via Injectable Hydrogel Microspheres. Nano Lett. 2021;21(6):2690-2698. [22] YANG J, LIANG J, ZHU Y, et al. Fullerol-hydrogel microfluidic spheres for in situ redox regulation of stem cell fate and refractory bone healing. Bioact Mater. 2021;6(12):4801-4815. [23] ZHANG C, GULLBRAND SE, SCHAER TP, et al. Combined Hydrogel and Mesenchymal Stem Cell Therapy for Moderate-Severity Disc Degeneration in Goats. Tissue Eng Part A. 2021;27(1-2):117-128. [24] SAKAI D, ANDERSSON GB. Stem cell therapy for intervertebral disc regeneration: obstacles and solutions. Nat Rev Rheumatol. 2015;11(4):243-256. [25] MWALE F, ROUGHLEY P, ANTONIOU J. Distinction between the extracellular matrix of the nucleus pulposus and hyaline cartilage: a requisite for tissue engineering of intervertebral disc. Eur Cell Mater. 2004;8:58-63. [26] HUGHES SP, FREEMONT AJ, HUKINS DW, et al. The pathogenesis of degeneration of the intervertebral disc and emerging therapies in the management of back pain. J Bone Joint Surg Br. 2012;94(10):1298-1304. [27] LE MAITRE CL, POCKERT A, BUTTLE DJ, et al. Matrix synthesis and degradation in human intervertebral disc degeneration. Biochem Soc Trans. 2007;35(Pt 4):652-655. [28] LIU T, LI X, WANG T, et al. Kartogenin mediates cartilage regeneration by stimulating the IL-6/Stat3-dependent proliferation of cartilage stem/progenitor cells. Biochem Biophys Res Commun. 2020;532(3):385-392. [29] BAHARLOU HOUREH A, MASAELI E, NASR-ESFAHANI MH. Chitosan/polycaprolactone multilayer hydrogel: A sustained Kartogenin delivery model for cartilage regeneration. Int J Biol Macromol. 2021;177:589-600. [30] ZHANG M, HU W, CAI C, et al. Advanced application of stimuli-responsive drug delivery system for inflammatory arthritis treatment. Mater Today Bio. 2022;14:100223. [31] HUANG Y, JIANG T, CHEN J, et al. Effects of kartogenin on the attenuated nucleus pulposus cell degeneration of intervertebral discs induced by interleukin-1β and tumor necrosis factor-α. Int J Mol Med. 2018;41(2): 749-756. [32] HOU M, ZHANG Y, ZHOU X, et al. Kartogenin prevents cartilage degradation and alleviates osteoarthritis progression in mice via the miR-146a/NRF2 axis. Cell Death Dis. 2021;12(5):483. [33] FENG Q, LI D, LI Q, et al. Dynamic Nanocomposite Microgel Assembly with Microporosity, Injectability, Tissue-Adhesion, and Sustained Drug Release Promotes Articular Cartilage Repair and Regeneration. Adv Healthc Mater. 2022;11(8):e2102395. [34] ZHANG YJ, YIN WL, LIU Y, et al. Dynamic protein hydrogel with supramolecularly enveloped kartogenin promotes cartilage regeneration through mitochondrial activation. Compos B Eng. 2022;246:1359-8368. [35] MA CJ, LIU X, CHE L, et al. Stem Cell Therapies for Intervertebral Disc Degeneration: Immune Privilege Reinforcement by Fas/FasL Regulating Machinery. Curr Stem Cell Res Ther. 2015;10(4):285-295. [36] XU H, SUN M, WANG C, et al. Growth differentiation factor-5-gelatin methacryloyl injectable microspheres laden with adipose-derived stem cells for repair of disc degeneration. Biofabrication. 2020;13(1):015010. [37] RUOSLAHTI E. RGD and other recognition sequences for integrins. Annu Rev Cell Dev Biol. 1996;12:697-715. [38] RUTGES J, CREEMERS LB, DHERT W, et al. Variations in gene and protein expression in human nucleus pulposus in comparison with annulus fibrosus and cartilage cells: potential associations with aging and degeneration. Osteoarthritis Cartilage. 2010;18(3):416-423. |
| [1] | Weng Rui, Lin Dongxin, Guo Haiwei, Zhang Wensheng, Song Yuke, Lin Hongheng, Li Wenchao, Ye Linqiang. Abnormal types of intervertebral disc structure and related mechanical loading with biomechanical factors [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(9): 1436-1442. |
| [2] | Mo Jun, Luo Zongping. Effects of static traction on the nucleus pulposus and annulus fibrosus of the rat intervertebral disc [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(8): 1180-1185. |
| [3] | Liu Xin, Hu Man, Zhao Wenjie, Zhang Yu, Meng Bo, Yang Sheng, Peng Qing, Zhang Liang, Wang Jingcheng. Cadmium promotes senescence of annulus fibrosus cells via activation of PI3K/Akt signaling pathway [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(8): 1217-1222. |
| [4] | Liu Hanfeng, Wang Jingjing, Yu Yunsheng. Artificial exosomes in treatment of myocardial infarction: current status and prospects [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(7): 1118-1123. |
| [5] | Kang Zhijie, Cao Zhenhua, Xu Yangyang, Zhang Yunfeng, Jin Feng, Su Baoke, Wang Lidong, Tong Ling, Liu Qinghua, Fang Yuan, Sha Lirong, Liang Liang, Li Mengmeng, Du Yifei, Lin Lin, Wang Haiyan, Li Xiaohe, Li Zhijun. Finite element model establishment and stress analysis of lumbar-sacral intervertebral disc in ankylosing spondylitis [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(6): 840-846. |
| [6] | Li Yangfeng, Tian Xin, He Fan, Yang Huilin. Melatonin-loaded gelatin methacryloyl microspheres delay nucleus pulposus degeneration [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(5): 676-681. |
| [7] | Zhu Liwei, Wang Jiangyue, Bai Ding. Application value of nanocomposite gelatin methacryloyl hydrogels in different bone defect environments [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(5): 753-758. |
| [8] | Yin Tong, Yang Jilei, Li Yourui, Liu Zhuoran, Jiang Ming. Application of core-shell structured nanofibers in oral tissue regeneration [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(5): 766-770. |
| [9] | Cao Sheng, Kong Lingwei, Xu Kun, Sun Zhijie. Effect of gelatin methacryloyl hydrogel loaded with salvianolic acid B on intervertebral disc degeneration [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(3): 380-386. |
| [10] | Zhou Shuliang, Xu Liang, Qian Xuefeng, Zeng Jincai, Zhu Lifan. Correlation between the expression of miRNA-142-3p, mixed lineage kinase 3 and interleukin-1beta in nucleus pulposus and the degree of lumbar intervertebral disc degeneration [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(2): 165-171. |
| [11] | Wang Qian, Lu Ziang, Li Lihe, Lyu Chaoliang, Wang Meng, Zhang Cunxin. Sinomenine effectively inhibits interleukin-1beta-induced apoptosis in nucleus pulposus cells [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(2): 224-230. |
| [12] | Xie Wenguan, Liu Yutao, Cui Wenbo, Kuang Mingye. Melatonin inhibits hydrogen peroxide-induced injury of human nucleus pulposus cells [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(14): 2180-2185. |
| [13] | Han Zhongxiao, Ou Yaying, Zhuang Xinqing, Zhang Xiang, Li Biaoping, Jiang Zhirui, Zhang Jingyi, Yang Jiashun, Tang Ling, Xiao Wei. Mechanical puncture combined with tumor necrosis factor alpha and complete Freund’s adjuvant to construct a rat discogenic low back pain model [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(11): 1672-1677. |
| [14] | Cheng Haotian, Zhao Xiaofeng, Lu Xiangdong, Zhao Yibo, Fan Zhifeng, Qi Detai, Wang Xiaonan, Zhou Runtian, Jin Xinjie, Zhao Bin. Single-cell RNA sequencing and the pathogenesis of intervertebral disc degeneration [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(1): 93-99. |
| [15] | Cao Sheng, Kong Lingwei, Xu Kun, Sun Zhijie. Correlation of cervical sagittal force line parameters with degenerative segment and Pfirrmann classification in patients with cervical intervertebral disc degeneration [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(9): 1319-1324. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||