Chinese Journal of Tissue Engineering Research ›› 2024, Vol. 28 ›› Issue (9): 1436-1442.doi: 10.12307/2024.002
Previous Articles Next Articles
Abnormal types of intervertebral disc structure and related mechanical loading with biomechanical factors
Weng Rui1, 2, Lin Dongxin3, Guo Haiwei1, 2, Zhang Wensheng1, 2, Song Yuke1, 2, Lin Hongheng1, 2, Li Wenchao1, 2, Ye Linqiang4
- 1Department of Orthopedics, Third Affiliated Hospital of Guangzhou University of Chinese Medicine, Guangzhou 510000, China; 2Guangdong Research Institute for Orthopedics & Traumatology of Chinese Medicine, Guangzhou 510000, China; 3School of Basic Medical Sciences, Southern Medical University, Guangzhou 510000, China; 4Department of Orthopedics, Dongguan Hospital of Guangzhou University of Chinese Medicine, Dongguan 523000, China
-
Received:2023-01-16Accepted:2023-02-24Online:2024-03-28Published:2023-07-26 -
Contact:Ye Linqiang, MD, Attending TCM physician, Department of Orthopedics, Dongguan Hospital of Guangzhou University of Chinese Medicine, Dongguan 523000, Guangdong Province, China -
About author:Weng Rui, Master, Physician, Department of Orthopedics, Third Affiliated Hospital of Guangzhou University of Chinese Medicine, Guangzhou 510000, Guangdong Province, China; Guangdong Research Institute for Orthopedics & Traumatology of Chinese Medicine, Guangzhou 510000, Guangdong Province, China -
Supported by:Guangdong Basic and Applied Basic Research Foundation, No. 2019A1515110717 (to YLQ); Dongguan Science and Technology of Social Development Program, No. 202050715002180 (to YLQ)
CLC Number:
Cite this article
Weng Rui, Lin Dongxin, Guo Haiwei, Zhang Wensheng, Song Yuke, Lin Hongheng, Li Wenchao, Ye Linqiang. Abnormal types of intervertebral disc structure and related mechanical loading with biomechanical factors[J]. Chinese Journal of Tissue Engineering Research, 2024, 28(9): 1436-1442.
share this article
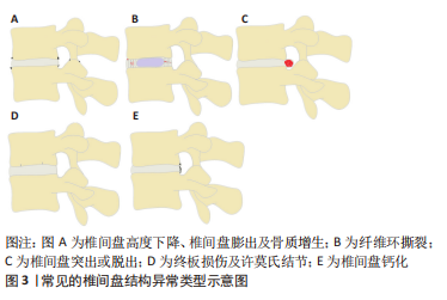
2.1 椎间盘的解剖及生理特点 椎间盘主要由3部分组成,其中包括髓核、纤维环及与上下椎体相连的软骨终板[9]。髓核是由Ⅱ型胶原蛋白和其他蛋白质组成的胶质样结构[10],由于其含有大量的带阴离子的蛋白聚糖,具有较强的渗透性,因此能保持必要的静水压力以抵抗重力作用的压缩并保持椎骨分离。纤维环是包绕在髓核外围的高度排列的纤维结缔组织,其两端分别锚定在上下软骨终板。纤维环由15-25层的同心环组成,每层同心环由交错排列的斜向胶原纤维束组成,这些胶原纤维束被由弹性蛋白、原纤维蛋白和其他蛋白组成的弹性纤维鞘包裹,从而使纤维环具有一定的弹性[11]。这样的结构既能限制脊柱过度屈伸及旋转活动,又有利于脊柱在活动后恢复原来的结构排列。软骨终板是椎体骨组织和髓核与纤维环软组织的过渡结构,避免了骨性终板与髓核或纤维环的直接接触,有利于缓冲骨性终板对椎间盘造成直接损伤。另外,软骨终板也是椎间盘营养物质、代谢产物及水分交换的主要通道,其对椎间盘的生理代谢具有重要作用[12-13]。 2.2 常见的椎间盘结构异常类型 通过对已发表文献的检索和回顾,总结了以下几种常见的已明确椎间盘结构异常的类型,其中包括:椎间盘高度下降或椎间盘膨出及骨质增生、纤维环撕裂、椎间盘突出或脱出、终板损伤及许莫氏结节、椎间盘钙化等类型,见图3。"
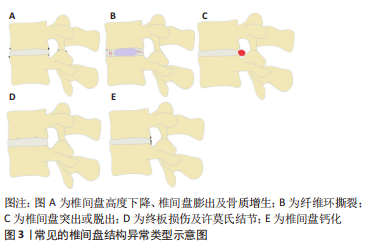
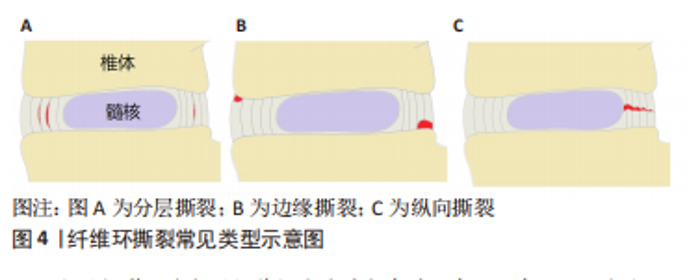
2.2.1 椎间盘高度下降、椎间盘膨出及骨质增生 健康的髓核由于饱含大量的蛋白聚糖,具有较强的结合水的能力,这使髓核内部产生足够的压力,不仅能保持足够的椎间盘高度,使相邻的两个椎体分离,而且能使周围的纤维环拉紧,将压力均匀地分布在上下的软骨终板[14]。长期异常压力载荷下或随着年龄的增长,髓核结合水的能力逐渐减弱,髓核内部压力减少,此时,椎间盘就像一个“瘪气的轮胎”[14-15]。由于髓核的含水量和内压减少,垂直压力载荷直接作用在纤维环,纤维环向外膨出,偶尔也向内膨出,导致椎间隙高度下降以及椎间盘膨出,后期可导致纤维环撕裂甚至累及整个椎间盘结构的病变[16]。椎间盘高度下降及椎间盘膨出与骨质增生具有相关性[17-18]。椎间隙的高度正常不仅能使相邻椎体相互分离,而且能正常地传递和分散压缩载荷。当髓核内压降低,椎间隙高度下降时,会导致50%以上的脊柱压力转移到椎弓根上。POLLINTINE等[19]在尸体腰椎运动节段施加2 000 N的压缩载荷,并测量椎弓根受到的压力,证明了这一点。椎弓根应力的额外增加导致应力分布异常,与关节突关节和椎体边缘的骨质增生有关[18],这也解释了椎间盘高度下降及椎间盘膨出与骨质增生相关。 2.2.2 纤维环撕裂 纤维环撕裂常见的3种类型包括分层撕裂、边缘撕裂和纵向撕裂[20],见图4。"
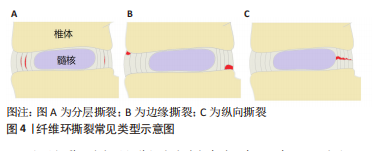
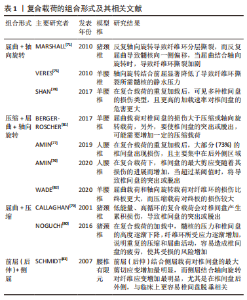
分层撕裂可能与层间剪切应力过大有关。有一项有限元研究发现,在L3-4的运动节段施加200-2 000 N的轴向压缩载荷,可见完整椎间盘后外侧层间剪切应力最大,该处的椎间盘突出和位移最明显,结果提示椎间盘后外侧分层撕裂的可能性较大[21]。另外,后外侧损伤的椎间盘的层间剪切应力随着损伤程度的增加而增加,可能导致纤维环进一步撕裂。纤维环分层撕裂之后,孤立的纤维环片层由于没有支撑而向内或向外塌陷,同时,也可能破坏它们锚定的软骨终板[22]。边缘撕裂是纤维环外层的部分撕裂,有时撕裂的程度可以到达纤维环的中间层,好发于纤维环前方的边缘撕裂主要与创伤有关[23],而好发于纤维环后方的撕裂与年龄和椎间盘退变有关[24]。纵向撕裂主要从椎间盘内部向外发展,好发于纤维环的后侧或后外侧,与后续髓核从椎间盘脱出有关[25],这一过程可以在体外通过椎间盘承受压缩和弯曲的循环载荷来模拟[26]。当纵向撕裂存在时,在轴向压力的作用下促使髓核沿着纤维环裂隙向外挤出,髓核内压下降,导致椎间盘传递和分散压力的能力下降,压力直接作用在纤维环上,由于纤维环对直接压力的耐受性较差,导致纤维环弹性纤维层紊乱和进一步受损。另外,纤维环撕裂促使蛋白多糖和胶原蛋白等物质暴露容易诱发免疫炎症反应,导致纤维环基质降解,使纤维环进一步受损。在力学因素和代谢因素的双重影响下,加剧纤维环撕裂和椎间盘损伤[7]。 2.2.3 椎间盘突出或脱出 椎间盘突出或脱出是纤维环撕裂损伤的进一步发展,当纵向撕裂的裂隙达到一定程度,在机械载荷作用下,髓核移动嵌入裂隙,此时纤维环撕裂逐渐发展为椎间盘突出或脱出。解剖研究发现突出的椎间盘组织主要是髓核也验证了这种椎间盘的退变模式[27]。椎间盘脱出也可以在体外通过椎间盘承受压缩和弯曲载荷来模拟[28],然而,与纤维环撕裂不同的是,这两种载荷其中至少一种载荷应超过生理承受极限,或者施加较强的重复载荷。值得注意的是,严重退变的椎间盘在体外模拟时很难脱出,这是因为髓核的静水压不足以推动髓核往纤维环裂隙移动[29]。 2.2.4 终板损伤及许莫氏结节 软骨终板是椎间盘中最脆弱的部分,在受压时最容易发生小梁微损伤,当这种微损伤不断累积达到一定程度时,会造成终板损伤[30],椎间盘内部的髓核会向终板损伤的位置迁移,随着年龄的增长,髓核通过受损的终板往椎体突出,后续形成“许莫氏结节”[31-32]。同时,终板损伤使髓核向椎体迁移导致髓核内压减少,椎间盘所受应力转移到纤维环上。软骨终板是椎间盘营养物质、代谢产物及水分交换的主要通道[33],当其受损时,不仅影响椎体与椎间盘间的应力传导,也可能影响椎间盘营养物质和代谢产物的流通,会导致椎间盘进一步退变和损伤,这说明软骨终板结构的完整性对维持椎间盘结构和功能正常的重要意义。另外,有研究也发现,软骨终板的异常骨化会导致椎间盘营养供给的部分孔隙闭塞,使椎间盘营养相对不足进而造成椎间盘退变。在一项对30名受试者颈椎的尸检研究中,软骨终板孔隙通道数量与椎间盘退变程度、年龄呈较强的负相关,而终板孔隙通道数量的减少主要是由于终板骨化导致这些孔隙闭塞所致[34]。 2.2.5 椎间盘钙化 椎间盘钙化在老龄或椎间盘退变和脊柱侧弯中并不少见[35-36],但容易被忽视[37]。导致椎间盘钙化的因素有很多种,生物力学的改变是其中重要的因素。过度的机械载荷或脊柱不稳定会导致椎间盘的损伤塌陷以及骨质增生,刺激椎间盘组织分泌促炎细胞因子,该因子可促进骨祖细胞分化和血管生成,最终激活异位骨化,促使椎间盘钙化[38]。ILLIEN-JüNGER等[39]对不同退变程度的人类椎间盘进行组织学分析,并确定了椎间盘内的钙化区域,结果发现这些钙化区域通常位于退变组织区域的裂隙附近,并与椎间盘退变程度增加有关,该结果也验证了前面的理论。另外,也有研究表明,脊柱骨盆对齐参数可以预测椎间盘钙化[40],这也表明生物力学的改变可能诱发椎间盘钙化,从而影响椎间盘的功能。异常的机械载荷已被证明会增加X型胶原蛋白的水平,这是一个已知的软骨内骨化的标志[41]。其纤维环细胞具有祖细胞的特性,在适当的刺激下,可在体内外分化为软骨细胞和成骨细胞[42-43]。由此可见,椎间盘钙化与椎间盘内机械载荷的改变和应力分布异常明显相关。 以上研究介绍了不同的椎间盘结构异常类型,然而,在临床实践中,这些不同类型可能同时存在且有联系。终板损伤和纤维环撕裂是早期常见的类型,早期出现终板损伤与臀部摔倒或过度负重造成脊柱的轴向压缩损伤有关,随后髓核往受损终板迁移钙化形成许莫氏结节;早期纤维环撕裂与脊柱长期姿势不当或重复弯曲有关,随后发展为椎间盘突出或脱出,这些改变进一步造成了椎间盘生理和功能的变化,可能导致椎间盘进一步损伤,出现新的异常结构。由此可见,椎间盘的损伤退变呈渐进式的结构破坏,在临床实践中前期的预防和保护尤为重要。 2.3 导致椎间盘结构异常的机械载荷类型 正常的机械载荷对维持椎间盘的结构功能具有积极作用,有利于刺激椎间盘细胞功能的维持和椎间盘的营养代谢[44]。过度的机械载荷会对椎间盘造成急性或慢性损伤,产生炎症反应或基质降解等,从而导致椎间盘结构损伤,同时,也会影响椎间盘营养物质和代谢产物的流通,引起力学-生物学效应而造成椎间盘细胞凋亡或功能障碍[45-46]。正常的椎间盘在机械载荷作用下髓核通过形变将压力转换成对终板均匀的压力和对纤维环的张力从而分散压力,此时,髓核内通过蛋白聚糖渗透吸收的水分在压力作用下通过终板进入椎体;当机械载荷解除时,蛋白聚糖又重新吸收水分恢复原状,在此循环过程中,水分的流动带动了椎间盘和椎体之间的营养物质和代谢产物的交换[47]。正常日常活动引起的这种载荷变化有助于蛋白聚糖通过渗透作用吸入营养和排出废物,然而,过度的循环载荷或长时间的持续载荷则容易导致椎间盘的损伤和退变[48-49]。 2.3.1 压缩载荷 压缩载荷是椎间盘所受机械载荷的常见类型之一。受重力的影响,人体直立行走、保持或改变姿势、各种运动等均受到压缩载荷的影响。体外研究发现其对椎间盘的影响因压缩载荷大小、作用形式、持续时间、循环次数等因素的不同而不同。PAUL等[50]研究了不同压缩载荷大小和作用形式对椎间盘的影响,他们将羊椎间盘置于生物反应器中培养,分别模拟生理压缩载荷、超生理动态压缩载荷和超生理静态压缩载荷,并对髓核和纤维环的前、后、内、外侧进行压力测定,结果发现在不同压缩载荷条件下,椎间盘高度均下降,但超生理载荷的高度下降更明显;另外,超生理动态载荷导致髓核和纤维环的所有区域都出现细胞死亡和基质破坏,而超生理静态载荷主要破坏纤维环后部区域的细胞和基质。这说明了过度的压缩载荷对椎间盘的结构功能有不利影响,超生理动态载荷对椎间盘的破坏最大,而超生理静态载荷容易使椎间盘向后突出或者脱出。EMANUEL等[51]和ZHU等[52]研究了持续的压缩载荷对椎间盘的影响,EMANUEL等[51]对36例人体腰椎间盘进行模拟生理持续压缩加载,发现随着时间的推移,椎间隙高度下降,椎间盘膨出,且其结合水的能力不断降低,由此导致的髓核静水压力丢失可能会导致椎间盘进一步改变。ZHU等[52]对兔腰椎间盘在特定设备中分别在恒定的压缩载荷下和无载荷条件下培养14 d,发现两种条件下蛋白聚糖含量均下降,但压缩载荷条件下下降明显,其蛋白聚糖基因表达也明显下调,且在培养3 d后就出现组织形态的破坏。他们的研究说明了持续性的压缩载荷导致了蛋白聚糖含量的减少,与髓核静水压力丢失和椎间盘高度下降以及进一步的损伤退变相关。当髓核的静水压力逐渐丢失时,载荷逐渐作用在纤维环上,由于纤维环不具备髓核的形变能力难以将载荷分散转移,导致其承受更大的载荷,这种情况下容易产生局部损伤,当组织损伤和微损伤的积累超过了椎间盘的自我修复能力,会进一步导致椎间盘的损伤退变[53]。 WADE等[54]研究了不同循环周期的压缩载荷对椎间盘的影响,其将成熟绵羊腰椎间盘运动节段前屈7°,以5 Hz的频率和(1 300±500) N的压缩载荷循环加载,运行20 000-48 000个周期和70 000-120 000个周期,结果发现两种运行周期的椎间盘高度呈轻度下降,纤维环有膨出趋势,纤维环-终板连接处明显变形和纤维环中内层出现分层撕裂和纵向撕裂,且高运行周期的椎间盘更严重。WADE等[55]的另一项研究用高速率压缩载荷作用于椎间盘直至其损伤,发现大部分椎间盘终板-纤维环的连接断裂,该研究表明纤维环和终板连接之处是椎间盘较为脆弱的位置,可能是因为纤维环和终板之间的力学结构不平衡导致的。有其他学者则认为在压缩载荷下,终板才是椎间盘最脆弱的位置[56]。终板位于椎体和椎间盘的之间,一方面承受了椎体骨性结构的压力,另一方面髓核的静水压力往终板膨胀[57]。DOLAN等[58]对人体椎间盘进行可控制的超生理的压缩载荷,结果发现平均3.4 kN的压缩载荷可导致终板断裂,且责任节段的椎间盘高度丢失平均约1.88 mm;终板断裂后,纤维环后部的应力明显增加。这也表明了终板断裂会造成椎间盘的异常应力分布,增加了椎间盘退变和纤维环后方撕裂的风险。VERES等[59]在绵羊椎间盘上逐步施加压缩载荷至椎间盘结构出现严重损伤,并用电子显微镜进行观察,结果发现每个椎间盘均出现后部纤维环断裂,且部分髓核通过裂隙挤压脱出,其结果也验证了DOLAN等[58]的推测。综上所述,过度或持续的压缩载荷容易造成椎间盘的损伤或退变,其中包括椎间盘高度下降或椎间盘膨出、纤维环撕裂、椎间盘突出或脱出、终板损伤。 2.3.2 屈曲载荷 屈曲载荷作用引起的脊柱活动包括前屈、后伸和左右侧屈,其对椎间盘会产生相似但局部位置不同的变形模式,髓核在这种情况下会偏向一侧移动[60]。静态持续长时间或动态重复的屈曲活动虽然不会直接引起脊髓和神经的损伤,但会增加周围肌肉劳损和椎间盘损伤的风险[61-62]。WADE等[62]的一项研究将成熟绵羊腰椎间盘运动节段分别处于中立位或向前屈曲10°,并施加压缩载荷至椎间盘出现结构损伤,结果发现屈曲状态下的椎间盘结构损伤的平均载荷比中立位的低18%,这说明屈曲状态下椎间盘更容易发生损伤。有学者认为,屈曲引起椎间盘更容易损伤可能与椎间盘组织的蠕变有关,这种蠕变引起椎间盘高度和黏弹性的暂时性改变,导致短时间内椎间关节的松弛和椎间异常活动,在此期间,椎间盘相对较脆弱[63]。MCGILL等[64]发现深度屈曲20 min,即使休息50 min,椎间盘的蠕变也尚未完全恢复,其认为椎间盘的蠕变和黏弹性的恢复有滞后性。因此建议长时间弯曲姿势的工作者,如长时间弯腰或伏案工作等,应该适当休息,否认可能因过度屈曲,增加椎间盘损伤的风险[65]。 静态的屈曲载荷对椎间盘的影响主要与屈曲程度、作用时间和休息间隔时间有关,而动态重复的屈曲载荷对椎间盘的影响与载荷大小[66]、循环周期及频率[67]、屈曲幅度[68]、年龄等因素相关[69]。WADE等[62]从微观角度解释了屈伸活动对椎间盘的损伤原理,他们注意到在终板起伏的边缘,纤维环在此处与上下终板的距离最短,在反复屈伸的活动中,此处受到的应变最大,最容易发生断裂。SOLOMONOW等[66]也解释了重复的屈曲载荷会导致纤维环纤维的微损伤,微损伤的累积会进一步导致纤维环的撕裂以及椎间盘的退变。SCHOLLUM等[70]将羊腰椎间盘置于特定生物试验机上,以0.5 Hz的低频率和亚急性的屈曲载荷进行5 000次的循环活动,结果发现有30%的椎间盘出现损伤,主要表现在纤维环-终板连接处出现变形和纤维环内层出现撕裂,SCHOLLUM认为在更多的循环周期或更大的屈曲载荷会导致椎间盘的脱出。MARSHALL等[71]在单纯重复的屈曲载荷研究中发现了髓核沿纤维环裂隙脱出,其结果与SCHOLLUM等[70]的观点一致。综上所述,持续长时间或重复的屈曲会导致椎间盘黏弹性的改变以及纤维环的损伤,对纤维环的损伤主要表现为纤维环撕裂和椎间盘的突出或脱出。 2.3.3 轴向旋转 MARSHALL等[71]对猪颈椎间盘进行不同载荷类型的测试,结果发现单纯重复的屈曲运动会导致髓核通过纤维环后方撕裂缝隙脱出,而单纯重复的轴向旋转运动却没有发现髓核脱出,但在单纯轴向旋转中发现纤维环的撕裂。HARVEY-BURGESS等[72]对比牛椎间盘进行轴向旋转和未进行轴向旋转测试的区别,结果发现整体椎间盘结构的受损较小,但进行轴向旋转的椎间盘纤维环层内基质强度和刚度明显下降。纤维环层内基质将纤维环内的胶原纤维黏附在同一纤维环纤维层内,当纤维环层内基质损伤时,纤维环纤维撕裂的风险增加。单纯的旋转运动对椎间盘的损伤相对较小,有学者认为这是因为有双侧关节突关节的阻挡保护[73]。旋转运动时,一侧关节突关节相互分离,另一侧相互挤压,起到阻挡作用,关节突关节的阻挡作用能够限制脊柱的过度旋转运动[74]。 2.3.4 复合载荷 尽管单纯循环旋转运动对椎间盘的损伤较小,但当旋转结合前屈时,椎间盘损伤的风险将显著提高[71,75]。MARSHALL等[71]通过对比单纯轴向旋转和轴向旋转结合前屈的力学测试,发现轴向旋转结合前屈对椎间盘的损伤比单纯轴向旋转明显更大。VERES等[75]将30例羊腰椎间盘运动节段屈曲7°并轴向旋转2°,通过注射特定凝胶增强髓核内压力至难以再注入,发现有25例椎间盘出现明显损伤,其中17例出现终板断裂,8例出现纤维环撕裂,对比单纯屈曲的研究[59],发现轴向旋转结合前屈显著降低了导致纤维环撕裂所需髓核的静水压力。轴向旋转和前屈的联合运动在日常生活很常见,如伏案工作,弯腰搬运重物等,然而,这种联合运动往往与椎间盘的损伤退变有关[74-75]。AMIN等[76-77]为模拟搬运重物的过程,将人腰椎间盘进行不同程度的预压缩,然后进行屈曲和旋转的循环运动,结果发现低强度预压缩组在预压缩过程中有20%的标本出现终板破裂,高强度预压缩组有67%出现终板破裂,在循环运动后均有超过一半的终板未破裂椎间盘出现突出或脱出,主要集中在椎间盘后侧或后外侧区域,且高强度预压缩组对椎间盘的损伤更严重。该研究也表明搬运重物应该掌握安全的技巧,比如靠近重物进行搬运、避免弯腰、避免过重等。SHAN等[78]将成熟绵羊腰椎间盘以不同速率模拟做屈曲和旋转复合运动,在多次循环运动后,发现所有椎间盘都出现损伤,其中以终板断裂和纤维环撕裂为主,并且高速率组的平均破坏载荷相对较低,该研究表明在高速率的复合载荷下,椎间盘的承载能力相对较低。综上所述,屈曲结合旋转的复合运动对椎间盘损伤的风险比单纯旋转运动或单纯屈曲运动更大,尤其是在快速运动中。 由于人类活动的多样化,也存在着其他的复合运动形式,如屈曲和压缩载荷结合[79-80],屈曲与轴向旋转和压缩载荷结合[81-82],前屈和侧屈载荷结合等[83],见表1。这些复合载荷与单一机械载荷相比,更容易导致椎间盘出现更高的应力,尤其是位于椎间盘的后外侧,该位置更容易发生椎间盘的损伤退变或椎间盘脱出,这似乎也解释了临床上椎间盘的损伤退变常见于椎间盘的后外侧,其损伤表现包括终板损伤、椎间盘高度下降或椎间盘膨出、纤维环撕裂、椎间盘突出或脱出等。"

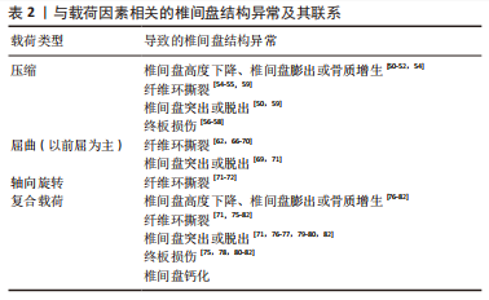
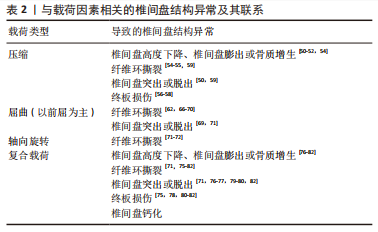
目前,尚无体外研究表明具体哪种机械载荷导致椎间盘钙化。然而,很多研究表明,椎间盘机械载荷的改变和应力分布的不均匀可能是椎间盘钙化的相关因素[37-38,40]。这是因为生物力学环境的改变可能诱发椎间盘中某些促炎细胞因子的产生,而这些促炎细胞因子可促进骨祖细胞分化和血管生成,最终激活异位骨化[38]。有研究表明,椎间盘的钙化潜能与椎间盘的退变严重程度有关,主要与钙感应受体、转录因子(如osterix和Runx)以及蛋白(如骨形态发生蛋白2和骨钙素)的表达增强有关,这些都能诱导椎间盘钙化[84]。HRISTOVA等[85]发现退行性椎间盘疾病和脊柱侧弯患者的椎间盘存在钙沉淀和X型胶原蛋白,而正常组几乎没有,其它研究也发现在退行性椎间盘机械载荷改变的过程中,纤维环细胞高表达Runx2,纤维环细胞具有祖细胞的特性,在适当的刺激下,可在体内外分化为软骨细胞和成骨细胞[42-43]。另外,椎间盘钙化又会影响椎间盘应力的分布及其运动学特征,可能进一步导致椎间盘的损伤退变[86]。研究表明钙化的椎间盘比非钙化的椎间盘更容易破裂和突出,这可能是因为钙化产生异常的应力集中,导致纤维环撕裂或周围组织损伤或造成炎症反应进一步削弱椎间盘,最终导致椎间盘突出或脱出[87]。综上所述,椎间盘钙化似乎并不是由机械载荷直接作用形成,而是因为机械载荷导致生物力学环境的改变或椎间盘损伤退变间接导致椎间盘钙化。 椎间盘的结构异常已经被证实发生在不同类型的重复载荷下,表2将各种类型的载荷与椎间盘结构异常类型的关系联系起来进行归纳总结。 以上研究介绍了不同机械载荷类型对椎间盘产生的不同影响,压缩载荷容易导致蛋白聚糖基因表达下调,使蛋白聚糖含量减少和椎间盘结合水的能力降低,导致椎间盘高度下降或膨出以及椎间盘进一步的损伤退变。另外,过度的压缩载荷容易导致终板损伤断裂,也会进一步导致整个椎间盘的损伤退变。屈曲载荷和轴向旋转载荷对纤维环的损伤比终板更大,持续长时间或重复的屈曲载荷会导致纤维环撕裂和椎间盘的突出或脱出,而单纯的轴向旋转载荷对椎间盘的损伤较小,只会引起纤维环撕裂。然而,当不同的载荷类型结合作用时更容易导致椎间盘出现高应力,椎间盘损伤的风险更大。"
| [1] NEIDLINGER-WILKE C, GALBUSERA F, PRATSINIS H, et al. Mechanical loading of the intervertebral disc: from the macroscopic to the cellular level. Eur Spine J. 2014;23 Suppl 3:S333-S343. [2] AMIN DB, TAVAKOLI J, FREEMAN BJC, et al. Mechanisms of failure following simulated repetitive lifting: a clinically relevant biomechanical cadaveric study. Spine (Phila Pa 1976). 2020;45(6):357-367. [3] JO M, CHAE SW. Stress analysis of intervertebral disc during occupational activities. Comput Methods Programs Biomed. 2021;208:106298. [4] ROHLMANN A, PETERSEN R, SCHWACHMEYER V, et al. Spinal loads during position changes. Clin Biomech (Bristol, Avon). 2012;27(8):754-758. [5] LI JQ, KWONG WH, CHAN YL, et al. Comparison of in vivo intradiscal pressure between sitting and standing in human lumbar spine: a systematic review and meta-analysis. Life (Basel). 2022;12(3):457. [6] STOKES IA, IATRIDIS JC. Mechanical conditions that accelerate intervertebral disc degeneration: overload versus immobilization. Spine (Phila Pa 1976). 2004;29(23):2724-2732. [7] VERGROESEN PP, KINGMA I, EMANUEL KS, et al. Mechanics and biology in intervertebral disc degeneration: a vicious circle. Osteoarthritis Cartilage. 2015;23(7):1057-1070. [8] HSIEH AH, TWOMEY JD. Cellular mechanobiology of the intervertebral disc: new directions and approaches. J Biomech. 2010;43(1):137-145. [9] FRANCISCO V, PINO J, GONZÁLEZ-GAY MÁ, et al. A new immunometabolic perspective of intervertebral disc degeneration. Nat Rev Rheumatol. 2022; 18(1):47-60. [10] LAMA P, TEWARI J, ADAMS MA, et al. Degenerative physiochemical events in the pathological intervertebral disc. Histol Histopathol. 2022;37(1):11-20. [11] GHEZELBASH F, SHIRAZI-ADL A, BAGHANI M, et al. On the modeling of human intervertebral disc annulus fibrosus: elastic, permanent deformation and failure responses. J Biomech. 2020;102:109463. [12] GRUNHAGEN T, SHIRAZI-ADL A, FAIRBANK JC, et al. Intervertebral disk nutrition: a review of factors influencing concentrations of nutrients and metabolites. Orthop Clin North Am. 2011;42(4):465-477. [13] FUJIWARA T, AKEDA K, YAMADA J, et al. Endplate and intervertebral disc injuries in acute and single level osteoporotic vertebral fractures: is there any association with the process of bone healing? BMC Musculoskelet Disord. 2019;20(1):336. [14] VERGROESEN PP, VAN DER VEEN AJ, VAN ROYEN BJ, et al. Intradiscal pressure depends on recent loading and correlates with disc height and compressive stiffness. Eur Spine J. 2014;23(11):2359-2368. [15] STEFANAKIS M, LUO J, POLLINTINE P, et al. ISSLS Prize winner: Mechanical influences in progressive intervertebral disc degeneration. Spine (Phila Pa 1976). 2014;39(17):1365-1372. [16] MCMORRAN JG, GREGORY DE. The influence of axial compression on the cellular and mechanical function of spinal tissues; emphasis on the nucleus pulposus and annulus fibrosus: a review. J Biomech Eng. 2021;143(5):050802. [17] RAVIKANTH R. Magnetic resonance evaluation of lumbar disc degenerative disease as an implication of low back pain: a prospective analysis. Neurol India. 2020;68(6):1378-1384. [18] VIDEMAN T, BATTIÉ MC, GILL K, et al. Magnetic resonance imaging findings and their relationships in the thoracic and lumbar spine. Insights into the etiopathogenesis of spinal degeneration. Spine (Phila Pa 1976). 1995;20(8):928-935. [19] POLLINTINE P, PRZYBYLA AS, DOLAN P, et al. Neural arch load-bearing in old and degenerated spines. J Biomech. 2004;37(2):197-204. [20] DESMOULIN GT, PRADHAN V, MILNER TE. Mechanical aspects of intervertebral disc injury and implications on biomechanics. Spine (Phila Pa 1976). 2020;45(8):E457-E464. [21] GOEL VK, MONROE BT, GILBERTSON LG, et al. Interlaminar shear stresses and laminae separation in a disc. Finite element analysis of the L3-L4 motion segment subjected to axial compressive loads. Spine (Phila Pa 1976). 1995; 20(6):689-698. [22] SCHMIDT H, HEUER F, WILKE HJ. Dependency of disc degeneration on shear and tensile strains between annular fiber layers for complex loads. Med Eng Phys. 2009;31(6):642-649. [23] OSTI OL, VERNON-ROBERTS B, MOORE R, et al. Annular tears and disc degeneration in the lumbar spine. A post-mortem study of 135 discs. J Bone Joint Surg Br. 1992;74(5):678-682. [24] VERNON-ROBERTS B, MOORE RJ, FRASER RD. The natural history of age-related disc degeneration: the pathology and sequelae of tears. Spine (Phila Pa 1976). 2007;32(25):2797-2804. [25] SENCK S, TRIEB K, KASTNER J, et al. Visualization of intervertebral disc degeneration in a cadaveric human lumbar spine using microcomputed tomography. J Anat. 2020;236(2):243-251. [26] WANG W, PEI B, PEI Y, et al. Biomechanical effects of over lordotic curvature after spinal fusion on adjacent intervertebral discs under continuous compressive load. Clin Biomech (Bristol, Avon). 2020;73:149-156. [27] KRYSTKIEWICZ K, MAŚLANKA M, SKADORWA T, et al. Meningovertebral ligaments could be a barrier for migration of a herniated intervertebral disc: an anatomical study. Front Surg. 2022;9:969244. [28] HEESWIJK VM, THAMBYAH A, ROBERTSON PA, et al. Posterolateral disc prolapse in flexion initiated by lateral inner annular failure: an investigation of the herniation pathway. Spine (Phila Pa 1976). 2017;42(21):1604-1613. [29] ADAMS MA, ROUGHLEY PJ. What is intervertebral disc degeneration, and what causes it? Spine (Phila Pa 1976). 2006;31(18):2151-2161. [30] LOTZ JC, FIELDS AJ, LIEBENBERG EC. The role of the vertebral end plate in low back pain. Global Spine J. 2013;3(3):153-164. [31] MOK FP, SAMARTZIS D, KARPPINEN J, et al. ISSLS prize winner: prevalence, determinants, and association of Schmorl nodes of the lumbar spine with disc degeneration: a population-based study of 2449 individuals. Spine (Phila Pa 1976). 2010;35(21):1944-1952. [32] VON FORELL GA, NELSON TG, SAMARTZIS D, et al. Changes in vertebral strain energy correlate with increased presence of Schmorl’s nodes in multi-level lumbar disk degeneration. J Biomech Eng. 2014;136(6): 061002. [33] GIERS MB, MUNTER BT, EYSTER KJ, et al. Biomechanical and endplate effects on nutrient transport in the intervertebral disc. World Neurosurg. 2017;99:395-402. [34] TOMASZEWSKI KA, ADAMEK D, KONOPKA T, et al. Endplate calcification and cervical intervertebral disc degeneration: the role of endplate marrow contact channel occlusion. Folia Morphol (Warsz). 2015;74(1):84-92. [35] ZEHRA U, BOW C, CHEUNG JPY, et al. The association of lumbar intervertebral disc calcification on plain radiographs with the UTE Disc Sign on MRI. Eur Spine J. 2018;27(5):1049-1057. [36] SHAO J, YU M, JIANG L, et al. Differences in calcification and osteogenic potential of herniated discs according to the severity of degeneration based on Pfirrmann grade: a cross-sectional study. BMC Musculoskelet Disord. 2016;17:191. [37] ZEHRA U, TRYFONIDOU M, IATRIDIS JC, et al. Mechanisms and clinical implications of intervertebral disc calcification. Nat Rev Rheumatol. 2022; 18(6):352-362. [38] KRZYZANOWSKA AK, FRAWLEY RJ, DAMLE S, et al. Activation of nuclear factor-kappa B by TNF promotes nucleus pulposus mineralization through inhibition of ANKH and ENPP1. Sci Rep. 2021;11(1):8271. [39] ILLIEN-JÜNGER S, TORRE OM, KINDSCHUH WF, et al. AGEs induce ectopic endochondral ossification in intervertebral discs. Eur Cell Mater. 2016;32: 257-270. [40] ZEHRA U, CHEUNG JPY, BOW C, et al. Spinopelvic alignment predicts disc calcification, displacement, and modic changes: evidence of an evolutionary etiology for clinically-relevant spinal phenotypes. JOR Spine. 2020;3(1):e1083. [41] FONTES RBV, BAPTISTA JS, RABBANI SR, et al. Normal aging in human lumbar discs: An ultrastructural comparison. PLoS One. 2019;14(6):e0218121. [42] JIN L, LIU Q, SCOTT P, et al. Annulus fibrosus cell characteristics are a potential source of intervertebral disc pathogenesis. PLoS One. 2014;9(5): e96519. [43] FENG G, YANG X, SHANG H, et al. Multipotential differentiation of human anulus fibrosus cells: an in vitro study. J Bone Joint Surg Am. 2010;92(3):675-685. [44] RUIZ WILLS C, FOATA B, GONZÁLEZ BALLESTER MÁ, et al. Theoretical explorations generate new hypotheses about the role of the cartilage endplate in early intervertebral disk degeneration. Front Physiol. 2018;9: 1210. [45] HOFMANN UK, STEIDLE J, DANALACHE M, et al. Chondrocyte death after mechanically overloading degenerated human intervertebral disk explants is associated with a structurally impaired pericellular matrix. J Tissue Eng Regen Med. 2018;12(9):2000-2010. [46] PAUL CP, SCHOORL T, ZUIDERBAAN HA, et al. Dynamic and static overloading induce early degenerative processes in caprine lumbar intervertebral discs. PLoS One. 2013;8(4):e62411. [47] GULLBRAND SE, PETERSON J, AHLBORN J, et al. ISSLS prize winner: dynamic loading-induced convective transport enhances intervertebral disc nutrition. Spine (Phila Pa 1976). 2015;40(15):1158-1164. [48] QASIM M, NATARAJAN RN, AN HS, et al. Damage accumulation location under cyclic loading in the lumbar disc shifts from inner annulus lamellae to peripheral annulus with increasing disc degeneration. J Biomech. 2014; 47(1):24-31. [49] 解志锋,刘清,刘冰,等.腰椎间盘疲劳损伤的生物力学特性[J].中国组织工程研究,2021,25(3):339-343. [50] PAUL CP, DE GRAAF M, BISSCHOP A, et al. Static axial overloading primes lumbar caprine intervertebral discs for posterior herniation. PLoS One. 2017;12(4):e0174278. [51] EMANUEL KS, VERGROESEN PP, PEETERS M, et al. Poroelastic behaviour of the degenerating human intervertebral disc: a ten-day study in a loaded disc culture system. Eur Cell Mater. 2015;29:330-341. [52] ZHU LG, FENG MS, ZHAN JW, et al. Effect of static load on the nucleus pulposus of rabbit intervertebral disc motion segment in ex vivo organ culture. Chin Med J (Engl). 2016;129(19):2338-2346. [53] POLLINTINE P, PRZYBYLA AS, DOLAN P, et al. Neural arch load-bearing in old and degenerated spines. J Biomech. 2004;37(2):197-204. [54] WADE KR, SCHOLLUM ML, ROBERTSON PA, et al. ISSLS prize winner: vibration really does disrupt the disc: a microanatomical investigation. Spine (Phila Pa 1976). 2016;41(15):1185-1198. [55] WADE KR, ROBERTSON PA, THAMBYAH A, et al. “Surprise” loading in flexion increases the risk of disc herniation due to annulus-endplate junction failure: a mechanical and microstructural investigation. Spine (Phila Pa 1976). 2015;40(12):891-901. [56] ADAMS MA, DOLAN P. Intervertebral disc degeneration: evidence for two distinct phenotypes. J Anat. 2012;221(6):497-506. [57] DUDLI S, ENNS-BRAY W, PAUCHARD Y, et al. Larger vertebral endplate concavities cause higher failure load and work at failure under high-rate impact loading of rabbit spinal explants. J Mech Behav Biomed Mater. 2018;80:104-110. [58] DOLAN P, LUO J, POLLINTINE P, et al. Intervertebral disc decompression following endplate damage: implications for disc degeneration depend on spinal level and age. Spine (Phila Pa 1976). 2013;38(17):1473-1481. [59] VERES SP, ROBERTSON PA, BROOM ND. ISSLS prize winner: microstructure and mechanical disruption of the lumbar disc annulus: part II: how the annulus fails under hydrostatic pressure. Spine (Phila Pa 1976). 2008; 33(25):2711-2720. [60] SETTON LA, CHEN J. Mechanobiology of the intervertebral disc and relevance to disc degeneration. J Bone Joint Surg Am. 2006;88 Suppl 2:52-57. [61] PETERSSON M, ABBOTT A. Lumbar interspinous pressure pain threshold values for healthy young men and women and the effect of prolonged fully flexed lumbar sitting posture: an observational study. World J Orthop. 2020;11(3):158-166. [62] WADE KR, ROBERTSON PA, THAMBYAH A, et al. How healthy discs herniate: a biomechanical and microstructural study investigating the combined effects of compression rate and flexion. Spine (Phila Pa 1976). 2014;39(13):1018-1028. [63] ALESSA F, NING X. Changes of lumbar posture and tissue loading during static trunk bending. Hum Mov Sci. 2018;57:59-68. [64] MCGILL SM, BROWN S. Creep response of the lumbar spine to prolonged full flexion. Clin Biomech (Bristol, Avon). 1992;7(1):43-46. [65] PETERSSON M, ABBOTT A. Lumbar interspinous pressure pain threshold values for healthy young men and women and the effect of prolonged fully flexed lumbar sitting posture: an observational study. World J Orthop. 2020;11(3):158-166. [66] SOLOMONOW M. Neuromuscular manifestations of viscoelastic tissue degradation following high and low risk repetitive lumbar flexion. J Electromyogr Kinesiol. 2012;22(2):155-175. [67] GALE NC, ZEIGLER SL, TOWLER C, et al. Increased lumbar spinal column laxity due to low-angle, low-load cyclic flexion may predispose to acute injury. JOR Spine. 2018;1(4):e1038. [68] GALLAGHER S, MARRAS WS, LITSKY AS, et al. Torso flexion loads and the fatigue failure of human lumbosacral motion segments. Spine (Phila Pa 1976). 2005;30(20):2265-2273. [69] GALLAGHER S, MARRAS WS, LITSKY AS, et al. A comparison of fatigue failure responses of old versus middle-aged lumbar motion segments in simulated flexed lifting. Spine (Phila Pa 1976). 2007;32(17):1832-1839. [70] SCHOLLUM ML, WADE KR, ROBERTSON PA, et al. A Microstructural Investigation of Disc Disruption Induced by Low Frequency Cyclic Loading. Spine (Phila Pa 1976). 2018;43(3):E132-E142. [71] MARSHALL LW, MCGILL SM. The role of axial torque in disc herniation. Clin Biomech (Bristol, Avon). 2010;25(1):6-9. [72] HARVEY-BURGESS M, GREGORY DE. The effect of axial torsion on the mechanical properties of the annulus fibrosus. Spine (Phila Pa 1976). 2019; 44(4):E195-E201. [73] RONG X, LIU Z, WANG B, et al. The facet orientation of the subaxial cervical spine and the implications for cervical movements and clinical conditions. Spine (Phila Pa 1976). 2017;42(6):E320-E325. [74] 翁汭,叶林强,姚珍松,等.颈椎关节突关节矢状角不对称对颈椎间盘纤维环应力影响的三维有限元研究[J].中国脊柱脊髓杂志,2022,32(2): 149-159. [75] VERES SP, ROBERTSON PA, BROOM ND. The influence of torsion on disc herniation when combined with flexion. Eur Spine J. 2010;19(9): 1468-1478. [76] AMIN DB, TAVAKOLI J, FREEMAN BJC, et al. Mechanisms of failure following simulated repetitive lifting: a clinically relevant biomechanical cadaveric study. Spine (Phila Pa 1976). 2020;45(6):357-367. [77] AMIN DB, MOAWAD CM, COSTI JJ. New findings confirm regional internal disc strain changes during simulation of repetitive lifting motions. Ann Biomed Eng. 2019;47(6):1378-1390. [78] SHAN Z, WADE KR, SCHOLLUM ML, et al. A more realistic disc herniation model incorporating compression, flexion and facet-constrained shear: a mechanical and microstructural analysis. Part II: high rate or ‘surprise’ loading. Eur Spine J. 2017;26(10):2629-2641. [79] CALLAGHAN JP, MCGILL SM. Intervertebral disc herniation: studies on a porcine model exposed to highly repetitive flexion/extension motion with compressive force. Clin Biomech (Bristol, Avon). 2001;16(1):28-37. [80] NOGUCHI M, GOOYERS CE, KARAKOLIS T, et al. Is intervertebral disc pressure linked to herniation? An in-vitro study using a porcine model. J Biomech. 2016;49(9):1824-1830. [81] BERGER-ROSCHER N, CASAROLI G, RASCHE V, et al. Influence of complex loading conditions on intervertebral disc failure. Spine (Phila Pa 1976). 2017;42(2):E78-E85. [82] WADE K, BERGER-ROSCHER N, RASCHE V, et al. Disc wall structural abnormalities can act as initiation sites for herniation. Eur Cell Mater. 2020; 40:227-238. [83] SCHMIDT H, KETTLER A, HEUER F, et al. Intradiscal pressure, shear strain, and fiber strain in the intervertebral disc under combined loading. Spine (Phila Pa 1976). 2007;32(7):748-755. [84] WANG Z, HUTTON WC, YOON ST. ISSLS Prize winner: effect of link protein peptide on human intervertebral disc cells. Spine (Phila Pa 1976). 2013; 38(17):1501-1507. [85] HRISTOVA GI, JARZEM P, OUELLET JA, et al. Calcification in human intervertebral disc degeneration and scoliosis. J Orthop Res. 2011;29(12): 1888-1895. [86] EYVAZOV K, SAMARTZIS D, CHEUNG JP. The association of lumbar curve magnitude and spinal range of motion in adolescent idiopathic scoliosis: a cross-sectional study. BMC Musculoskelet Disord. 2017;18(1):51. [87] IATRIDIS JC, AP GWYNN I. Mechanisms for mechanical damage in the intervertebral disc annulus fibrosus. J Biomech. 2004;37(8):1165-1175. [88] 祝永刚,孙旗,陈江,等. 退变椎间盘生物力学研究[J].中国矫形外科杂志,2018,26(15):1400-1404. |
| [1] | Cao Yue, Ye Xinjian, Li Biyao, Zhang Yining, Feng Jianying. Effect of extracellular vesicles for diagnosis and therapy of oral squamous cell carcinoma [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1523-1530. |
| [2] | Lu Jieming, Li Yajing, Du Peijie, Xu Dongqing. Effects of artificial turf versus natural grass on biomechanical performance of the lower limbs in young females during jump-landing [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(6): 1101-1107. |
| [3] | Wang X, Wang Hm, Chen Sh, Feng Tx, Bu Hm, Zhu Lg, Chen Dd, Wei X. Stress and morphological characteristics of intervertebral foramen of cervical rotation-traction manipulation for treating cervical spondylotic radiculopathy: a three-dimensional finite element analysis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(3): 441-447. |
| [4] | Zhao Yuxin, Liang Liang, Jin Feng, Xu Yangyang, Kang Zhijie, Fang Yuan, He Yujie, Wang Xing, Wang Haiyan, Li Xiaohe. Establishment and stress analysis of a finite element model for adolescent cervical disc herniation [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(3): 448-454. |
| [5] | Li Zhenggang, Shang Xuehong, Wu Zhang, Li Hong, Sun Chaojun, Chen Huadong, Sun Zhe, Yang Yi. Finite element analysis of three internal fixation modalities for treatment of Pauwels type III femoral neck fractures under different loading conditions [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(3): 455-463. |
| [6] | Liu Mengfei, Chen Gang, Shi Yihan, Zeng Lin, Jiang Kan, Yilihamujiang•Wusiman. Finite element analysis of optimization of femoral prosthesis implantation position in unicompartmental knee arthroplasty in osteoporotic patients [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(3): 464-470. |
| [7] | Wang Zilong, Meng Xin, Zhang Zhiqi, Xie Yu, Meng Lingyue, Zhang Qiuxia, Kong Lingyu. Biomechanical characteristics of lower extremities during counter movement jump in male patients with functional ankle instability [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(3): 478-485. |
| [8] | Liang Jiyao, Zhou Honghai, Wei Guikang, Su Shaoting, Chen Longhao, He Xinyu, Liu Liangpu. Quantification of in vivo biomechanics and analysis of influencing factors in cervical spine fixed-point rotation manipulation [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(3): 486-492. |
| [9] | Su Dejun, Dong Wanpeng, Dong Yuefu, Zhang Jichao, Zhang Zhen. Design of asymmetric prosthesis and mechanical analysis of total knee arthroplasty [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(3): 510-516. |
| [10] | He Kai, Xing Wenhua, Liu Shengxiang, Bai Xianming, Zhou Chen, Gao Xu, Qiao Yu, He Qiang, Gao Zhiyu, Guo Zhen, Bao Aruhan, Li Chade. Constructing a model of degenerative scoliosis using finite element method: biomechanical analysis in etiology and treatment [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(3): 572-578. |
| [11] | Gao Xilin, Wu Si Zhang Chao Zhu Liguo, Fu Bifeng, Wang Ping. Mechanotransduction proteins in intervertebral disc degeneration [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(3): 579-589. |
| [12] | Wei Mengli, Zhong Yaping, Gui Huixian, Zhou Yiwen, Guan Yeming, Yu Shaohua. Sports injury prediction model based on machine learning [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(2): 409-418. |
| [13] | Mawulanjiang · Abudurenmu, Zilalai · Julaiti, Baibujiafu · Yelisi, Gulizainu · Yibulayin, Nijiati · Tuerxun. Stress analysis of angled abutments of maxillary central incisor implant crown in different implant spacing [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(16): 3351-3359. |
| [14] | Cao Yilin, Wang Xinyu, Zhuang Yan, Wang Yaru Jiang Zhixiu, Liu Danyu, Men Jiuxu, Ding Yuansheng. Three-dimensional finite element analysis of three-dimensional printed personalized orthodontic appliances for vertical movement of single teeth [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(16): 3360-3368. |
| [15] | Chen Guangneng, Luo Siyang, Wang Mei, Ye Bin, Chen Jiawen, Liu Yin, Zuo Yuwen, He Xianyu, Shen Jiajin, Ma Minxian. Finite element analysis of various root shield thicknesses in maxillary central incisor socket-shield technique [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(10): 2052-2060. |
| Viewed | ||||||||||||||||||||||||||||||||||||||||||||||||||
|
Full text 216
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||
|
Abstract 318
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||