Chinese Journal of Tissue Engineering Research ›› 2024, Vol. 28 ›› Issue (10): 1526-1532.doi: 10.12307/2024.249
Previous Articles Next Articles
Prussian blue nanoparticles promote wound healing of diabetic skin
Bei Ying1, Li Wenjing2, Li Meiyun1, Su Meng1, Zhang Jin1, Huang Yu1, Zhu Yanzhao1, Li Jiali1, Wu Yan1
- 1College of Life Sciences, Mudanjiang Medical University, Mudanjiang 157000, Heilongjiang Province, China; 2Department of Chemical Engineering and Materials Science, Soochow University, Suzhou 215123, Jiangsu Province, China
-
Received:2023-01-30Accepted:2023-03-06Online:2024-04-08Published:2023-08-18 -
Contact:Wu Yan, Associate professor, College of Life Sciences, Mudanjiang Medical University, Mudanjiang 157000, Heilongjiang Province, China -
About author:Bei Ying, Master, College of Life Sciences, Mudanjiang Medical University, Mudanjiang 157000, Heilongjiang Province, China -
Supported by:Natural Science Foundation of Heilongjiang Province, No. LH2020H076 (to WY); Mudanjiang City Guiding Science and Technology Plan Project, No. HT2022JG125 (to WY)
CLC Number:
Cite this article
Bei Ying, Li Wenjing, Li Meiyun, Su Meng, Zhang Jin, Huang Yu, Zhu Yanzhao, Li Jiali, Wu Yan. Prussian blue nanoparticles promote wound healing of diabetic skin[J]. Chinese Journal of Tissue Engineering Research, 2024, 28(10): 1526-1532.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
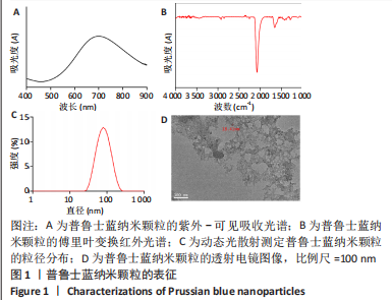
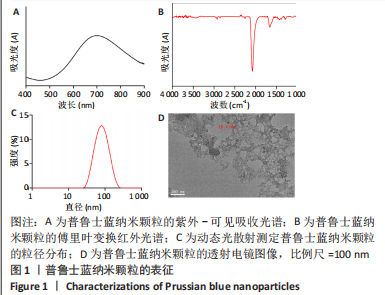
2.1 普鲁士蓝纳米颗粒的合成与表征 通过三氯化铁和亚铁氰化钾的常规反应合成了普鲁士蓝纳米颗粒,蓝色的颗粒出现表明普鲁士蓝纳米颗粒的形成。纳米颗粒的吸收光谱显示了典型的普鲁士蓝纳米颗粒的紫外光谱,由于二价铁和三价铁之间的价间电荷转移,在700 nm附近有一个峰值,见图1A。普鲁士蓝纳米颗粒的红外光谱在2 075 cm?1处有一个主峰,这是?C≡N?从Fe(Ⅱ)-CN-Fe(Ⅲ)键延伸出来的特征峰,见图1B。在3 443 cm?1附近的宽峰可归属于羟基(O-H)的伸缩,而1 660 cm?1处的峰来自H-O-H的弯曲,表明普鲁士蓝纳米颗粒中存在间隙水。在动态光散射分析中,普鲁士蓝纳米颗粒的平均粒径约为80 nm,多分散性指数为0.2,见图1C。普鲁士蓝纳米颗粒的Zeta电位为-12.9 mV,表面带负电荷。透射电子显微镜成像显示,普鲁士蓝纳米颗粒呈直径为20 nm左右的立方体形状,见图1D。动态光散射和透射电镜测量结果之间的差异是因为动态光散射表示水动力尺寸,而透射电镜表示纳米颗粒的干燥尺寸。"
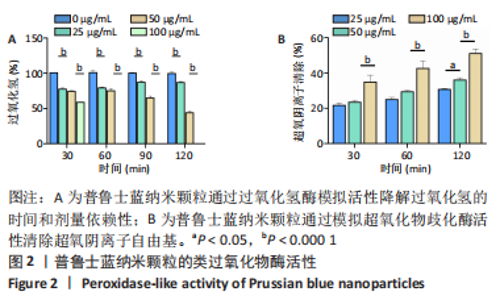
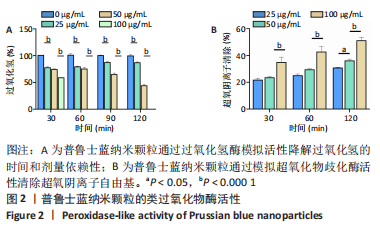
2.2 体外实验结果 2.2.1 普鲁士蓝纳米颗粒清除活性氧活性的结果 通过二甲酚橙法分析了普鲁士蓝纳米颗粒对过氧化氢的总降解能力,结果表明:普鲁士蓝纳米颗粒对过氧化氢的降解具有时间和剂量依赖性(图2A),过氧化氢的降解率随着普鲁士蓝纳米颗粒质量浓度的增加而显著增加,在反应体系中加入100 μg/mL的普鲁士蓝纳米颗粒,60 min内过氧化氢几乎完全被降解。 通过测定黄嘌呤/黄嘌呤氧化酶反应体系产生的超氧阴离子自由基的歧化程度,定量测定了普鲁士蓝纳米颗粒对超氧化物歧化酶的模拟活性。与过氧化氢的降解类似,普鲁士蓝纳米颗粒也显示出很强的清除超氧化物的能力,并呈剂量和时间依赖关系(图2B)。25 μg/mL的普鲁士蓝纳米颗粒可在反应60 min和120 min内分别清除(25±1)%和(31±1)%的超氧阴离子自由基;当普鲁士蓝纳米颗粒质量浓度增加到100 μg/mL时,超氧化物歧化能力增强,120 min内可清除50%以上的超氧化物。这些结果表明,合成的普鲁士蓝纳米颗粒具有呈剂量和时间依赖的有效清除活性氧的能力。"
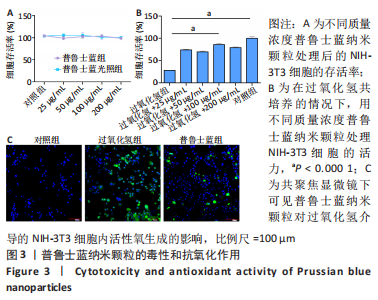
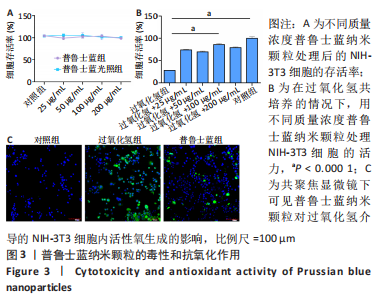
2.2.2 普鲁士蓝纳米颗粒的体外细胞保护作用 以NIH-3T3细胞为模型细胞,评价了普鲁士蓝纳米颗粒的生物相容性,结果显示:普鲁士蓝纳米颗粒无明显的细胞毒性,且在200 μg/mL普鲁士蓝纳米颗粒存在下细胞存活率将近100%(图3A);此外,普鲁士蓝光照组细胞存活率和普鲁士蓝组无明显差异。 接下来,探索了普鲁士蓝纳米颗粒在氧化应激环境下对细胞的保护作用,将细胞与过氧化氢(100 μmol/L)孵育,模拟氧化应激环境,结果显示:仅用过氧化氢处理导致细胞损伤,细胞存活率仅(28±1)%,然而随着普鲁士蓝纳米颗粒质量浓度的增加,细胞存活率显著提高,均达到60%以上;其中,加入100 μg/mL的普鲁士蓝纳米颗粒时细胞存活率高达(86±3)%(图3B)。 为了验证这种细胞保护作用是由于普鲁士蓝纳米颗粒清除活性氧减少氧化应激,使用DCFH-DA作为荧光探针检测了细胞内活性氧水平,如图3C所示,过氧化氢处理导致细胞内高荧光信号,与细胞内活性氧水平增强相对应;在普鲁士蓝纳米颗粒的存在下荧光信号显著减弱,表明纳米颗粒具有有效清除活性氧的活性。"
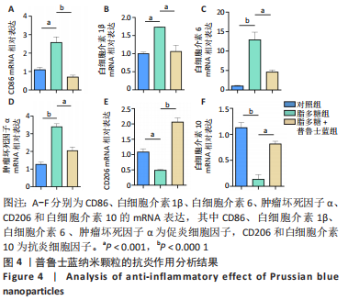
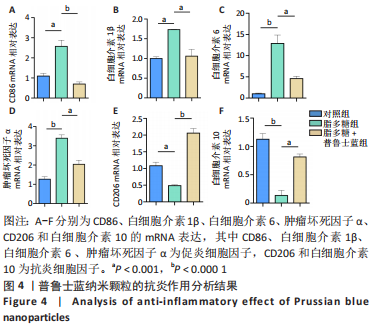
2.2.3 普鲁士蓝纳米颗粒的体外抗炎作用 通过炎性基因表达分析来评估普鲁士蓝纳米颗粒的抗炎作用。在炎性环境中,巨噬细胞中促炎基因的表达可能显著增加,而抗炎基因的表达则减少。如图4所示,脂多糖刺激后巨噬细胞中促炎症细胞因子(CD86、白细胞介素1β、白细胞介素6及肿瘤坏死因子α)的表达显著增加;与脂多糖单独处理的细胞相比,脂多糖和普鲁士蓝纳米颗粒共同处理的细胞显示出明显的CD86、白细胞介素1β、白细胞介素6及肿瘤坏死因子α的表达减少;此外,在脂多糖刺激后,细胞CD206和白细胞介素10的表达减少;相比之下,在脂多糖和普鲁士蓝纳米颗粒共同处理的细胞中,CD206和白细胞介素10的表达显著增加(P < 0.05)。结果表明,普鲁士蓝纳米颗粒抑制了脂多糖刺激巨噬细胞后所引发的炎症反应。"
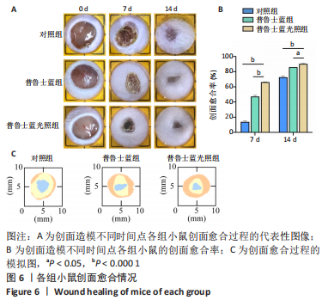
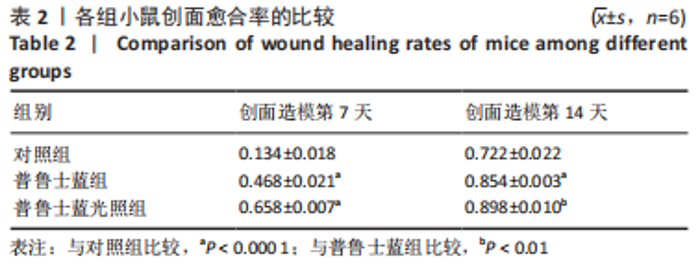
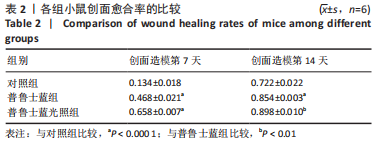
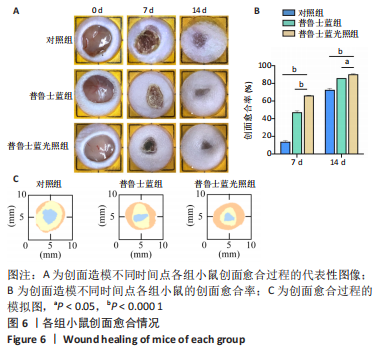
2.3 体内实验结果 2.3.1 实验动物数量分析 实验过程中无小鼠死亡,36只小鼠全部进入结果分析。 2.3.2 各组小鼠创面宏观愈合情况观察 使用全层糖尿病创面模型检测了普鲁士蓝纳米颗粒的促创面愈合能力。 图6A,B显示了不同时间点的创面愈合情况。创面造模第7天,与对照组相比较,普鲁士蓝组和普鲁士蓝光照组小鼠创面面积显著缩小,创面愈合率分别为(46.8±2.1)%和(65.8±0.7)%,创面造模第14天,各组创面面积进一步缩小,其中普鲁士蓝光照组愈合最快,创面愈合率高达(89.8±1.0)%,见图6C、表2。结果表明,普鲁士蓝纳米颗粒明显改善了糖尿病小鼠创面修复,且光照进一步加强了这一作用。"
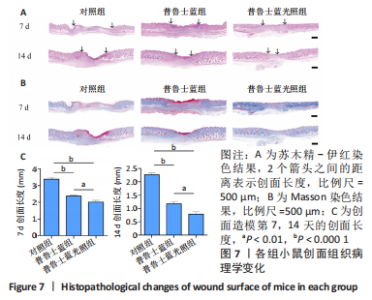
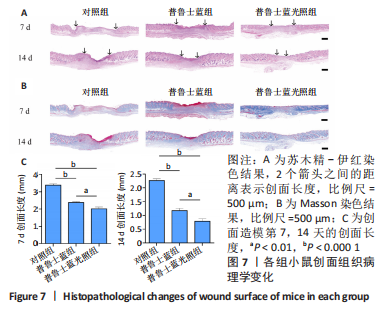
2.3.3 各组小鼠创面组织学变化分析 通过组织学分析评价不同处理组的创面愈合效果。如图7A所示,创面造模第7天,苏木精-伊红染色结果显示,普鲁士蓝组和普鲁士蓝光照组小鼠创面肉芽组织明显增厚,且创面长度开始有明显差距,其中对照组创面长度最长,为(3.38±0.10) mm;普鲁士蓝光照组的创面长度最短,为(2.01±0.10) mm(图7C)。创面造模第14天,各组小鼠创面长度进一步缩短,普鲁士蓝组和普鲁士蓝光照组创面长度分别为(1.17±0.10) mm和(0.79±0.10) mm,与对照组(2.26±0.10) mm相比创面愈合更快(图7C)。此外,普鲁士蓝光照组的组织中可见大量皮肤附属器,而其他组中均未见或见少量。 在创面愈合过程中胶原是细胞外基质的主要成分,创面部位胶原的形成对创面的最终修复至关重要。如图7B所示,Masson染色结果显示,创面造模第7天,除对照组外,其余两组创面均产生了丰富的胶原纤维,其中普鲁士蓝光照组最多。创面造模第14 天,各组相对于第7天均显示出更多的胶原沉积,尤其以普鲁士蓝光照组胶原沉积最多。此外,普鲁士蓝光照组中也清晰可见大量的皮肤附属器形成,这证实了上述苏木精-伊红染色结果。"

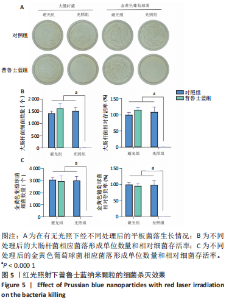
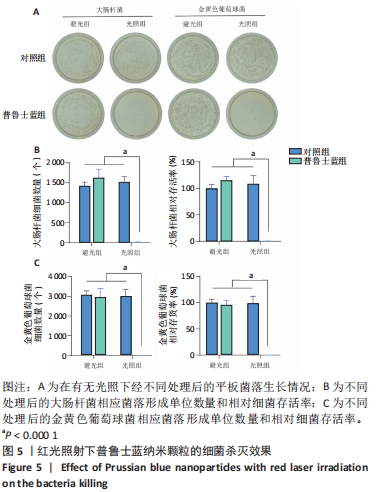
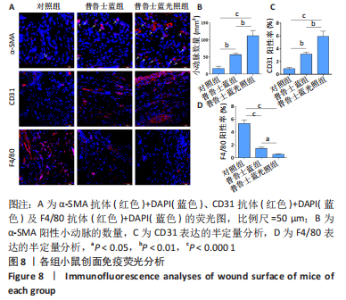
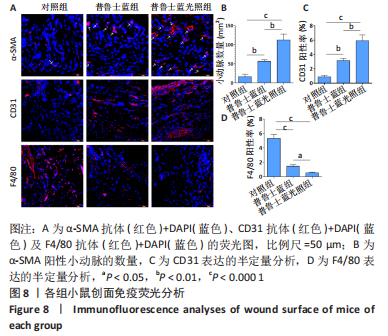
通过α-SMA、CD31染色来分析创面区域血管形成情况,F4/80以分析创面区域炎症情况。然而,α-SMA 不仅在血管平滑肌细胞中表达,也在肌成纤维细胞中表达,因此仅分析了代表新生小动脉的 α-SMA 阳性圆形结构(如图8A中的白色箭头所示),以估计创面区域内的血管形成情况。与对照组相比较,普鲁士蓝组和普鲁士蓝光照组α-SMA表达显著增加;与普鲁士蓝组相比,普鲁士蓝光照组α-SMA表达显然更多[(16±7),(57±4),(112±16)个/mm2](图8B)。此外,采用CD31抗体染色来检测创面区域的毛细血管,结果显示:普鲁士蓝光照组CD31的表达[(5.9±0.1)%]显著高于其他组,几乎是对照组[(0.8±0.2)%]的7倍(图8C)。 普鲁士蓝纳米颗粒可能会减弱创面处巨噬细胞的募集[28]。为了分析巨噬细胞募集的程度,检测了表达在巨噬细胞表面的F4/80糖蛋白的免疫荧光染色,这种糖蛋白通常用于识别组织巨噬细胞。如图8D所示,对照组 F4/80阳性区域的百分比[(5.3±0.5)%]显著高于其他组,普鲁士蓝组F4/80阳性率较对照组明显降低,为(1.4±0.2)%,普鲁士蓝光照组F4/80阳性率最低,为(0.5±0.1)%。 2.4 材料生物相容性 由体外实验与体内实验结果可知,普鲁士蓝纳米颗粒具有良好的生物相容性。"

| [1] BURGESS JL, WYANT WA, ABDO ABUJAMRA B, et al. Diabetic Wound-Healing Science. Medicina (Kaunas). 2021;57(10):1072. [2] DUNNILL C, PATTON T, BRENNAN J, et al. Reactive oxygen species (ROS) and wound healing: the functional role of ROS and emerging ROS-modulating technologies for augmentation of the healing process. Int Wound J. 2017;14(1):89-96. [3] DENG L, DU C, SONG P, et al. The Role of Oxidative Stress and Antioxidants in Diabetic Wound Healing. Oxid Med Cell Longev. 2021;2021:8852759. [4] KUNKEMOELLER B, KYRIAKIDES TR. Redox Signaling in Diabetic Wound Healing Regulates Extracellular Matrix Deposition. Antioxid Redox Signal. 2017;27(12): 823-838. [5] CHANG M, NGUYEN TT. Strategy for Treatment of Infected Diabetic Foot Ulcers. Acc Chem Res. 2021;54(5):1080-1093. [6] PATEL S, SRIVASTAVA S, SINGH MR, et al. Mechanistic insight into diabetic wounds: Pathogenesis, molecular targets and treatment strategies to pace wound healing. Biomed Pharmacother. 2019;112:108615. [7] OYEBODE O, HOURELD NN, ABRAHAMSE H. Photobiomodulation in diabetic wound healing: A review of red and near-infrared wavelength applications. Cell Biochem Funct. 2021;39(5):596-612. [8] EZHILARASU H, VISHALLI D, DHEEN ST, et al. Nanoparticle-Based Therapeutic Approach for Diabetic Wound Healing. Nanomaterials (Basel). 2020;10(6):1234. [9] WANG F, ZHANG W, LI H, et al. How Effective are Nano-Based Dressings in Diabetic Wound Healing? A Comprehensive Review of Literature. Int J Nanomedicine. 2022;17:2097-2119. [10] BAI Q, HAN K, DONG K, et al. Potential Applications of Nanomaterials and Technology for Diabetic Wound Healing. Int J Nanomedicine. 2020;15:9717-9743. [11] SABAREES G, VELMURUGAN V, TAMILARASI GP, et al. Recent Advances in Silver Nanoparticles Containing Nanofibers for Chronic Wound Management. Polymers (Basel). 2022;14(19):3994. [12] MASOOD N, AHMED R, TARIQ M, et al. Silver nanoparticle impregnated chitosan-PEG hydrogel enhances wound healing in diabetes induced rabbits. Int J Pharm. 2019;559:23-36. [13] ZHAO X, CHANG L, HU Y, et al. Preparation of Photocatalytic and Antibacterial MOF Nanozyme Used for Infected Diabetic Wound Healing. ACS Appl Mater Interfaces. 2022;14(16):18194-18208. [14] CHEN YH, RAO ZF, LIU YJ, et al. Multifunctional Injectable Hydrogel Loaded with Cerium-Containing Bioactive Glass Nanoparticles for Diabetic Wound Healing. Biomolecules. 2021; 11(5):702. [15] LI S, WANG X, CHEN J, et al. Calcium ion cross-linked sodium alginate hydrogels containing deferoxamine and copper nanoparticles for diabetic wound healing. Int J Biol Macromol. 2022;202:657-670. [16] GAN J, LIU C, LI H, et al. Accelerated wound healing in diabetes by reprogramming the macrophages with particle-induced clustering of the mannose receptors. Biomaterials. 2019; 219:119340. [17] 吴海能,耿康,王静,等.富血小板纤维蛋白联合姜黄素纳米颗粒水凝胶促糖尿病小鼠创面愈合[J].中国组织工程研究,2022,26(27):4300-4307. [18] WANG Y, PANG X, WANG J, et al. Magnetically-targeted and near infrared fluorescence/magnetic resonance/photoacoustic imaging-guided combinational anti-tumor phototherapy based on polydopamine-capped magnetic Prussian blue nanoparticles. J Mater Chem B. 2018;6(16):2460-2473. [19] LU L, ZHANG C, ZOU B, et al. Hollow Prussian Blue Nanospheres for Photothermal/Chemo-Synergistic Therapy. Int J Nanomedicine. 2020;15: 5165-5177. [20] WANG S, ZENG N, ZHANG Q, et al. Nanozyme Hydrogels for Self-Augmented Sonodynamic/Photothermal Combination Therapy. Front Oncol. 2022;12:888855. [21] XU M, GAO H, JI Q, et al. Construction multifunctional nanozyme for synergistic catalytic therapy and phototherapy based on controllable performance. J Colloid Interface Sci. 2022; 609:364-374. [22] MAAOUI H, JIJIE R, PAN GH, et al. A 980nm driven photothermal ablation of virulent and antibiotic resistant Gram-positive and Gram-negative bacteria strains using Prussian blue nanoparticles. J Colloid Interface Sci. 2016;480:63-68. [23] BORZENKOV M, CHIRICO G, PALLAVICINI P, et al. Nanocomposite Sprayed Films with Photo-Thermal Properties for Remote Bacteria Eradication. Nanomaterials (Basel). 2020;10(4):786. [24] LI Y, YU P, WEN J, et al. Nanozyme‐Based Stretchable Hydrogel of Low Hysteresis with Antibacterial and Antioxidant Dual Functions for Closely Fitting and Wound Healing in Movable Parts. Adv Funct Mater. 2021;32(13):2110720. [25] ESTELRICH J, BUSQUETS MA. Prussian Blue: A Nanozyme with Versatile Catalytic Properties. Int J Mol Sci. 2021;22(11):5993. [26] 王青,刘学,孙亚娟,等.负载普鲁士蓝的CoOOH纳米片用于抗氧化活性检测的研究[J].现代化工,2023,43(2):234-238. [27] WANG Y, LIANG Z, LIANG Z, et al. Advancements of Prussian blue-based nanoplatforms in biomedical fields: Progress and perspectives. J Control Release. 2022;351:752-778. [28] SAHU A, JEON J, LEE MS, et al. Antioxidant and anti-inflammatory activities of Prussian blue nanozyme promotes full-thickness skin wound healing. Mater Sci Eng C Mater Biol Appl. 2021;119:111596. [29] GANTWERKER EA, HOM DB. Skin: histology and physiology of wound healing. Clin Plast Surg. 2012;39(1):85-97. [30] VELNAR T, BAILEY T, SMRKOLJ V. The wound healing process: an overview of the cellular and molecular mechanisms. J Int Med Res. 2009;37(5):1528-1542. [31] MARTIN P, NUNAN R. Cellular and molecular mechanisms of repair in acute and chronic wound healing. Br J Dermatol. 2015;173(2):370-378. [32] BURANASIN P, MIZUTANI K, IWASAKI K, et al. High glucose-induced oxidative stress impairs proliferation and migration of human gingival fibroblasts. PLoS One. 2018;13(8):e0201855. [33] GONG F, ZHANG Y, CHENG S, et al. Inhibition of TGFbeta1/Smad pathway by NF-kappaB induces inflammation leading to poor wound healing in high glucose. Cells Dev. 2022;172:203814. [34] QIN W, WU Y, LIU J, et al. A Comprehensive Review of the Application of Nanoparticles in Diabetic Wound Healing: Therapeutic Potential and Future Perspectives. Int J Nanomedicine. 2022;17:6007-6029. [35] SAYED FA, EISSA NG, SHEN Y, et al. Morphologic design of nanostructures for enhanced antimicrobial activity. J Nanobiotechnology. 2022;20(1):536. [36] SAHOO BM, BANIK BK, BORAH P, et al. Reactive Oxygen Species (ROS): Key Components in Cancer Therapies. Anticancer Agents Med Chem. 2022; 22(2):215-222. [37] NIKITINA VN, ZAVOLSKOVA MD, KARYAKIN AA. Protein-sized nanozymes <<artificial peroxidase>> based on template catalytic synthesis of Prussian Blue. Bioelectrochemistry. 2023;149:108275. [38] LI Z, GUO X, QIN J, et al. Size-effect on the intracellular antioxidative activity of Prussian blue nanoparticles investigated by atomic force microscopy. Anal Chim Acta. 2022;1227:340321. [39] AITCHESON SM, FRENTIU FD, HURN SE, et al. Skin Wound Healing: Normal Macrophage Function and Macrophage Dysfunction in Diabetic Wounds. Molecules. 2021;26(16):4917. [40] RAZIYEVA K, KIM Y, ZHARKINBEKOV Z, et al. Immunology of Acute and Chronic Wound Healing. Biomolecules. 2021;11(5):700. [41] LOUISELLE AE, NIEMIEC SM, ZGHEIB C, et al. Macrophage polarization and diabetic wound healing. Transl Res. 2021;236:109-116. [42] LI D, LIU M, LI W, et al. Synthesis of Prussian Blue Nanoparticles and Their Antibacterial, Antiinflammation and Antitumor Applications. Pharmaceuticals (Basel). 2022;15(7):769. [43] SAHU A, JEON J, LEE MS, et al. Nanozyme Impregnated Mesenchymal Stem Cells for Hepatic Ischemia-Reperfusion Injury Alleviation. ACS Appl Mater Interfaces. 2021;13(22):25649-25662. [44] TONG C, ZHONG X, YANG Y, et al. PB@PDA@Ag nanosystem for synergistically eradicating MRSA and accelerating diabetic wound healing assisted with laser irradiation. Biomaterials. 2020;243:119936. [45] TAN L, LI J, LIU X, et al. Rapid Biofilm Eradication on Bone Implants Using Red Phosphorus and Near-Infrared Light. Adv Mater. 2018;30(31):e1801808. [46] SUN J, SONG L, FAN Y, et al. Synergistic Photodynamic and Photothermal Antibacterial Nanocomposite Membrane Triggered by Single NIR Light Source. ACS Appl Mater Interfaces. 2019;11(30):26581-26589. [47] WANG X, SUN X, BU T, et al. Construction of a photothermal hydrogel platform with two-dimensional PEG@zirconium-ferrocene MOF nanozymes for rapid tissue repair of bacteria-infected wounds. Acta Biomater. 2021; 135:342-355. |
| [1] | Zhang Ya, Mu Qiuju, Wang Zilin, Liu Hongjie, Zhu Lili. Hydrogel loaded with platelet-rich plasma promotes wound healing in diabetic rats [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(5): 690-696. |
| [2] | Jiao Wencheng, Dai Jing, Yan Wenrui, Shen Jintao, Hu Jinglu, Jin Yiguang, Du Lina. 3D-printed multifunctional wound dressing for combined radiation and wound injury [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(10): 1562-1567. |
| [3] | Deng Rui, Huang Keming, Luo Jian, Chen Gong, Feng Jian, Huang Weiyi, Wei Gang. Effect of heme oxygenase-1-mediated atorvastatin on macrophage polarization and cholesterol accumulation [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(1): 62-67. |
| [4] | Li Li, Li Xiao, Li Duchenhui, Zhang Jie, Xiao Tianjiao, Kang Jiabing, Tian Ai. Regulation of interleukin-4 on osteoclast differentiation during bone regeneration guided by bone replacement materials [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(34): 5455-5461. |
| [5] | Zhou Jie, Pei Xibo, Wan Qianbing. Advances and biological application of asymmetric dressings [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(3): 434-440. |
| [6] | Tian Yuyi, Liu Lihong. Osteoimmunological effects of macrophages [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(29): 4712-4722. |
| [7] | Liu Jichao, Zhao Jinlong, Yu Yang. Silk fibroin collagen composite scaffold combined with platelet-rich plasma for repairing skin injury [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(25): 3971-3976. |
| [8] | Zhang Jie, Tian Ai. Advances in the signaling pathway of M2 macrophages involved in bone regeneration [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(2): 314-321. |
| [9] | Zhu Hong, Lin Ziheng, He Rouye, Pan Jinbin, Liu Xiaochuan, He Xiaoling, Zhang Jingying. Antibacterial and hemostatic properties of chitosan collagen sponge [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(16): 2525-2533. |
| [10] | Chen Di, Xue Yu, Tang Yu, Fei Xiaoming, Zhuang Qin, Zhou Wenwen, Lyu Demin, Shi Wentao, Zhang Zhijian, Zheng Wenjuan, Jiang Yu. Ecto-mesenchymal stem cell-conditioned medium lyophilized powder combined with fibrin glue to repair skin injury in rats [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(15): 2350-2355. |
| [11] | Xu Mingkui, Xu Riming, Lin Yewu, Luo Yuantai, Zhang Xihui, Zhou Li, Yuan Shiguo. Naringenin repairs skeletal muscle injury by regulating polarization of macrophages and proliferation of muscle satellite cells [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(14): 2133-2138. |
| [12] | Ma Hongfeng, Ren Qing, Cheng Wanmin, Wang Jiguo, Meng Qingyan. Effects of Sanhuang gel on wound healing, basic fibroblast growth factor and interleukin 17 levels of auricular skin defect in rabbits [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(12): 1842-1847. |
| [13] | Ling Huajun, Cui Ruiwen, Wang Qiyou. 3D extracellular matrix hydrogel loaded with exosomes promotes wound repair [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(12): 1900-1905. |
| [14] | Hu Jinlong, Quan Huahong, Wang Jingcheng, Zhang Pei, Zhang Jiale, Chen Pengtao, Liang Yuan. Effect of copper sulfide nanoparticles loaded thermosensitive hydrogel Pluronic F127 on infected wound healing in rats [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(12): 1927-1931. |
| [15] | Zhang Rui, Liu Lan, Xie Defu, Lin Yingxi, Ren Hongjing, Yan Hong. Effect of adipose-derived stem cells stimulated by direct current electric field on refractory wound healing in diabetic rats [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(10): 1484-1491. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||