Chinese Journal of Tissue Engineering Research ›› 2024, Vol. 28 ›› Issue (10): 1519-1525.doi: 10.12307/2024.317
Previous Articles Next Articles
Tabersonine alleviates wear particle-induced inflammatory osteolysis by inhibiting osteoclast activation
Zhang Wei, Yu Lei, Yang Peng, Geng Dechun
- Department of Orthopedics, First Affiliated Hospital of Soochow University, Suzhou 215006, Jiangsu Province, China
-
Received:2023-03-24Accepted:2023-05-15Online:2024-04-08Published:2023-08-18 -
Contact:Geng Dechun, Researcher, Department of Orthopedics, First Affiliated Hospital of Soochow University, Suzhou 215006, Jiangsu Province, China -
About author:Zhang Wei, Master, Attending physician, Department of Orthopedics, First Affiliated Hospital of Soochow University, Suzhou 215006, Jiangsu Province, China -
Supported by:National Natural Science Foundation of China, No. 8227091833 (to GDC)
CLC Number:
Cite this article
Zhang Wei, Yu Lei, Yang Peng, Geng Dechun. Tabersonine alleviates wear particle-induced inflammatory osteolysis by inhibiting osteoclast activation[J]. Chinese Journal of Tissue Engineering Research, 2024, 28(10): 1519-1525.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
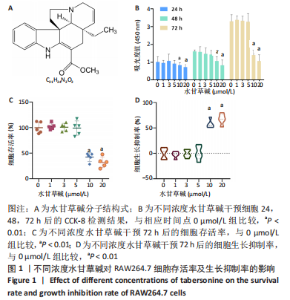
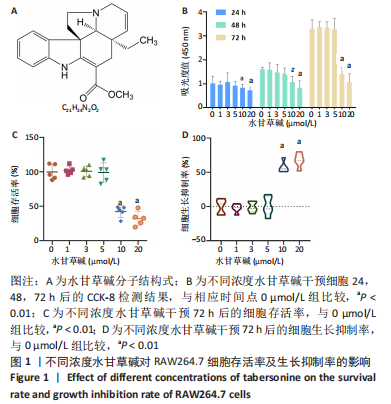
2.1 水甘草碱对RAW264.7细胞增殖的影响及细胞毒性作用 水甘草碱是一种菊科长春花提取物质(分子结构式见图1A),将RAW264.7细胞分别暴露于0-20 μmol/L的水甘草碱中24,48,72 h,并进行CCK-8检测。检测结果显示,当水甘草碱浓度低于5 μmol/L时,细胞吸光度值均未受到显著影响;当水甘草碱浓度上升至10,20 μmol/L时,细胞吸光度值急剧降低,见图1B,提示水甘草碱浓度≤5 μmol/L时对RAW264.7细胞的增殖活性无影响。另外,使用水甘草碱干预72 h,若水甘草碱浓度≤5 μmol/L时,细胞存活率与对照组无明显差异(P > 0.05),见图1C;当水甘草碱浓度为10,20 μmol/L时,细胞抑制率分别达到(57.41±8.25)%和(67.60±10.40)%,见图1D,提示10 μmol/L及以上浓度的水甘草碱影响RAW264.7细胞的增殖,对RAW264.7细胞具有毒性作用。鉴于以上细胞抑制率检测结果,课题组选用1,5 μmol/L的水甘草碱进行细胞实验。"
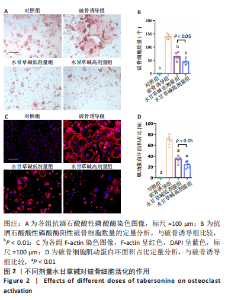
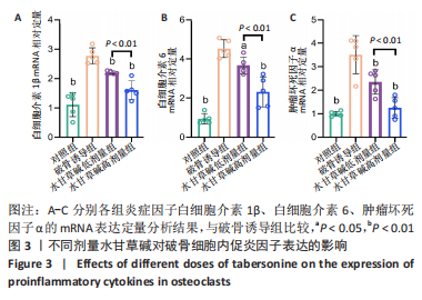
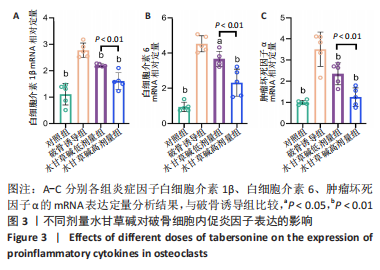
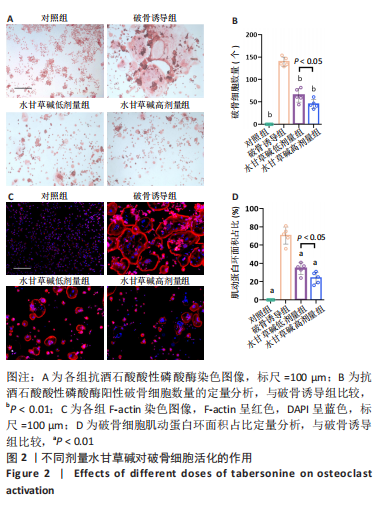
2.2 体外实验结果 2.2.1 水甘草碱对破骨细胞活化的影响 抗酒石酸酸性磷酸酶染色显示,破骨诱导组细胞融合形成抗酒石酸酸性磷酸酶阳性多核破骨细胞,与破骨诱导组比较,使用水甘草碱干预后破骨细胞形成显著减少,并且水甘草碱高剂量组对破骨细胞活化的抑制作用强于水甘草碱低剂量组(P < 0.05),见图2A、B。 F-actin环的形成和破骨细胞的迁移、侵袭、黏附能力有关,是反映破骨细胞活性的重要指标。F-actin染色显示,在破骨诱导过程中破骨细胞F-actin环逐步融合形成,使用水甘草碱干预后破骨细胞F-actin环面积占比较破骨诱导组均降低,并且水甘草碱高剂量组对F-actin的形成抑制作用强于水甘草碱低剂量组(P < 0.05),见图2C、D。"
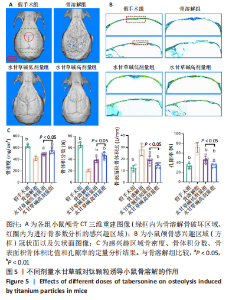
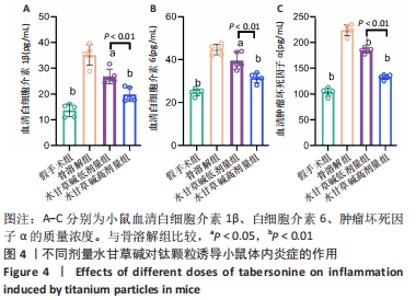
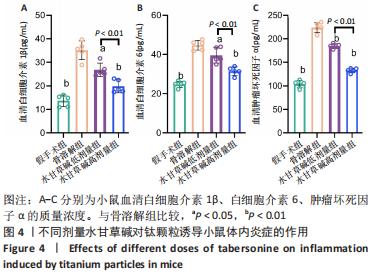
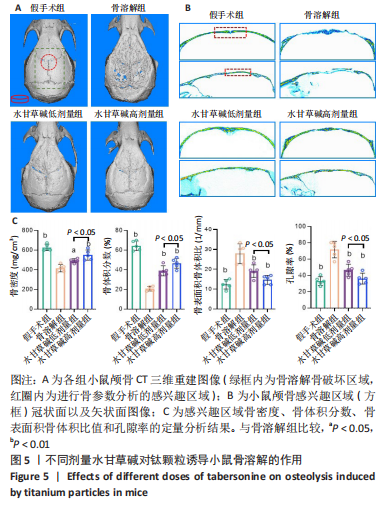
2.3.3 水甘草碱对钛颗粒诱导骨质破坏的影响 使用micro-CT对颅骨进行扫描,并进行2D和3D重建,对颅骨感兴趣区域(矢状缝和冠状缝交界处直径200 mm、高度20 mm的圆柱体区域)进行骨溶解度和骨参数分析。2D和3D重建图像显示,骨溶解组小鼠颅骨受到广泛侵蚀,颅腔封闭性丢失;与骨溶解组比较,水甘草碱干预组明显缓解了小鼠颅骨的骨质侵蚀破坏,见图5A、B。基于感兴趣区域的骨参数分析发现,相较于假手术组,骨溶解组小鼠颅骨感兴趣区域的骨密度和骨体积分数降低(P < 0.01)、骨表面积骨体积比值和颅骨孔隙率升高(P < 0.01),成功构建骨溶解模型;与骨溶解组比较,水甘草碱干预逆转了钛颗粒导致的骨参数变化(P < 0.05,P < 0.01),其中水甘草碱高剂量组的疗效好于水甘草碱低剂量组(P < 0.05),见图5C。"
| [1] SHETH NP, ROZELL JC,PAPROSKY WG. Evaluation and Treatment of Patients With Acetabular Osteolysis After Total Hip Arthroplasty. J Am Acad Orthop Surg. 2019;27(6):e258-e267. [2] KURTZ S, ONG K, LAU E, et al. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785. [3] MAHOMED NN, BARRETT JA, KATZ JN, et al. Rates and outcomes of primary and revision total hip replacement in the United States medicare population. J Bone Joint Surg Am. 2003;85(1):27-32. [4] ONG KL, LAU E, SUGGS J, et al. Risk of subsequent revision after primary and revision total joint arthroplasty. Clin Orthop Relat Res. 2010;468(11):3070-3076. [5] SCHWARTZ AM, FARLEY KX, GUILD GN, et al. Projections and Epidemiology of Revision Hip and Knee Arthroplasty in the United States to 2030. J Arthroplasty. 2020;35(6S):S79-S85. [6] GOODMAN SB, MA T. Cellular chemotaxis induced by wear particles from joint replacements. Biomaterials. 2010;31(19): 5045-5050. [7] OLLIVERE B, WIMHURST JA, CLARK IM, et al. Current concepts in osteolysis. J Bone Joint Surg Br. 2012;94(1):10-15. [8] BITAR D, PARVIZI J. Biological response to prosthetic debris. World J Orthop. 2015;6(2):172-189. [9] HOLT G, MURNAGHAN C, REILLY J, et al. The biology of aseptic osteolysis. Clin Orthop Relat Res. 2007;460:240-252. [10] HODGES NA, SUSSMAN EM, STEGEMANN JP. Aseptic and septic prosthetic joint loosening: Impact of biomaterial wear on immune cell function, inflammation, and infection. Biomaterials. 2021;278:121127. [11] LI Y, LING J, JIANG Q. Inflammasomes in Alveolar Bone Loss. Front Immunol. 2021;12:691013. [12] NEJAT N, VALDIANI A, CAHILL D, et al. Ornamental exterior versus therapeutic interior of Madagascar periwinkle (Catharanthus roseus): the two faces of a versatile herb. ScientificWorldJournal. 2015;2015: 982412. [13] QU Y, EASSON ML, FROESE J, et al. Completion of the seven-step pathway from tabersonine to the anticancer drug precursor vindoline and its assembly in yeast. Proc Natl Acad Sci U S A. 2015;112(19): 6224-6229. [14] ZHANG D, LI X, HU Y, et al. Tabersonine attenuates lipopolysaccharide-induced acute lung injury via suppressing TRAF6 ubiquitination. Biochem Pharmacol. 2018;154:183-192. [15] DAI C, LUO W, CHEN Y, et al. Tabersonine attenuates Angiotensin II-induced cardiac remodeling and dysfunction through targeting TAK1 and inhibiting TAK1-mediated cardiac inflammation. Phytomedicine. 2022;103:154238. [16] 张云鸽,宋科官.假体周围磨损颗粒诱导骨溶解:钙调磷酸酶/活化T细胞核因子信号通路作用的研究进展[J].中国组织工程研究, 2017;21(7):1115-1122. [17] SUN KY, WU Y, XU J, et al. Niobium carbide (MXene) reduces UHMWPE particle-induced osteolysis. Bioact Mater. 2022;8:435-448. [18] CALLAGHAN JJ, HENNESSY DW, LIU SS, et al. Cementing acetabular liners into secure cementless shells for polyethylene wear provides durable mid-term fixation. Clin Orthop Relat Res. 2012;470(11): 3142-3147. [19] LU Y, XU X, YANG C, et al. Copper modified cobalt-chromium particles for attenuating wear particle induced-inflammation and osteoclastogenesis. Biomater Adv. 2023;147:213315. [20] YIN J, YIN Z, LAI P, et al. Pyroptosis in Periprosthetic Osteolysis. Biomolecules. 2022;12(12):1733. [21] ZHAO F, CANG D, ZHANG J, et al. Chemerin/ChemR23 signaling mediates the effects of ultra-high molecular weight polyethylene wear particles on the balance between osteoblast and osteoclast differentiation. Ann Transl Med. 2021;9(14):1149. [22] FERREIRA P, BATES P, DAOUB A, et al. Is bisphosphonate use a risk factor for atypical periprosthetic/peri-implant fractures? - A metanalysis of retrospective cohort studies and systematic review of the current evidence. Orthop Traumatol Surg Res. 2023;109(2):103475. [23] FIORILLO L, CICCIU M, TOZUM TF, et al. Impact of bisphosphonate drugs on dental implant healing and peri-implant hard and soft tissues: a systematic review. BMC Oral Health. 2022;22(1):291. [24] KAWAHARA M, KUROSHIMA S, SAWASE T. Clinical considerations for medication-related osteonecrosis of the jaw: a comprehensive literature review. Int J Implant Dent. 2021;7(1):47. [25] QIAN C, WANG J, LIN W, et al. Tabersonine attenuates obesity-induced renal injury via inhibiting NF-kappaB-mediated inflammation. Phytother Res. 2023. doi: 10.1002/ptr.7756. [26] SHI J, WANG C, SANG C, et al. Tabersonine Inhibits the Lipopolysaccharide-Induced Neuroinflammatory Response in BV2 Microglia Cells via the NF-kappaB Signaling Pathway. Molecules. 2022; 27(21):7521. [27] SUN X, GAN L, LI N, et al. Tabersonine ameliorates osteoblast apoptosis in rats with dexamethasone-induced osteoporosis by regulating the Nrf2/ROS/Bax signalling pathway. AMB Express. 2020;10(1):165. [28] XU HW, LI WF, HONG SS, et al. Tabersonine, a natural NLRP3 inhibitor, suppresses inflammasome activation in macrophages and attenuate NLRP3-driven diseases in mice. Acta Pharmacol Sin. 2023. doi: 10.1038/s41401-022-01040-z. [29] KIM JM, LIN C, STAVRE Z, et al. Osteoblast-Osteoclast Communication and Bone Homeostasis. Cells. 2020;9(9):2073. [30] 谢冰洁,冯捷,韩向龙.破骨细胞生物学特征的研究与进展[J].中国组织工程研究,2017,21(11):1770-1775. [31] YAO Y, CAI X, REN F, et al. The Macrophage-Osteoclast Axis in Osteoimmunity and Osteo-Related Diseases. Front Immunol. 2021;12: 664871. [32] WEI L, CHEN W, HUANG L, et al. Alpinetin ameliorates bone loss in LPS-induced inflammation osteolysis via ROS mediated P38/PI3K signaling pathway. Pharmacol Res. 2022;184:106400. [33] WEN Z, LIN S, LI C, et al. MiR-92a/KLF4/p110delta regulates titanium particles-induced macrophages inflammation and osteolysis. Cell Death Discov. 2022;8(1):197. [34] HU X, PING Z, GAN M, et al. Theaflavin-3,3’-digallate represses osteoclastogenesis and prevents wear debris-induced osteolysis via suppression of ERK pathway. Acta Biomater. 2017;48:479-488. [35] PING Z, WANG Z, SHI J, et al. Inhibitory effects of melatonin on titanium particle-induced inflammatory bone resorption and osteoclastogenesis via suppression of NF-kappaB signaling. Acta Biomater. 2017;62:362-371. [36] GREEN JM, HALLAB NJ, LIAO YS, et al. Anti-oxidation treatment of ultra high molecular weight polyethylene components to decrease periprosthetic osteolysis: evaluation of osteolytic and osteogenic properties of wear debris particles in a murine calvaria model. Curr Rheumatol Rep. 2013;15(5):325. |
| [1] | Wang Weiqing, Zhou Yue. Chronic inflammation regulates adipose tissue fibrosis [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(8): 1307-1312. |
| [2] | Li Long, Li Guangdi, Shi Hao, Deng Keqi. Circular RNA as a competing endogenous RNA is involved in the regulation of osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(5): 751-757. |
| [3] | Ma Suilu, He Zhijun, Liu Tao, Li Jinpeng, Li Yan, He Bo, Wei Xiaotao, Wang Weiwei. Traditional Chinese medicine regulates nuclear factor-kappa B signaling pathway against ischemia-reperfusion injury of skin flaps [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(35): 5721-5726. |
| [4] | Xiong Bo, Wang Bin, Liu Jinfu, Lu Guanyu, Chen Cai, Huang Yue, Chen Lihua. Role of cuproptosis regulator in diagnosis and subtype of osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(34): 5530-5537. |
| [5] | Gu Yingchu, Gu Ye, Wu Zerui, Fang Tao, Wang Qiufei, Chen Bingqian, Peng Yuqin, Geng Dechun, Xu Yaozeng. Signaling pathway of osteoblast autophagy in periprosthetic osteolysis [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(34): 5561-5569. |
| [6] | Tang Li, Pan Yao, Zhu Guochen. Epidermal neural crest stem cells of hair follicles regulate the local expression of inflammatory factors after facial nerve injury [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(33): 5249-5255. |
| [7] | Xie Na, Ma Run, Wang Lian, Shu Yuanhui, He Ping, Zhou Yan, Xiang Yining, Wang Yuping. Effect of formononetin on glucose oxidase-induced oxidative stress in rat hepatic stellate cells [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(33): 5309-5313. |
| [8] | Zhang Huiyu, Yu Jingwen, Bai Zhenjun, Li Liang, Mu Bingtao, Zhang Jinfeng, Xie Jiawei. Triptolide protects damaged neurons by regulating microglial polarization [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(33): 5342-5347. |
| [9] | Feng Ruibing, Huang Yong, Hu Hao, Wu Gang, Duan Xiaofeng, Li Chaowen. Analgesic effect and mechanism of icariin in a rat model of post-traumatic knee arthritis [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(32): 5108-5113. |
| [10] | Yue Jing, Wang Shijun. Antiinflammatory and analgesic effects and mechanisms of coix seed and its components in adjuvant arthritis rats [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(26): 4187-4192. |
| [11] | Hou Zhenyang, Sun Yiling, Su Changzheng, Li Zhen, Xu Zhengwen, Li Wenming, Bai Jiaxiang. Theaflavin-3,3'-digallate alleviates osteogenesis inhibition induced by wear particles in artificial prostheses [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(25): 4069-4074. |
| [12] | Chen Zili, Cao Ning, Xu Meng, Jiang Yan, Ji Meichao, Zheng Yangyang, Yang Lili. Biological characteristics of tumor necrosis factor-alpha-primed human umbilical cord mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(24): 3780-3787. |
| [13] | Liu Haichao, Wang Hao, Wang Shizhong, Lin Jianping, Jin Jing, Chen Shaoqing. Effects of point-pressing of the spleen meridian on inflammatory factors and calcium homeostasis in the skeletal muscle of acute blunt contusion rats [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(20): 3123-3128. |
| [14] | Zhang Houjun, Jiang Shengyuan, Deng Bowen, Liu Gang, Bai Huizhong, Tao Jingwei, Fan Xiao, Zhao Yi, Ren Jingpei, Xu Lin, Mu Xiaohong. Mechanism by which tetramethylpyrazine improves inflammatory microenvironment after spinal cord injury in rats [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(11): 1701-1707. |
| [15] | Wang Xinxin, Wang Jingxin. Mesenchymal stem cells-derived exosomes in treatment of secondary lymphedema [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(10): 1603-1609. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||