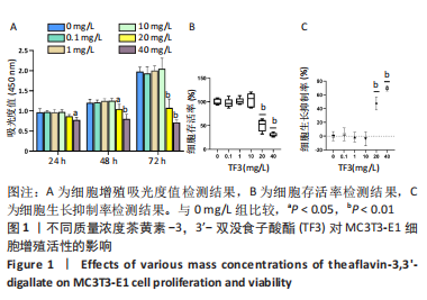
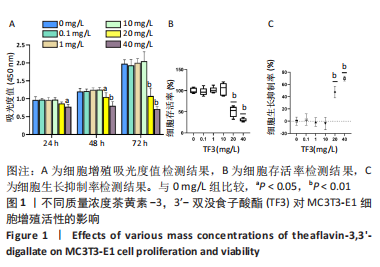
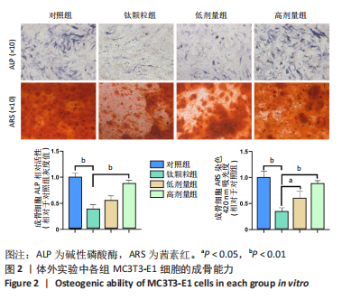
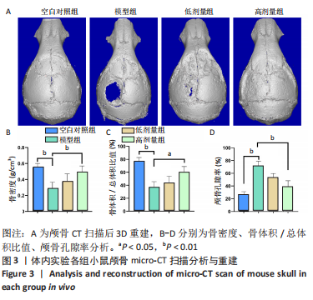
[1] JACOBS JJ, HALLAB NJ, URBAN RM, et al. Wear particles. J Bone Joint Surg Am. 2006;88 Suppl 2:99-102.
[2] OLLIVERE B, WIMHURST JA, CLARK IM, et al. Current concepts in osteolysis. J Bone Joint Surg Br. 2012;94(1):10-15.
[3] BITAR D, PARVIZI J. Biological response to prosthetic debris. World J Orthop. 2015;6(2):172-189.
[4] HOLT G, MURNAGHAN C, REILLY J, et al. The biology of aseptic osteolysis. Clin Orthop Relat Res. 2007;460:240-252.
[5] HODGES NA, SUSSMAN EM, STEGEMANN JP. Aseptic and septic prosthetic joint loosening: Impact of biomaterial wear on immune cell function, inflammation, and infection. Biomaterials. 2021;278:121127.
[6] GOODMAN SB, GALLO J. Periprosthetic Osteolysis: Mechanisms, Prevention and Treatment. J Clin Med. 2019;8(12):2091.
[7] FLECHER X, ROLLAND C, RIXRATH E, et al. Local and systemic activation of the mononuclear phagocyte system in aseptic loosening of total hip arthroplasty. J Clin Immunol. 2009;29(5):681-690.
[8] GALLO J, GOODMAN SB, KONTTINEN YT, et al. Osteolysis around total knee arthroplasty: a review of pathogenetic mechanisms. Acta Biomater. 2013;9(9):8046-8058.
[9] NAKASHIMA T, HAYASHI M, FUKUNAGA T, et al. Evidence for osteocyte regulation of bone homeostasis through RANKL expression. Nat Med. 2011;17(10):1231-1234.
[10] NAKAHAMA K. Cellular communications in bone homeostasis and repair. Cell Mol Life Sci. 2010;67(23):4001-4009.
[11] PAJARINEN J, LIN TH, NABESHIMA A, et al. Mesenchymal stem cells in the aseptic loosening of total joint replacements. J Biomed Mater Res A. 2017;105(4):1195-1207.
[12] APOSTU D, LUCACIU O, MESTER A, et al. Cannabinoids and bone regeneration. Drug Metab Rev. 2019;51(1):65-75.
[13] ZHANG L, HADDOUTI EM, WELLE K, et al. The Effects of Biomaterial Implant Wear Debris on Osteoblasts. Front Cell Dev Biol. 2020;8:352.
[14] KHAN N, MUKHTAR H. Tea Polyphenols in Promotion of Human Health. Nutrients. 2018;11(1):39.
[15] HU X, PING Z, GAN M, et al. Theaflavin-3,3’-digallate represses osteoclastogenesis and prevents wear debris-induced osteolysis via suppression of ERK pathway. Acta Biomater. 2017;48:479-488.
[16] AI Z, WU Y, YU M, et al. Theaflavin-3, 3’-Digallate Suppresses RANKL-Induced Osteoclastogenesis and Attenuates Ovariectomy-Induced Bone Loss in Mice. Front Pharmacol. 2020;11:803.
[17] OKA Y, IWAI S, AMANO H, et al. Tea polyphenols inhibit rat osteoclast formation and differentiation. J Pharmacol Sci. 2012;118(1):55-64.
[18] NASH LA, WARD WE. Comparison of black, green and rooibos tea on osteoblast activity. Food Funct. 2016;7(2):1166-1175.
[19] SHALAN NA, MUSTAPHA NM, MOHAMED S. Noni leaf and black tea enhance bone regeneration in estrogen-deficient rats. Nutrition. 2017; 33:42-51.
[20] NASH LA, WARD WE. Tea and bone health: Findings from human studies, potential mechanisms, and identification of knowledge gaps. Crit Rev Food Sci Nutr. 2017;57(8):1603-1617.
[21] 尚希福,路玉峰,张文志,等.茶黄素对人骨髓基质干细胞的成骨诱导作用[J].山东医药,2010,50(19):7-9.
[22] 刘名,王凯.茶黄素-3,3’-没食子酸酯修饰的纳米羟基磷灰石/聚已内酯复合多孔支架修复骨缺损[J].中国组织工程研究,2022, 26(22):3480-3486.
[23] UKIL A, MAITY S, DAS PK. Protection from experimental colitis by theaflavin-3,3’-digallate correlates with inhibition of IKK and NF-kappaB activation. Br J Pharmacol. 2006;149(1):121-131.
[24] HARRIS WH. Wear and periprosthetic osteolysis: the problem. Clin Orthop Relat Res. 2001;(393):66-70.
[25] PURDUE PE, KOULOUVARIS P, POTTER HG, et al. The cellular and molecular biology of periprosthetic osteolysis. Clin Orthop Relat Res. 2007;454:251-261.
[26] JASTY M, SMITH E. Wear particles of total joint replacements and their role in periprosthetic osteolysis. Curr Opin Rheumatol. 1992;4(2):204-249.
[27] XIONG L, LIU Y, ZHU F, et al. Acetyl-11-keto-β-boswellic acid attenuates titanium particle-induced osteogenic inhibition via activation of the GSK-3β/β-catenin signaling pathway. Theranostics. 2019;9(24):7140-7155.
[28] WANG N, MASKOMANI S, MEENASHISUNDARAM GK, et al. A study of Titanium and Magnesium particle-induced oxidative stress and toxicity to human osteoblasts. Mater Sci Eng C Mater Biol Appl. 2020; 117:111285.
[29] KALBACOVA M, ROESSLER S, HEMPEL U, et al. The effect of electrochemically simulated titanium cathodic corrosion products on ROS production and metabolic activity of osteoblasts and monocytes/macrophages. Biomaterials. 2007;28(22):3263-3272.
[30] DENG Z, WANG Z, JIN J, et al. SIRT1 protects osteoblasts against particle-induced inflammatory responses and apoptosis in aseptic prosthesis loosening. Acta Biomater. 2017;49:541-554.
[31] KIM KH, LEE MS. Autophagy--a key player in cellular and body metabolism. Nat Rev Endocrinol. 2014;10(6):322-337.
[32] YIN X, ZHOU C, LI J, et al. Autophagy in bone homeostasis and the onset of osteoporosis. Bone Res. 2019;7:28.
[33] NOLLET M, SANTUCCI-DARMANIN S, BREUIL V, et al. Autophagy in osteoblasts is involved in mineralization and bone homeostasis. Autophagy. 2014;10(11):1965-1977.
[34] WANG Z, LIU N, LIU K, et al. Autophagy mediated CoCrMo particle-induced peri-implant osteolysis by promoting osteoblast apoptosis. Autophagy. 2015;11(12):2358-2369.
[35] PAN MH, LIN-SHIAU SY, HO CT, et al. Suppression of lipopolysaccharide-induced nuclear factor-kappaB activity by theaflavin-3,3’-digallate from black tea and other polyphenols through down-regulation of IkappaB kinase activity in macrophages. Biochem Pharmacol. 2000;59(4):357-367.
[36] GE G, YANG S, HOU Z, et al. Theaflavin-3,3’-Digallate Promotes the Formation of Osteoblasts Under Inflammatory Environment and Increases the Bone Mass of Ovariectomized Mice. Front Pharmacol. 2021;12:648969.
[37] LI Z, ZHU J, WAN Z, et al. Theaflavin ameliorates renal ischemia/reperfusion injury by activating the Nrf2 signalling pathway in vivo and in vitro. Biomed Pharmacother. 2021;134:111097.
[38] SHEN Z, CHEN Q, JIN T, et al. Theaflavin 3,3’-digallate reverses the downregulation of connexin 43 and autophagy induced by high glucose via AMPK activation in cardiomyocytes. J Cell Physiol. 2019; 234(10):17999-18016. |