Chinese Journal of Tissue Engineering Research ›› 2023, Vol. 27 ›› Issue (33): 5348-5356.doi: 10.12307/2023.714
Previous Articles Next Articles
Macrophage migration inhibitory factor promotes the differentiation of human embryonic stem cells into ventral midbrain dopaminergic neural progenitor cells
Zheng Xiaohan1, 2, 3, Feng Xiaoli1, Hu Lan4, Gao Shijun1, 2, 3, Wei Yanzhao1, 2, 3, Huang Ting1, 2, 3, Sun Shengtong2, Wei Xufang2, Wang Tan1, 2, 3, Zhao Zhenqiang1, 2, 3
- 1Department of Neurology, 4Department of Clinical Laboratory, First Affiliated Hospital of Hainan Medical University, Haikou 570100, Hainan Province, China; 2Hainan Medical University, Haikou 570100, Hainan Province, China; 3Hainan Provincial Key Laboratory of Tropical Brain Research and Translation, Haikou 570100, Hainan Province, China
-
Received:2022-09-24Accepted:2022-11-16Online:2023-11-28Published:2023-03-30 -
Contact:Zhao Zhenqiang, MD, Chief physician, Department of Neurology, First Affiliated Hospital of Hainan Medical University, Haikou 570100, Hainan Province, China; Hainan Medical University, Haikou 570100, Hainan Province, China; Hainan Provincial Key Laboratory of Tropical Brain Research and Translation, Haikou 570100, Hainan Province, China Wang Tan, MD, Chief physician, Department of Neurology, First Affiliated Hospital of Hainan Medical University, Haikou 570100, Hainan Province, China; Hainan Medical University, Haikou 570100, Hainan Province, China; Hainan Provincial Key Laboratory of Tropical Brain Research and Translation, Haikou 570100, Hainan Province, China -
About author:Zheng Xiaohan, Master candidate, Department of Neurology, First Affiliated Hospital of Hainan Medical University, Haikou 570100, Hainan Province, China; Hainan Medical University, Haikou 570100, Hainan Province, China; Hainan Provincial Key Laboratory of Tropical Brain Research and Translation, Haikou 570100, Hainan Province, China -
Supported by:the National Natural Science Foundation of China, No. 81860238 (to ZZQ); Hainan Key Research & Development Program, No. ZDYF2018233 (to ZZQ); Hainan Natural Science Foundation Project, No. 821RC694 (to ZZQ); Hainan Clinical Medical Center Construction Project, No. QWYH[2021]276; Hainan Health Industry Scientific Research Project, No. 21A200354 (to FXL); Hainan Postgraduate Innovation Research Project, No. QJG[2021]116 (to ZXH)
CLC Number:
Cite this article
Zheng Xiaohan, Feng Xiaoli, Hu Lan, Gao Shijun, Wei Yanzhao, Huang Ting, Sun Shengtong, Wei Xufang, Wang Tan, Zhao Zhenqiang. Macrophage migration inhibitory factor promotes the differentiation of human embryonic stem cells into ventral midbrain dopaminergic neural progenitor cells[J]. Chinese Journal of Tissue Engineering Research, 2023, 27(33): 5348-5356.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
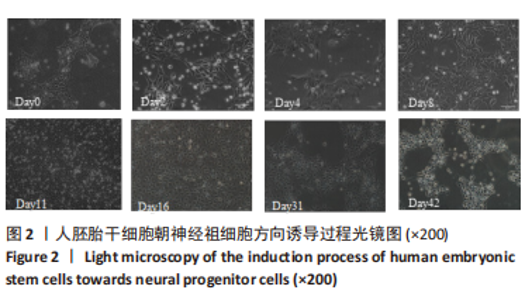
2.1 分化方案成功复现 根据NOLBRANT等[10]的方案稍作修改后,培养了人胚胎干细胞H9并成功诱导了细胞分化,见图2。Day0为胚胎干细胞阶段,细胞整体的体积略小,而细胞核的体积略大,且细胞内部的核质比较高,核型相对正常,内含单个或以上轮廓较显著的核仁。细胞为集落样存活,较高密度排列,整体类似鸟巢的形象,边界较糊。Day2-Day16为神经祖细胞阶段,细胞逐渐失去了胚胎干细胞形态,最初由圆形变成放射状或梭形。随着细胞的快速增殖,细胞之间的间隔密度也变得更加密切,而后细胞的形态日趋变得更加圆润,体积更小,形成大小均一的神经祖细胞形态。在Day31,细胞间产生了丝状结构。在Day42,细胞呈明显的神经元样形态。"
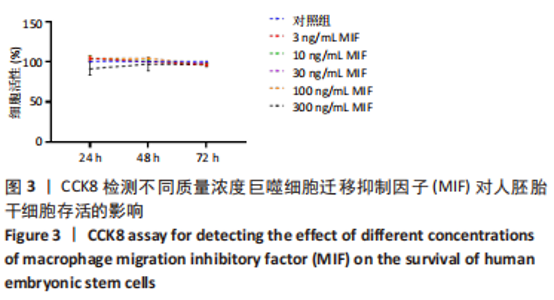
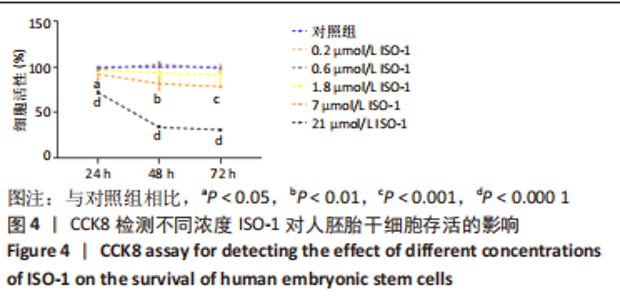
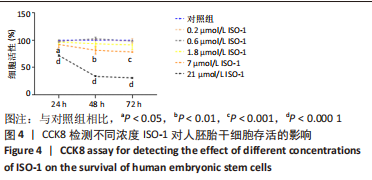
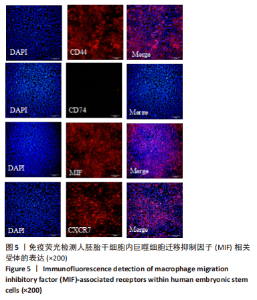
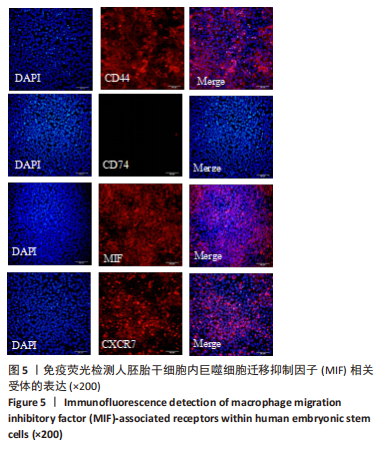
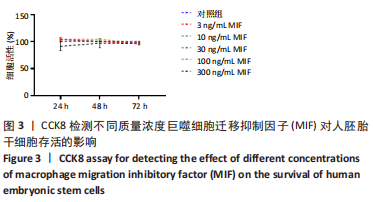
2.2 MIF或ISO-1对人胚胎干细胞活力的影响 如图3所示,不同质量浓度的MIF对细胞存活率影响没有明显差异。如图4所示,7,21 μmol/L ISO-1均显著降低了细胞的生存能力(P < 0.05)。作者选择处理H9的MIF质量浓度为3,10,30 ng/mL,处理人胚胎干细胞的ISO-1浓度为0.2,0.6,1.8 μmol/L。在接下来的实验中,用上述浓度的MIF和ISO-1来处理H9,并在光镜下观察H9的分化情况。MIF可以通过G蛋白偶联途径与细胞膜上的CD74、CXCR2、CXCR4和CXCR7受体相互作用而激活PI3K/AKT途径[25-26]。如图5所示,H9明显表达MIF、CD44和CXCR7,但不表达CD74。"
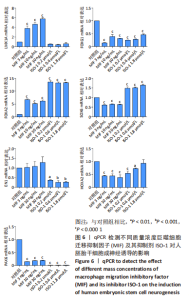
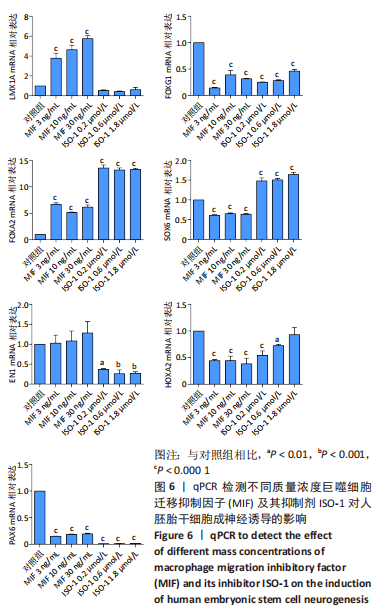
2.3 MIF增强了不同脑区神经元标志物的mRNA表达 如图6所示,对于用各质量浓度MIF处理的细胞,中脑神经元标志物(LMX1A和FOXA2)的mRNA表达水平相较对照组显著上升(P < 0.000 1)。对于另一中脑标记物(EN1)则无显著差异。MIF组的后脑神经元标志物(HOXA2)、前脑神经元标志物(FOXG1)、神经上皮层标志物(PAX6)表达水平相较对照组显著下降(P < 0.000 1)。每个组都表达Wnt1/β-catenin信号通路转录因子SOX6,但是没有显著性差异。对于用ISO-1处理的细胞,qRT-PCR显示各浓度ISO-1明显降低了EN1、FOXG1和PAX6的mRNA表达水平(P < 0.001),0.2 μmol/L与0.6 μmol/L ISO-1显著降低了PAX6的mRNA表达,1.8 μmol/L ISO-1则对PAX6无显著性差异。而各浓度ISO-1提高了FOXA2和SOX6的mRNA表达水平(P < 0.000 1)。"
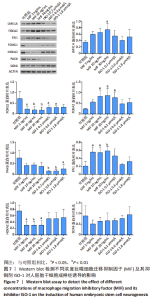
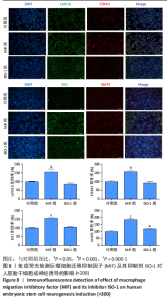
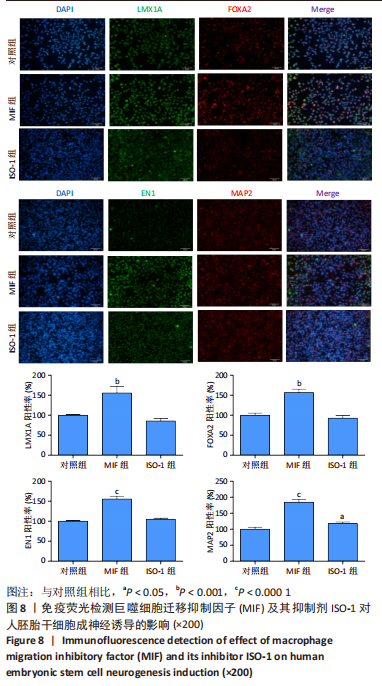
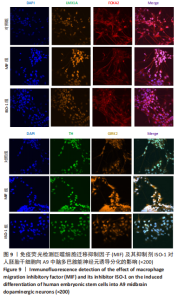
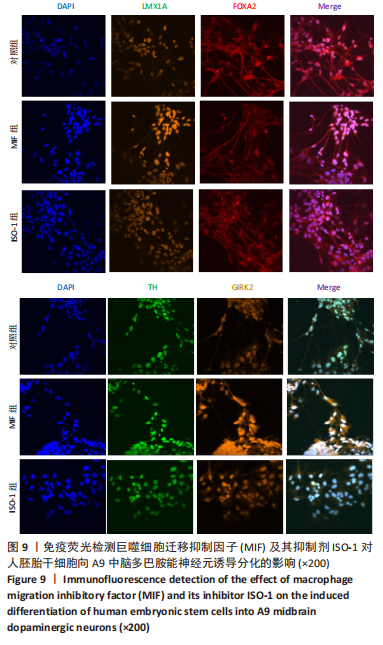
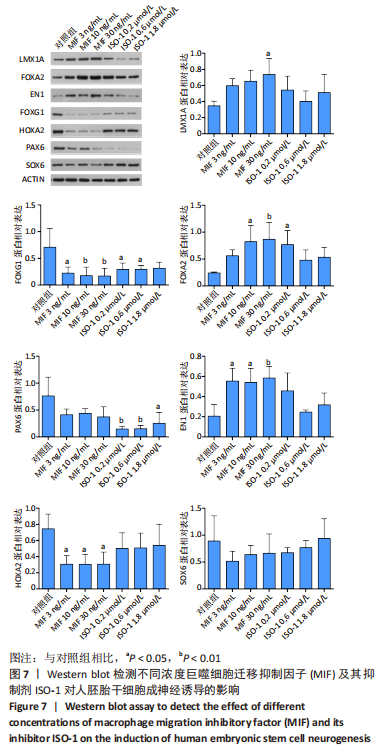
2.4 MIF增强了不同脑区神经元标志物的蛋白水平 上述标记物在蛋白水平上也观察到类似的表达变化。如图7所示,与对照组相比,MIF组的LMX1A、EN1和FOXA2蛋白水平升高,尤其是30 ng/mL MIF处理组(P < 0.05)。同时,各MIF组的FOXG1和HOXA2蛋白水平都有所下降(P < 0.05)。SOX6水平在各组中没有明显差异。对于用ISO-1处理的细胞,0.2 μmol/L的ISO-1明显提高了FOXA2的表达水平(P < 0.05),各浓度ISO-1显著降低了FOXG1和PAX6的表达水平(P < 0.05)。基于上述结果,研究确定30 ng/mL的MIF和0.2 μmol/L的ISO-1为H9分化的最佳浓度。"
| [1] TANG Y, HAN L, BAI X, et al. Intranasal Delivery of Bone Marrow Stromal Cells Preconditioned with Fasudil to Treat a Mouse Model of Parkinson’s Disease. Neuropsychiatr Dis Treat. 2020;16:249-262. [2] FAN Y, WINANTO, NG SY. Replacing what’s lost: a new era of stem cell therapy for Parkinson’s disease. Transl Neurodegener. 2020;9:2. [3] ARMSTRONG MJ, OKUN MS. Diagnosis and Treatment of Parkinson Disease: A Review. JAMA. 2020;323(6):548-560. [4] OLANOW CW, FACTOR SA, ESPAY AJ, et al. Apomorphine sublingual film for off episodes in Parkinson’s disease: a randomised, double-blind, placebo-controlled phase 3 study. Lancet Neurol. 2020;19(2):135-144. [5] GAO L, NATH SC, JIAO X, et al. Post-Passage rock inhibition induces cytoskeletal aberrations and apoptosis in Human embryonic stem cells. Stem Cell Res. 2019;41:101641. [6] 陈丽,胡兰,彭雅南,等.人多能干细胞向多巴胺能神经元分化:异质性的安全风险[J].中国组织工程研究,2019,23(1):118-124. [7] 李光然,王伟.小分子化合物在干细胞神经分化中的研究进展[J].中国生物工程杂志,2018,38(3):76-80. [8] KIRKEBY A, NOLBRANT S, TIKLOVA K, et al. Predictive Markers Guide Differentiation to Improve Graft Outcome in Clinical Translation of hESC-Based Therapy for Parkinson’s Disease. Cell Stem Cell. 2017; 20(1):135-148. [9] SONG B, CHA Y, KO S, et al. Human autologous iPSC-derived dopaminergic progenitors restore motor function in Parkinson’s disease models. J Clin Invest. 2020;130(2):904-920. [10] NOLBRANT S, HEUER A, PARMAR M, et al. Generation of high-purity human ventral midbrain dopaminergic progenitors for in vitro maturation and intracerebral transplantation. Nat Protoc. 2017;12(9): 1962-1979. [11] SAMATA B, DOI D, NISHIMURA K, et al. Purification of functional human ES and iPSC-derived midbrain dopaminergic progenitors using LRTM1. Nat Commun. 2016;7:13097. [12] ZHAO Z, MA Y, CHEN Z, et al. Effects of Feeder Cells on Dopaminergic Differentiation of Human Embryonic Stem Cells. Front Cell Neurosci. 2016;10:291. [13] KEE N, VOLAKAKIS N, KIRKEBY A, et al. Single-Cell Analysis Reveals a Close Relationship between Differentiating Dopamine and Subthalamic Nucleus Neuronal Lineages. Cell Stem Cell. 2017;20(1):29-40. [14] XIA N, FANG F, ZHANG P, et al. A Knockin Reporter Allows Purification and Characterization of mDA Neurons from Heterogeneous Populations. Cell Rep. 2017;18(10):2533-2546. [15] BILSBORROW JB, DOHERTY E, TILSTAM PV, et al. Macrophage migration inhibitory factor (MIF) as a therapeutic target for rheumatoid arthritis and systemic lupus erythematosus. Expert Opin Ther Targets. 2019; 23(9):733-744. [16] AL-ABED Y, VANPATTEN S. MIF as a disease target: ISO-1 as a proof-of-concept therapeutic. Future Med Chem. 2011;3(1):45-63. [17] JANKAUSKAS SS, WONG DWL, BUCALA R, et al. Evolving complexity of MIF signaling. Cell Signal. 2019;57:76-88. [18] OHTA S, MISAWA A, FUKAYA R, et al. Macrophage migration inhibitory factor (MIF) promotes cell survival and proliferation of neural stem/progenitor cells. J Cell Sci. 2012;125(Pt 13):3210-3220. [19] MILLER EJ, LI J, LENG L, et al. Macrophage migration inhibitory factor stimulates AMP-activated protein kinase in the ischaemic heart. Nature. 2008;451(7178):578-582. [20] SANTOS LL, MORAND EF. The role of macrophage migration inhibitory factor in the inflammatory immune response and rheumatoid arthritis. Wien Med Wochenschr. 2006;156(1-2):11-18. [21] CONBOY L, VAREA E, CASTRO JE, et al. Macrophage migration inhibitory factor is critically involved in basal and fluoxetine-stimulated adult hippocampal cell proliferation and in anxiety, depression, and memory-related behaviors. Mol Psychiatry. 2011;16(5):533-547. [22] LIU F, UCHUGONOVA A, KIMURA H, et al. The bulge area is the major hair follicle source of nestin-expressing pluripotent stem cells which can repair the spinal cord compared to the dermal papilla. Cell Cycle. 2011;10(5):830-839. [23] OHTA S, MISAWA A, LEFEBVRE V, et al. Sox6 up-regulation by macrophage migration inhibitory factor promotes survival and maintenance of mouse neural stem/progenitor cells. PLoS One. 2013;8(9):e74315. [24] PANMAN L, PAPATHANOU M, LAGUNA A, et al. Sox6 and Otx2 control the specification of substantia nigra and ventral tegmental area dopamine neurons. Cell Rep. 2014;8(4):1018-1025. [25] OLIVEIRA CS, DE BOCK CE, MOLLOY TJ, et al. Macrophage migration inhibitory factor engages PI3K/Akt signalling and is a prognostic factor in metastatic melanoma. BMC Cancer. 2014;14:630. [26] WEI QT, LIU BY, JI HY, et al. Exosome-mediated transfer of MIF confers temozolomide resistance by regulating TIMP3/PI3K/AKT axis in gliomas. Mol Ther Oncolytics. 2021;22:114-128. [27] MOURTZI T, KAZANIS I. Endogenous versus exogenous cell replacement for Parkinson’s disease: where are we at and where are we going? Neural Regen Res. 2022;17(12):2637-2642. [28] MESMAN S, VON OERTHEL L, SMIDT MP. Mesodiencephalic dopaminergic neuronal differentiation does not involve GLI2A-mediated SHH-signaling and is under the direct influence of canonical WNT signaling. PLoS One. 2014;9(5):e97926. [29] NGUYEN HN, BYERS B, CORD B, et al. LRRK2 mutant iPSC-derived DA neurons demonstrate increased susceptibility to oxidative stress. Cell Stem Cell. 2011;8(3):267-280. [30] FROM THE AMERICAN ASSOCIATION OF NEUROLOGICAL SURGEONS (AANS), AMERICAN SOCIETY OF NEURORADIOLOGY (ASNR), CARDIOVASCULAR AND INTERVENTIONAL RADIOLOGY SOCIETY OF EUROPE (CIRSE), et al. Multisociety Consensus Quality Improvement Revised Consensus Statement for Endovascular Therapy of Acute Ischemic Stroke. Int J Stroke. 2018;13(6):612-632. [31] POULIN JF, GAERTNER Z, MORENO-RAMOS OA, et al. Classification of Midbrain Dopamine Neurons Using Single-Cell Gene Expression Profiling Approaches. Trends Neurosci. 2020;43(3):155-169. [32] LA MANNO G, GYLLBORG D, CODELUPPI S, et al. Molecular Diversity of Midbrain Development in Mouse, Human, and Stem Cells. Cell. 2016;167(2):566-580.e19. [33] MA T, WANG C, WANG L, et al. Subcortical origins of human and monkey neocortical interneurons. Nat Neurosci. 2013;16(11):1588-1597. [34] MANUEL MN, MI D, MASON JO, et al. Regulation of cerebral cortical neurogenesis by the Pax6 transcription factor. Front Cell Neurosci. 2015;9:70. [35] COOPER O, HARGUS G, DELEIDI M, et al. Differentiation of human ES and Parkinson’s disease iPS cells into ventral midbrain dopaminergic neurons requires a high activity form of SHH, FGF8a and specific regionalization by retinoic acid. Mol Cell Neurosci. 2010;45(3):258-266. [36] HÉBERT JM, FISHELL G. The genetics of early telencephalon patterning: some assembly required. Nat Rev Neurosci. 2008;9(9):678-685. [37] HETTIGE NC, ERNST C. FOXG1 Dose in Brain Development. Front Pediatr. 2019;7:482. [38] TÜMPEL S, MACONOCHIE M, WIEDEMANN LM, et al. Conservation and diversity in the cis-regulatory networks that integrate information controlling expression of Hoxa2 in hindbrain and cranial neural crest cells in vertebrates. Dev Biol. 2002;246(1):45-56. [39] DAVENNE M, MACONOCHIE MK, NEUN R, et al. Hoxa2 and Hoxb2 control dorsoventral patterns of neuronal development in the rostral hindbrain. Neuron. 1999;22(4):677-691. [40] XIE J, YANG L, TIAN L, et al. Macrophage Migration Inhibitor Factor Upregulates MCP-1 Expression in an Autocrine Manner in Hepatocytes during Acute Mouse Liver Injury. Sci Rep. 2016;6:27665. [41] NISHINO T, BERNHAGEN J, SHIIKI H, et al. Localization of macrophage migration inhibitory factor (MIF) to secretory granules within the corticotrophic and thyrotrophic cells of the pituitary gland. Mol Med. 1995;1(7):781-788. [42] EICKHOFF R, WILHELM B, RENNEBERG H, et al. Purification and characterization of macrophage migration inhibitory factor as a secretory protein from rat epididymis: evidences for alternative release and transfer to spermatozoa. Mol Med. 2001;7(1):27-35. [43] PYLE ME, KORBONITS M, GUEORGUIEV M, et al. Macrophage migration inhibitory factor expression is increased in pituitary adenoma cell nuclei. J Endocrinol. 2003;176(1):103-110. [44] 魏艳召,郑晓晗,高仕君,等.人胚胎干细胞自分泌巨噬细胞迁移抑制因子及受体表达[J].中国组织工程研究,2023,27(1):34-41. [45] 郑晓晗,魏艳召,黄婷,等.巨噬细胞迁移抑制因子对干细胞的作用[J].中国组织工程研究,2023,27(15):2395-2403. [46] CHATTERJEE M, BORST O, WALKER B, et al. Macrophage migration inhibitory factor limits activation-induced apoptosis of platelets via CXCR7-dependent Akt signaling. Circ Res. 2014;115(11):939-949. [47] AMIN MA, HAAS CS, ZHU K, et al. Migration inhibitory factor up-regulates vascular cell adhesion molecule-1 and intercellular adhesion molecule-1 via Src, PI3 kinase, and NFkappaB. Blood. 2006;107(6): 2252-2261. [48] BERNHAGEN J, KROHN R, LUE H, et al.MIF is a noncognate ligand of CXC chemokine receptors in inflammatory and atherogenic cell recruitment.Nat Med. 2007;13(5):587-596. [49] SCHWARTZ V, LUE H, KRAEMER S, et al.A functional heteromeric MIF receptor formed by CD74 and CXCR4.FEBS Lett. 2009;583(17):2749-2757. [50] ALAMPOUR-RAJABI S, EL BOUNKARI O, ROT A, et al. MIF interacts with CXCR7 to promote receptor internalization, ERK1/2 and ZAP-70 signaling, and lymphocyte chemotaxis. FASEB J. 2015;29(11): 4497-4511. [51] LEVOYE A, BALABANIAN K, BALEUX F, et al.CXCR7 heterodimerizes with CXCR4 and regulates CXCL12-mediated G protein signaling. Blood. 2009;113(24):6085-6093. [52] RAJAGOPAL S, KIM J, AHN S, et al.Beta-arrestin- but not G protein-mediated signaling by the “decoy” receptor CXCR7. Proc Natl Acad Sci U S A. 2010;107(2):628-632. |
| [1] | Yang Zhishan, Tang Zhenglong. YAP/TAZ, a core factor of the Hippo signaling pathway, is involved in bone formation [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(8): 1264-1271. |
| [2] | Liu Xiaolin, Mu Xinyue, Ma Ziyu, Liu Shutai, Wang Wenlong, Han Xiaoqian, Dong Zhiheng. Effect of hydrogel-loaded simvastatin microspheres on osteoblast proliferation and differentiation [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(7): 998-1003. |
| [3] | Liu Wentao, Feng Xingchao, Yang Yi, Bai Shengbin. Effect of M2 macrophage-derived exosomes on osteogenic differentiation of bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(6): 840-845. |
| [4] | Long Yanming, Xie Mengsheng, Huang Jiajie, Xue Wenli, Rong Hui, Li Xiaojie. Casein kinase 2-interaction protein-1 regulates the osteogenic ability of bone marrow mesenchymal stem cells in osteoporosis rats [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(6): 878-882. |
| [5] | Li Xinyue, Li Xiheng, Mao Tianjiao, Tang Liang, Li Jiang. Three-dimensional culture affects morphology, activity and osteogenic differentiation of human periodontal ligament stem cells [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(6): 846-852. |
| [6] | Yuan Wei, Liu Jingdong, Xu Guanghui, Kang Jian, Li Fuping, Wang Yingjie, Zhi Zhongzheng, Li Guanwu. Osteogenic differentiation of human perivascular stem cells and its regulation based on Wnt/beta-catenin signaling pathway [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(6): 866-871. |
| [7] | Qiao Luhui, Ma Ziyu, Guo Haoyu, Hou Yudong. Comparison of puerarin and icariin on the biological properties of mouse preosteoblasts [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(6): 872-877. |
| [8] | Hao Liufang, Duan Hongmei, Wang Zijue, Hao Fei, Hao Peng, Zhao Wen, Gao Yudan, Yang Zhaoyang, Li Xiaoguang. Spatiotemporal dynamic changes of ependymal cells after spinal cord injury in transgenic mice [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(6): 883-889. |
| [9] | Xie Yingchun, Xu Wenjuan, Li Yuwan. Schnurri3 regulates osteogenic differentiation of C3H10T1/2 cells induced by bone morphogenetic protein 2 [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(33): 5270-5276. |
| [10] | He Qin, Bu Yan, Lin Guanglei, Luo Jing, Yong Min, Huang Yongqing. Differential expression of RNA binding protein Lin28A regulates osteoblastic differentiation of periodontal ligament stem cells [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(33): 5283-5291. |
| [11] | Xu Rongwei, Wang Hao, Fu Qiuyue, Lan Xingming, Yang Kun. Bidirectional interaction between inflammatory factors and dental pulp stem cells during bone regeneration [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(33): 5385-5393. |
| [12] | Liu Chunli, Yan Yujuan, Mo Liwen, Wu Zhijie, Zhang Li. Effects of puerarin on osteoclast differentiation of RAW264.7 cells [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(32): 5114-5119. |
| [13] | Liao Zirui, Chen Jianquan. Effect of inhibitor of Wnt production 2 on osteoclast differentiation [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(31): 4990-4995. |
| [14] | Dai Xianglin, Zhang Wenfeng, Yao Xijun, Shang Jiaqi, Huang Qiujin, Ren Yifan, Deng Jiupeng. Barium titanate/polylactic acid piezoelectric composite film affects adhesion, proliferation, and osteogenic differentiation of MC3T3-E1 cells [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(3): 367-373. |
| [15] | Li Rui, Liu Zhen, Guo Zige, Lu Ruijie, Wang Chen. Aspirin-loaded chitosan nanoparticles and polydopamine modified titanium sheets improve osteogenic differentiation [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(3): 374-379. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||