Chinese Journal of Tissue Engineering Research ›› 2023, Vol. 27 ›› Issue (31): 4990-4995.doi: 10.12307/2023.448
Previous Articles Next Articles
Effect of inhibitor of Wnt production 2 on osteoclast differentiation
Liao Zirui, Chen Jianquan
- Institute of Orthopedics, Suzhou Medical College, Soochow University, Suzhou 215000, Jiangsu Province, China
-
Received:2022-04-11Accepted:2022-07-14Online:2023-11-08Published:2023-01-31 -
Contact:Chen Jianquan, Professor, Doctoral supervisor, Institute of Orthopedics, Suzhou Medical College, Soochow University, Suzhou 215000, Jiangsu Province, China -
About author:Liao Zirui, Master candidate, Institute of Orthopedics, Suzhou Medical College, Soochow University, Suzhou 215000, Jiangsu Province, China -
Supported by:the National Natural Science Foundation of China, No. 81974344 (to CJQ)
CLC Number:
Cite this article
Liao Zirui, Chen Jianquan. Effect of inhibitor of Wnt production 2 on osteoclast differentiation[J]. Chinese Journal of Tissue Engineering Research, 2023, 27(31): 4990-4995.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
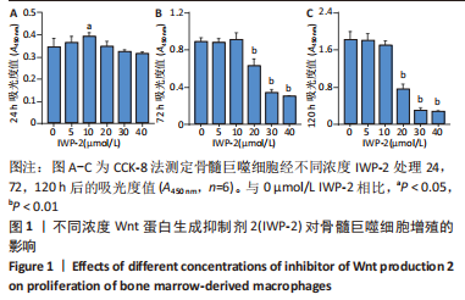
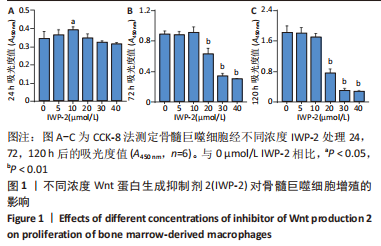
2.1 IWP-2对BMMs增殖的影响 首先为了明确IWP-2对BMMs增殖是否有影响,用不同浓度IWP-2(0,5,10,20,30,40 μmol/L)处理BMMs 24,72,120 h,并在每个时间点检测各组细胞的增殖情况。不同浓度IWP-2处理24 h,10 μmol/L IWP-2促进细胞增殖,差异有显著性意义(P < 0.05),见图1A。随着IWP-2处理时间延长至72 h和120 h,10 μmol/L及以下浓度的IWP-2对BMMs增殖无明显影响,而高浓度IWP-2(20,30,40 μmol/L)组细胞增殖明显受到抑制,差异有显著性意义(P < 0.01),见图1B,C。"
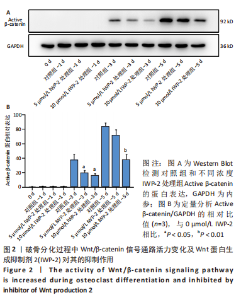
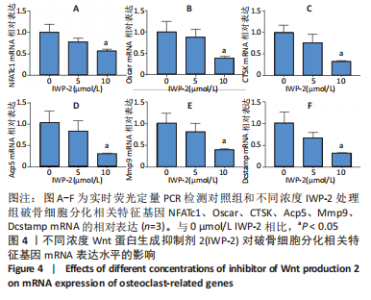
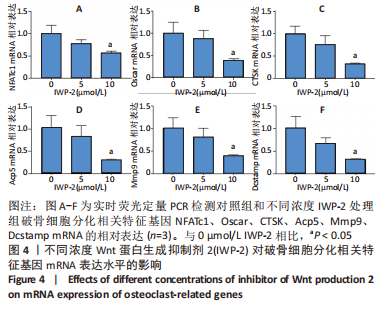
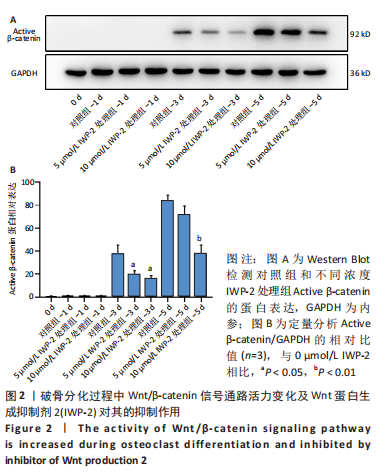
2.2 破骨分化过程中Wnt/β-catenin信号通路活力变化及IWP-2对其的抑制作用 为了进一步探讨Wnt/β-catenin信号通路在破骨分化过程中的作用,检测该信号通路在破骨分化过程中的活力变化。鉴于Active β-catenin的蛋白表达水平反映了Wnt/β-catenin信号通路的活力,因而利用Active β-catenin作为检测Wnt/β-catenin信号通路活力的指标。RANKL刺激1,3,5 d,对照组Active β-catenin的蛋白表达水平逐渐升高,见图2A,表明经典的Wnt/β-catenin信号通路随着破骨分化过程而逐步激活。随后,验证IWP-2对Wnt/β-catenin信号通路的抑制作用,选用5 μmol/L 和10 μmol/L IWP-2处理RANKL诱导的BMMs。与对照组相比,5 μmol/L 和10 μmol/L IWP-2处理组Active β-catenin的蛋白表达水平均受到抑制,且10 μmol/L IWP-2处理组Active β-catenin的蛋白表达水平较对照组均有显著性意义(P < 0.05,P < 0.01),见图2B,表明IWP-2可有效地抑制破骨分化过程中的Wnt/β-catenin信号通路。"
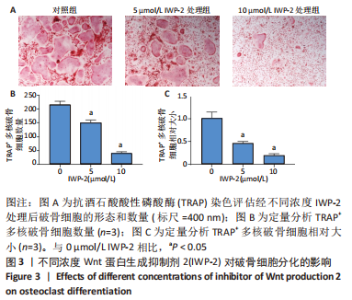
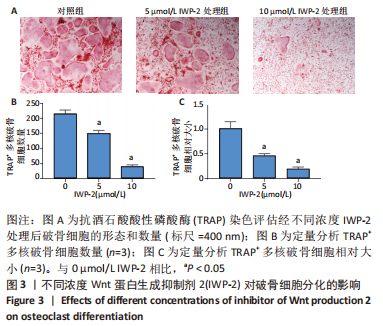
2.3 IWP-2抑制RANKL诱导的破骨细胞形成 由于细胞增殖影响破骨细胞分化,为了研究Wnt/β-catenin通路在破骨分化过程中的特异作用,选用对BMMs增殖无毒性的IWP-2浓度(0,5,10 μmol/L)来处理RANKL诱导的BMMs。诱导6 d后,通过抗酒石酸酸性磷酸酶染色鉴定评估破骨细胞的形成情况。结果显示,对照组可见许多大且细胞形态不规则的抗酒石酸酸性磷酸酶+多核成熟破骨细胞形成,表明成功诱导出破骨细胞,见图3A。与此相反,IWP-2处理组显示这些细胞的数量和大小呈剂量依赖性减少,且差异有显著性意义(P < 0.01),见图3B,C,表明IWP-2对RANKL诱导的破骨细胞分化具有抑制作用。由此可见,生理水平的Wnt/β-catenin通路对破骨分化至关重要。"
| [1] BUCKWALTER JA, GLIMCHER MJ, COOPER RR, et al. Bone biology. I: Structure, blood supply, cells, matrix, and mineralization. Instr Course Lect. 1996;45:371-386. [2] COHEN MM JR. The new bone biology: pathologic, molecular, and clinical correlates. Am J Med Genet A. 2006;140(23):2646-2706. [3] KULAR J, TICKNER J, CHIM SM, et al. An overview of the regulation of bone remodelling at the cellular level. Clin Biochem. 2012;45(12):863-873. [4] Zhu S, Yao F, Qiu H, et al. Coupling factors and exosomal packaging microRNAs involved in the regulation of bone remodelling. Biol Rev Camb Philos Soc. 2018; 93(1):469-480. [5] KRISHNAN V, BRYANT HU, MACDOUGALD OA. Regulation of bone mass by Wnt signaling. J Clin Invest. 2006;116(5):1202-1209. [6] LONG F. Building strong bones: molecular regulation of the osteoblast lineage. Nat Rev Mol Cell Biol. 2011;13(1):27-38. [7] CHARLES JF, ALIPRANTIS AO. Osteoclasts: more than ‘bone eaters’. Trends Mol Med. 2014;20(8):449-459. [8] NAKAGAWA N, KINOSAKI M, YAMAGUCHI K, et al. RANK is the essential signaling receptor for osteoclast differentiation factor in osteoclastogenesis. Biochem Biophys Res Commun. 1998;253(2):395-400. [9] YASUDA H, SHIMA N, NAKAGAWA N, et al. Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc Natl Acad Sci U S A. 1998;95(7):3597-3602. [10] Consensus development conference: diagnosis, prophylaxis, and treatment of osteoporosis. Am J Med. 1993;94(6):646-650. [11] DOWNEY PA, SIEGEL MI. Bone biology and the clinical implications for osteoporosis. Phys Ther. 2006;86(1):77-91. [12] FONSECA H, MOREIRA-GONÇALVES D, CORIOLANO HJ, et al. Bone quality: the determinants of bone strength and fragility. Sports Med. 2014;44(1):37-53. [13] GONG Y, SLEE RB, FUKAI N, et al. LDL receptor-related protein 5 (LRP5) affects bone accrual and eye development. Cell. 2001;107(4):513-523. [14] BOYDEN LM, MAO J, BELSKY J, et al. High bone density due to a mutation in LDL-receptor-related protein 5. N Engl J Med. 2002;346(20):1513-1521. [15] LITTLE RD, CARULLI JP, DEL MASTRO RG, et al. A mutation in the LDL receptor-related protein 5 gene results in the autosomal dominant high-bone-mass trait. Am J Hum Genet. 2002;70(1):11-19. [16] LIU J, XIAO Q, XIAO J, et al. Wnt/β-catenin signalling: function, biological mechanisms, and therapeutic opportunities. Signal Transduct Target Ther. 2022;7(1):3. [17] MACDONALD BT, TAMAI K, HE X. Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev Cell. 2009;17(1):9-26. [18] NUSSE R, CLEVERS H. Wnt/β-Catenin Signaling, Disease, and Emerging Therapeutic Modalities. Cell. 2017;169(6):985-999. [19] WEIVODA MM, RUAN M, HACHFELD CM, et al. Wnt Signaling Inhibits Osteoclast Differentiation by Activating Canonical and Noncanonical cAMP/PKA Pathways. J Bone Miner Res. 2019;34(8):1546-1548. [20] WEI W, ZEVE D, SUH JM, et al. Biphasic and dosage-dependent regulation of osteoclastogenesis by β-catenin. Mol Cell Biol. 2011;31(23):4706-4719. [21] RIOS-ESTEVES J, RESH MD. Stearoyl CoA desaturase is required to produce active, lipid-modified Wnt proteins. Cell Rep. 2013;4(6):1072-1081. [22] SHINDOU H, ETO M, MORIMOTO R, et al. Identification of membrane O-acyltransferase family motifs. Biochem Biophys Res Commun. 2009;383(3): 320-325. [23] LI Y, WU S, LI X, et al. Wnt signaling associated small molecules improve the viability of pPSCs in a PI3K/Akt pathway dependent way. J Cell Physiol. 2020; 235(7-8):5811-5822. [24] ANASTASILAKIS AD, POLYZOS SA, MAKRAS P. THERAPY OF ENDOCRINE DISEASE: Denosumab vs bisphosphonates for the treatment of postmenopausal osteoporosis. Eur J Endocrinol. 2018;179(1):R31-R45. [25] COSMAN F. Long-term treatment strategies for postmenopausal osteoporosis. Curr Opin Rheumatol. 2018;30(4):420-426. [26] DEEKS ED. Denosumab: A Review in Postmenopausal Osteoporosis. Drugs Aging. 2018;35(2):163-173. [27] YAVROPOULOU MP, MAKRAS P, ANASTASILAKIS AD. Bazedoxifene for the treatment of osteoporosis. Expert Opin Pharmacother. 2019;20(10):1201-1210. [28] BLACK DM, BAUER DC, SCHWARTZ AV, et al. Continuing bisphosphonate treatment for osteoporosis--for whom and for how long? N Engl J Med. 2012; 366(22):2051-2053. [29] RACHNER TD, KHOSLA S, HOFBAUER LC. Osteoporosis: now and the future. Lancet. 2011;377(9773):1276-1287. [30] BONE HG, WAGMAN RB, BRANDI ML, et al. 10 years of denosumab treatment in postmenopausal women with osteoporosis: results from the phase 3 randomised FREEDOM trial and open-label extension. Lancet Diabetes Endocrinol. 2017;5(7): 513-523. [31] ADLER RA. MANAGEMENT OF ENDOCRINE DISEASE: Atypical femoral fractures: risks and benefits of long-term treatment of osteoporosis with anti-resorptive therapy. Eur J Endocrinol. 2018;178(3):R81-R87. [32] FUJITA K, JANZ S. Attenuation of WNT signaling by DKK-1 and -2 regulates BMP2-induced osteoblast differentiation and expression of OPG, RANKL and M-CSF. Mol Cancer. 2007;6:71. [33] LACEY DL, TIMMS E, TAN HL, et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell. 1998;93(2):165-176. [34] BARON R, KNEISSEL M. WNT signaling in bone homeostasis and disease: from human mutations to treatments. Nat Med. 2013;19(2):179-192. [35] BENNETT CN, LONGO KA, WRIGHT WS, et al. Regulation of osteoblastogenesis and bone mass by Wnt10b. Proc Natl Acad Sci U S A. 2005;102(9):3324-3329. [36] NAKANISHI R, AKIYAMA H, KIMURA H, et al. Osteoblast-targeted expression of Sfrp4 in mice results in low bone mass. J Bone Miner Res. 2008;23(2):271-277. [37] BURGERS TA, WILLIAMS BO. Regulation of Wnt/β-catenin signaling within and from osteocytes. Bone. 2013;54(2):244-249. [38] ZHANG L, FU X, NI L, et al. Hedgehog Signaling Controls Bone Homeostasis by Regulating Osteogenic/Adipogenic Fate of Skeletal Stem/Progenitor Cells in Mice. J Bone Miner Res. 2022;37(3):559-576. |
| [1] | He Xi, Wan Yu, Tang Yuting, Yang Anning, Wu Kai, Jiao Yun, Bai Zhigang, Jiang Yideng, Shen Jiangyong. Erastin inhibits proliferation of hypertrophic scar fibroblasts [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(在线): 1-. |
| [2] | Li Xiaomin, Tian Xiangdong, Tan Yetong, Zhu Guangyu, Wang Rongtian, Wang Jian, Xue Zhipeng, Ma Sheng, Hu Yuanyi, Huang Ye, Ding Tiansong. Changes of lower limb force line and knee function after high tibial osteotomy in osteoporotic medial ventricular knee osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(9): 1325-1329. |
| [3] | Jiang Xiaocheng, Shi Lu, Wang Yinbin, Li Qiujiang, Xi Chuangzhen, Ma Zefeng, Cai Lijun. Systematical evaluation of bone fusion rate after interbody fusion in patients with osteoporosis and lumbar degenerative disease treated with teriparatide [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(9): 1427-1433. |
| [4] | Sun Jiajia, Zhu Haidi, Lu Yun, Zhang Kai. Comparison of bone metabolism markers between type 2 diabetes mellitus and non-type 2 diabetes mellitus patients with hip fracture [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(8): 1156-1160. |
| [5] | Zhao Lu, Zhao Yifei, Gao Da, Liu Yanfang, Fu Tingting, Xu Jiangyan. Expression of suppressor of Zeste 12 in kidney tissues of rats with diabetic nephropathy [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(8): 1179-1186. |
| [6] | Yang Zhishan, Tang Zhenglong. YAP/TAZ, a core factor of the Hippo signaling pathway, is involved in bone formation [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(8): 1264-1271. |
| [7] | Liu Xiaolin, Mu Xinyue, Ma Ziyu, Liu Shutai, Wang Wenlong, Han Xiaoqian, Dong Zhiheng. Effect of hydrogel-loaded simvastatin microspheres on osteoblast proliferation and differentiation [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(7): 998-1003. |
| [8] | Li Xinyue, Li Xiheng, Mao Tianjiao, Tang Liang, Li Jiang. Three-dimensional culture affects morphology, activity and osteogenic differentiation of human periodontal ligament stem cells [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(6): 846-852. |
| [9] | Yuan Wei, Liu Jingdong, Xu Guanghui, Kang Jian, Li Fuping, Wang Yingjie, Zhi Zhongzheng, Li Guanwu. Osteogenic differentiation of human perivascular stem cells and its regulation based on Wnt/beta-catenin signaling pathway [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(6): 866-871. |
| [10] | Qiao Luhui, Ma Ziyu, Guo Haoyu, Hou Yudong. Comparison of puerarin and icariin on the biological properties of mouse preosteoblasts [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(6): 872-877. |
| [11] | Hao Liufang, Duan Hongmei, Wang Zijue, Hao Fei, Hao Peng, Zhao Wen, Gao Yudan, Yang Zhaoyang, Li Xiaoguang. Spatiotemporal dynamic changes of ependymal cells after spinal cord injury in transgenic mice [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(6): 883-889. |
| [12] | Liu Wentao, Feng Xingchao, Yang Yi, Bai Shengbin. Effect of M2 macrophage-derived exosomes on osteogenic differentiation of bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(6): 840-845. |
| [13] | Long Yanming, Xie Mengsheng, Huang Jiajie, Xue Wenli, Rong Hui, Li Xiaojie. Casein kinase 2-interaction protein-1 regulates the osteogenic ability of bone marrow mesenchymal stem cells in osteoporosis rats [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(6): 878-882. |
| [14] | Li Long, Li Guangdi, Shi Hao, Deng Keqi. Circular RNA as a competing endogenous RNA is involved in the regulation of osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(5): 751-757. |
| [15] | Zhang Min, Zhang Xiaoming, Liu Tongbin. Application potential of naringin in bone tissue regeneration [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(5): 787-792. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||