Chinese Journal of Tissue Engineering Research ›› 2022, Vol. 26 ›› Issue (26): 4127-4135.doi: 10.12307/2022.814
Previous Articles Next Articles
Preventive and therapeutic effects of naringin on experimental autoimmune encephalomyelitis via regulating microglial polarization
Zeng Chunrong, Liu Menglan, Xie Yang, Li Zuoxiao
- Department of Neurology, the Affiliated Hospital of Southwest Medical University, Luzhou 646000, Sichuan Province, China
-
Received:2021-03-26Accepted:2021-05-12Online:2022-09-18Published:2022-03-08 -
Contact:Li Zuoxiao, Professor, Master’s supervisor, the Affiliated Hospital of Southwest Medical University, Luzhou 646000, Sichuan Province, China -
About author:Zeng Chunrong, Master, the Affiliated Hospital of Southwest Medical University, Luzhou 646000, Sichuan Province, China -
Supported by:Luzhou Municipal Government & Southwest Medical University Science and Technology Strategic Cooperation Fund Project, No. 2018LZXNYD-ZK17 (to LZX)
CLC Number:
Cite this article
Zeng Chunrong, Liu Menglan, Xie Yang, Li Zuoxiao. Preventive and therapeutic effects of naringin on experimental autoimmune encephalomyelitis via regulating microglial polarization[J]. Chinese Journal of Tissue Engineering Research, 2022, 26(26): 4127-4135.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
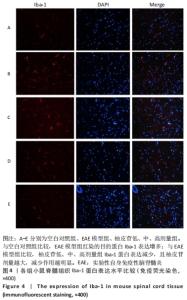
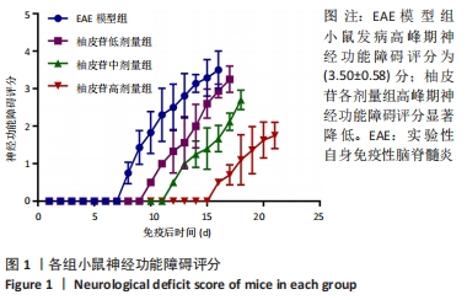
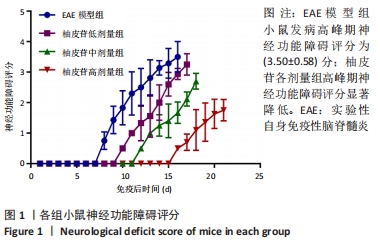
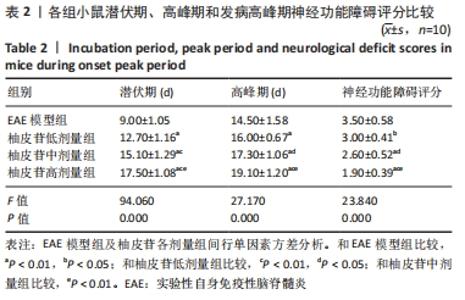
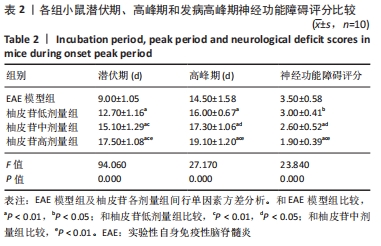
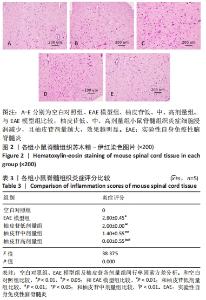
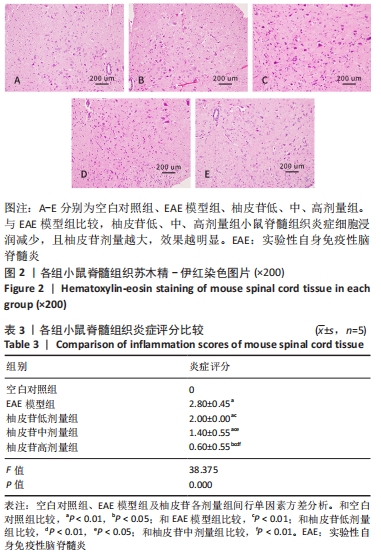
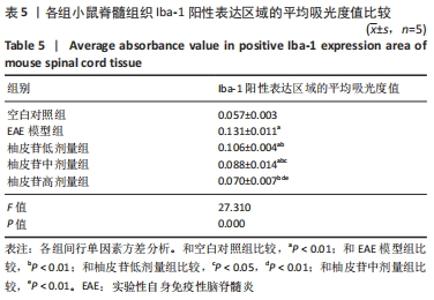
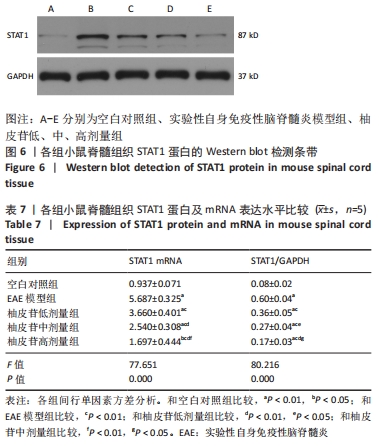
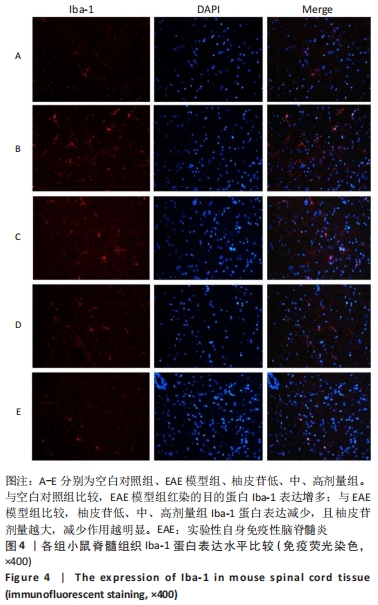
2.5 柚皮苷减少小胶质细胞激活 与空白对照组比较,EAE模型组红染的目的蛋白Iba-1表达增多,Merge图中Iba-1蛋白明显增多,呈聚集现象,脊髓组织Iba-1阳性表达区域的平均吸光度值增加(P < 0.01);与EAE模型组比较,柚皮苷各剂量组Iba-1蛋白表达减少,Merge图中Iba-1蛋白减少,Iba-1阳性表达区域的平均吸光度值减少(P < 0.01),柚皮苷各剂量组间Iba-1阳性表达区域的平均吸光度值差异均有显著性意义(P < 0.01或P < 0.05),见图4及表5。说明EAE小鼠发病高峰期小胶质细胞激活明显,柚皮苷干预后可减少小胶质细胞激活,且柚皮苷剂量越大,效果越明显。"

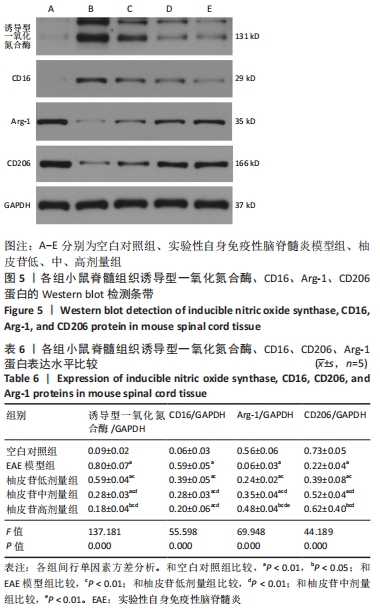
2.6 柚皮苷促进M1型小胶质细胞向M2型转化 与空白对照组比较,EAE模型组M1型标记物诱导型一氧化氮合酶、CD16明显增加(P < 0.01或P < 0.05),而M2型标记物Arg-1、CD206明显减少(P < 0.01或P < 0.05);与EAE模型组比较,柚皮苷各剂量组CD16、诱导型一氧化氮合酶减少(P < 0.01),Arg-1、CD206增加(P < 0.01);柚皮苷各剂量组间比较,差异均有显著性意义(P < 0.01或P < 0.05),见图5及表6。说明EAE小鼠脊髓组织中发病高峰期以M1型小胶质细胞浸润为主,柚皮苷可促进M1型小胶质细胞向M2型转化,且柚皮苷剂量越大,变化越明显。"
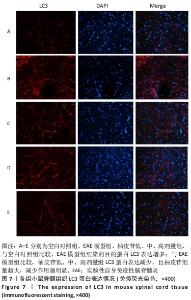
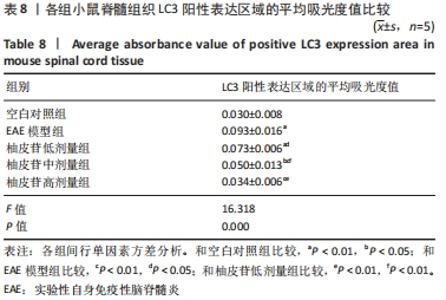
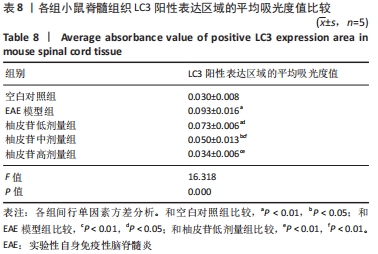
2.8 柚皮苷降低EAE模型中LC3表达 与空白对照组比较,EAE模型组红染的目的蛋白LC3表达增多,Merge图中LC3蛋白明显增多,呈聚集现象,脊髓组织LC3阳性表达区域的平均吸光度值增加(P < 0.01);与EAE模型组比较,柚皮苷各剂量组LC3表达减少,Merge图中LC3蛋白减少,LC3阳性表达区域的平均吸光度值减少(P < 0.01或P < 0.05);柚皮苷各剂量组间比较,除中、高剂量组间LC3阳性表达区域的平均吸光度值差异无显著性意义,其余组间差异均有显著性意义(P < 0.01或P < 0.05),见图7及表8。说明EAE小鼠发病高峰期存在自噬激活,柚皮苷干预后可下调自噬,且柚皮苷剂量越大,效果越明显。"


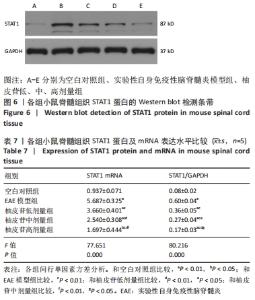
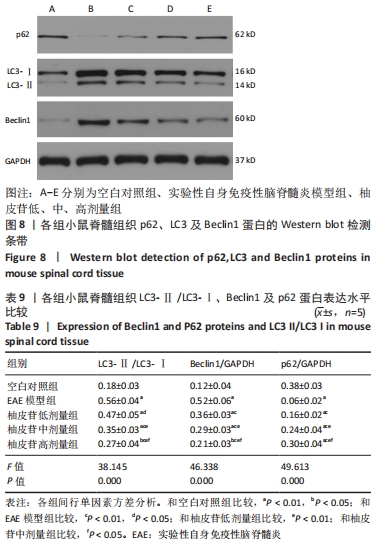
2.9 柚皮苷下调EAE模型中自噬通量 与空白对照组比较,EAE模型组脊髓组织LC3-Ⅱ/LC3-Ⅰ灰度值比值、Beclin1表达增加(P < 0.01),p62表达减少(P < 0.01);与EAE模型组比较,柚皮苷各剂量组LC3-Ⅱ/LC3-Ⅰ灰度值比值、Beclin1表达减少(P < 0.01或P < 0.05),p62表达增加(P < 0.01);柚皮苷各剂量组间比较,LC3-Ⅱ/LC3-Ⅰ灰度值比值、Beclin1及p62差异均有显著性意义(P < 0.01或P < 0.05),见图8及表9。说明EAE小鼠发病高峰期存在自噬通量增加,柚皮苷干预后可降低自噬通量,且柚皮苷剂量越大,作用越明显。"
| [1] Robinson AP, Harp CT, Noronha A, et al. The experimental autoimmune encephalomyelitis (EAE) model of MS: utility for understanding disease pathophysiology and treatment. Handb Clin Neurol. 2014;122:173-189. [2] Colonna M, Butovsky O. Microglia Function in the Central Nervous System During Health and Neurodegeneration. Ann Rev Immunol. 2017,35:441-468. [3] Geladaris A, Häusler D, Weber MS. Microglia: The Missing Link to Decipher and Therapeutically Control MS Progression? Int J Mol Sci. 2021;22(7):3461. [4] Bhattarai P, Thomas AK, Cosacak MI, et al. IL4/STAT6 Signaling Activates Neural Stem Cell Proliferation and Neurogenesis upon Amyloid-β42 Aggregation in Adult Zebrafish Brain. Cell Rep. 2016; 17(4):941-948. [5] Hu ZW, Zhou LQ, Yang S, et al. FTY720 Modulates Microglia Toward Anti-inflammatory Phenotype by Suppressing Autophagy via STAT1 Pathway. Cell Mol Neurobiol. 2021;41(2):353-364. [6] Feng J, Chen X, Lu S, et al. Naringin Attenuates Cerebral Ischemia-Reperfusion Injury Through Inhibiting Peroxynitrite-Mediated Mitophagy Activation. Mol Neurobiol. 2018;55(12):9029-9042. [7] Zhang YS, Wang F, Cui SX, et al. Natural dietary compound naringin prevents azoxymethane/dextran sodium sulfate-induced chronic colorectal inflammation and carcinogenesis in mice. Cancer Biol Ther. 2018;19(8):735-744. [8] Okuyama S, Yamamoto K, Mori H, et al. Neuroprotective effect of Citrus kawachiensis (Kawachi Bankan) peels, a rich source of naringin, against global cerebral ischemia/reperfusion injury in mice. Biosci Biotechnol Biochem. 2018;82(7):1216-1224. [9] Jagetia GC, Venkatesha VA, Reddy TK. Naringin, a citrus flavonone, protects against radiation-induced chromosome damage in mouse bone marrow. Mutagenesis. 2003;18(4):337-343. [10] Dijkstra S, Kooij G, Verbeek R, et al. Targeting the tetraspanin CD81 blocks monocyte transmigration and ameliorates EAE. Neurobiol Dis. 2008;31(3):413-421. [11] Kim SR. Control of Granule Cell Dispersion by Natural Materials Such as Eugenol and Naringin: A Potential Therapeutic Strategy Against Temporal Lobe Epilepsy. J Med Food. 2016;19(8):730-736. [12] Kim SR. Naringin as a beneficial natural product against degeneration of the nigrostriatal dopaminergic projection in the adult brain. Neural Regen Res. 2017;12(8):1375-1376. [13] Rong W, Pan YW, Cai X, et al. The mechanism of Naringin-enhanced remyelination after spinal cord injury. Neural Regen Res. 2017;12(3):470-477. [14] Singh N, Bansal Y, Bhandari R, et al. Naringin Reverses Neurobehavioral and Biochemical Alterations in Intracerebroventricular Collagenase-Induced Intracerebral Hemorrhage in Rats. Pharmacology. 2017;100(3-4):172-187. [15] Bai J, Li S, Wu G, et al. Naringin inhibits lipopolysaccharide-induced activation of microglia cells. Cell Mol Biol (Noisy-le-Grand, France). 2019;65(5):38-42. [16] Chen Q, Wu H, Tao J, et al. Effect of naringin on gp120-induced injury mediated by P2X7 receptors in rat primary cultured microglia. PloS one. 2017;12(8):e0183688. [17] Wolburg H, Wolburg-Buchholz K, Kraus J, et al. Localization of claudin-3 in tight junctions of the blood-brain barrier is selectively lost during experimental autoimmune encephalomyelitis and human glioblastoma multiforme. Acta Neuropathologica. 2003;105(6):586-592. [18] Sasaki Y, Ohsawa K, Kanazawa H, et al. Iba1 is an actin-cross-linking protein in macrophages/microglia. Biochem Biophys Res Commun. 2001;286(2):292-297. [19] Chu F, Shi M, Zheng C, et al. The roles of macrophages and microglia in multiple sclerosis and experimental autoimmune encephalomyelitis. J Neuroimmunol. 2018;318:1-7. [20] Hamilton JA. Colony-stimulating factors in inflammation and autoimmunity. Nat Rev Immunol. 2008;8(7):533-544. [21] Weber MS, Prod’homme T, Youssef S, et al. Type II monocytes modulate T cell-mediated central nervous system autoimmune disease. Nat Med. 2007;13(8):935-943. [22] Qu Z, Zheng N, Wei Y, et al. Effect of cornel iridoid glycoside on microglia activation through suppression of the JAK/STAT signalling pathway. J Neuroimmunol. 2019;330:96-107. [23] Thome R, Bonfanti AP, Rasouli J, et al. Chloroquine-treated dendritic cells require STAT1 signaling for their tolerogenic activity. Eur J Immunol. 2018;48(7):1228-1234. [24] Vezzani B, Carinci M, Patergnani S, et al. The Dichotomous Role of Inflammation in the CNS: A Mitochondrial Point of View. Biomolecules. 2020;10(10):1437. [25] Yin H, Wu H, Chen Y, et al. The Therapeutic and Pathogenic Role of Autophagy in Autoimmune Diseases. Front Immunol. 2018;9:1512. [26] Isogai S, Morimoto D, Arita K, et al. Crystal structure of the ubiquitin-associated (UBA) domain of p62 and its interaction with ubiquitin. J Biol Chem. 2011;286(36):31864-31874. [27] Raha S, Kim SM, Lee HJ, et al. Naringin Induces Lysosomal Permeabilization and Autophagy Cell Death in AGS Gastric Cancer Cells. Am J Chin Med. 2020;48(3):679-702. [28] Li W, Feng J, Gao C, et al. Nitration of Drp1 provokes mitophagy activation mediating neuronal injury in experimental autoimmune encephalomyelitis. Free Radic Biol Med. 2019;143:70-83. [29] Plaza-Zabala A, Sierra-Torre V, Sierra A. Autophagy and Microglia: Novel Partners in Neurodegeneration and Aging. Int J Mol Sci. 2017;18(3):598. [30] Su P, Zhang J, Wang D, et al. The role of autophagy in modulation of neuroinflammation in microglia. Neuroscience. 2016;319:155-167. |
| [1] | Cui Xing, Sun Xiaoqi, Zheng Wei, Ma Dexin. Huangqin Decoction regulates autophagy to intervene with intestinal acute graft-versus-host disease in mice [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1057-1062. |
| [2] | Lin Xuchen, Zhu Hainian, Wang Zengshun, Qi Tengmin, Liu Limin, Suonan Angxiu. Effect of xanthohumol on inflammatory factors and articular cartilage in a mouse mode of osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(5): 676-681. |
| [3] | Dong Miaomiao, Lai Han, Li Manling, Xu Xiuhong, Luo Meng, Wang Wenhao, Zhou Guoping. Effect of electroacupuncture on expression of nucleotide binding oligomerization domain-like receptor protein 3/cysteinyl aspartate specific proteinase 1 in rats with cerebral ischemia/reperfusion injury [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(5): 749-755. |
| [4] | Mi Jianguo, Qiao Rongqin, Liu Shaojin. Bushen Jianpi Huoxue Recipe improves bone metabolism, oxidative stress, and autophagy in osteoporotic rats [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(26): 4147-4152. |
| [5] | Wang Yuyin, Wei Wenyue, Guo Minfang, Li Hongxia, Zhang Jing, Gu Qingfang, Liu Xiaoqin, Guo Xiaoping, Song Lijuan, Chai Zhi, Ma Cungen, Wei Jiezhong. Bushen Yizhi Anti-aging Prescription improves cognitive function of APP/PS1 mice by regulating microglia and macrophage polarization [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(26): 4166-4172. |
| [6] | Zhang Gaofei, Wang Di, Li Jiamei, Lou Hanxiao, Zeng Yueqin, Liu Wenjun. Mesenchymal stem cells promote wound healing by regulating the autophagy [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(25): 4058-4063. |
| [7] | Jiang Jie, Zhao Baixiao, Chen Libin, Wen Li, Zhang Shanshan, Ma Jie, Zhao Hua. Effect of moxibustion pretreatment on autophagy and NLRP3 inflammasome expression in cerebral ischemia-reperfusion model rats [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(23): 3615-3619. |
| [8] | Li Jinglu, Wang Sainan, Wang Yangyang, Fu Guiqiang, Wang Ying, Hu Jianguo, Tang Jie, Lyu Hezuo. CRID3, a blocker of apoptosis-associated speck-like protein containing a card, influences local gene transcription in mice with acute spinal cord injury: a transcriptomic analysis [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(23): 3620-3632. |
| [9] | Fan Shuai, Wu Chunfei, Liang Zujian, Xu Zhaohui, Xie Pingjin, Zhu Genfu. Bushen Tiaogan Prescription containing serum in the treatment of osteoarthritis by promoting chondrocyte autophagy in rats [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(20): 3178-3183. |
| [10] | Cai Zhiguo, Du Shasha, Yang Kun, Zhao Na, Liu Qi. Lipopolysaccharides mediate autophagy of mouse insulinoma βtc6 cells in high glucose state [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(20): 3127-3132. |
| [11] | Wang Sihan, Chen Junji, Mo Weibin. Effects of Dendrobium officinale flavonoid on oxidative stress and autophagy in the liver of an exhaustive exercise rat model [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(20): 3212-3219. |
| [12] | Liu Yulu, Jia Weiwei, Dai Yaling, Xu Wenshan, Ding Yanyi, Liang Shengxiang, Liu Weilin, Chen Lidian. Effects of time-specific AMP-activated protein kinase alpha1/2 gene knockout on hippocampal energy metabolism and synaptic plasticity in mice [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(20): 3230-3235. |
| [13] | Lyu Yuhu, Cheng Lin, Lin Wentao, Peng Fenglin. Role and regulatory mechanism of glycophagy in glycogen metabolism [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(20): 3243-3249. |
| [14] | Sun Youqiang, Ma Chao, Liang Mengmeng, Xin Pengfei, Zhang Hua, Xiang Xiaobing. The pivotal role of autophagy in bone cells: bone-related cell activity and bone metabolism [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(2): 276-282. |
| [15] | Wang Jiajia, Liu Jie, Wang Min. Establishing a murine model of experimental apical periodontitis induced by Fusobacterium nucleatum [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(2): 176-181. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||