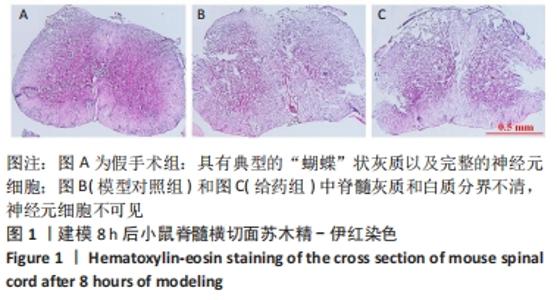
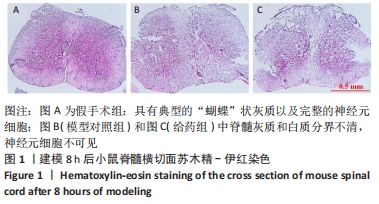
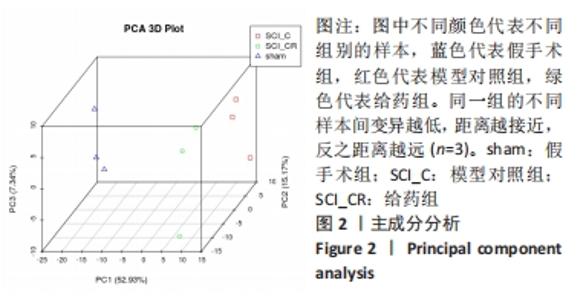
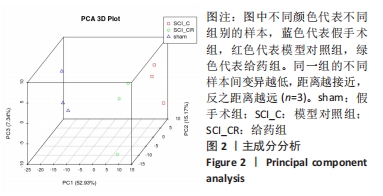
Chinese Journal of Tissue Engineering Research ›› 2022, Vol. 26 ›› Issue (23): 3620-3632.doi: 10.12307/2022.659
Previous Articles Next Articles
CRID3, a blocker of apoptosis-associated speck-like protein containing a card, influences local gene transcription in mice with acute spinal cord injury: a transcriptomic analysis
Li Jinglu1, 2, Wang Sainan1, 2, 3, Wang Yangyang1, 2, 3, Fu Guiqiang1, 2, 3, Wang Ying2, Hu Jianguo1, 2, Tang Jie1, 3, Lyu Hezuo1, 2, 3
- 1Clinical Laboratory, the First Affiliated Hospital of Bengbu Medical College, Bengbu 233004, Anhui Province, China; 2Key Laboratory of Tissue Transplantation, 3Department of Immunology, Bengbu Medical College, Bengbu 233000, Anhui Province, China
-
Received:2021-05-20Accepted:2021-07-21Online:2022-08-18Published:2022-02-15 -
Contact:Lyu Hezuo, MD, Master candidate, Clinical Laboratory, the First Affiliated Hospital of Bengbu Medical College, Bengbu 233004, Anhui Province, China; Key Laboratory of Tissue Transplantation, Bengbu Medical College, Bengbu 233000, Anhui Province, China; Department of Immunology, Bengbu Medical College, Bengbu 233000, Anhui Province, China -
About author:Li Jinglu, Master candidate, Clinical Laboratory, the First Affiliated Hospital of Bengbu Medical College, Bengbu 233004, Anhui Province, China; Key Laboratory of Tissue Transplantation, Bengbu Medical College, Bengbu 233000, Anhui Province, China -
Supported by:the National Natural Science Foundation of China, No. 81772321(to LHZ); the First-batch “512 Talent Training Program” of Bengbu Medical College, No. 51201109 (to LHZ)
CLC Number:
Cite this article
Li Jinglu, Wang Sainan, Wang Yangyang, Fu Guiqiang, Wang Ying, Hu Jianguo, Tang Jie, Lyu Hezuo. CRID3, a blocker of apoptosis-associated speck-like protein containing a card, influences local gene transcription in mice with acute spinal cord injury: a transcriptomic analysis[J]. Chinese Journal of Tissue Engineering Research, 2022, 26(23): 3620-3632.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
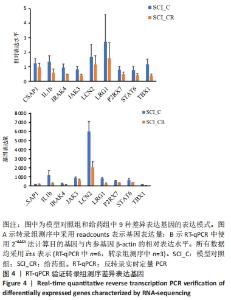
2.5 RT-qPCR验证转录组测序结果 为了验证转录组测序结果,从模型对照组和给药组中随机选择9个差异表达的基因,即半胱氨酸天冬氨酸蛋白酶1、白细胞介素1β、irak4、jak3、lcn2、lrg1、p2rx7、stat6和tbx1,采用RT-qPCR进行验证。结果表明,在模型对照组中,这些基因在转录组测序的相对表达量(readcounts)分别为162.00±28.21,1 188.00±528.32,240.00±46.29,859.33±1 34.35,6 015.33±1 085.22,825.33±97.57,523.67±72.67,632.00±172.50和161.67±16.29;其在RT-qPCR检测中的相对表达水平分别为1.23±0.33,1.33±0.42,0.95±0.23,0.82±0.21,1.67±0.88,2.72±1.87,0.79±0.23,0.77±0.26和1.16±0.36。在给药组中,这些基因在转录组测序的相对表达量(readcounts)分别为177.00±29.61,257.33±86.03,150.33±13.58,601.00±143.04,2 051.00±603.76,248.33±71.93,318.33±50.77,343.67±23.07和38.00±8.89;其在RT-qPCR检测中的相对表达水平分别为0.97±0.26,0.59±0.25,0.46±0.03, 0.41±0.08,1.15±0.60,1.59±1.06,0.48±0.15,0.40±0.12和0.37±0.13。整体来看这些差异基因的表达趋势是基本一致的,见图4。"


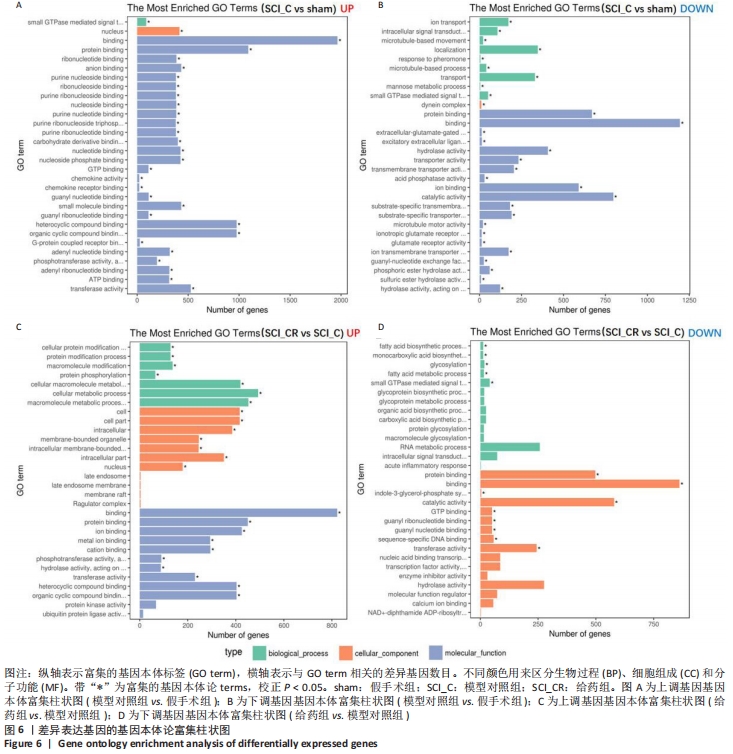
2.7 差异基因基因本体富集分析 基因本体数据库把基因的本体分为3种:生物过程、细胞组成和分子功能。差异基因的基因本体富集分析表明,与假手术相比,模型对照组上调的差异基因中有111个有意义的基因本体功能注释(校正后的P < 0.05),30个最为丰富的功能注释(有28个分子功能、1个生物过程和1个细胞组成),其中在蛋白结合、核苷酸结合、阴离子结合、趋化因子受体结合、G-蛋白偶联受体结合等方面最为丰富,见图6A;在下调的差异基因中有33个有意义的基因本体功能注释(校正后的P < 0.05),30个最为丰富的功能注释(有20个分子功能、9个生物过程和1个细胞组成),其中在蛋白结合、核苷酸结合、阴离子结合、趋化因子受体结合、G-蛋白偶联受体结合等方面最为丰富,见图6B。与模型对照组相比,给药组上调的差异基因有24个有意义的基因本体功能注释(校正后的P < 0.05,包括10个分子功能、7个生物过程和7个细胞组成),其中在结合、细胞膜内结合、金属离子结合、蛋白质结合、离子结合、阳离子结合等最为丰富,见图6C;下调的差异基因有14个有意义的基因本体功能注释(校正后的P < 0.05,包括5个生物过程和9个细胞组成),其中在结合、蛋白质结合、序列特异性DNA结合等方面最为丰富,见图6D。"

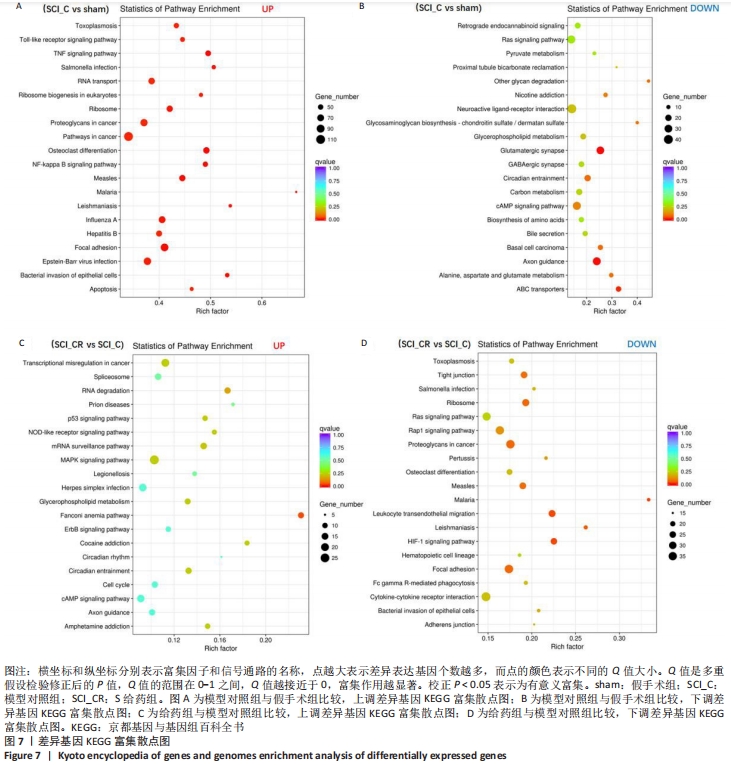
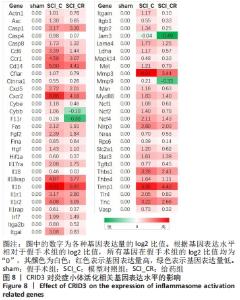
2.8 差异基因的KEGG富集分析 差异表达基因的KEGG富集分析表明:与假手术组相比,模型对照组上调的差异基因中富集出39个重要的信号通路(校正后的P < 0.05),前20个最主要的信号通路见图7A,包括Toll样受体信号通路、肿瘤坏死因子信号通路、核因子κB信号通路、焦点粘连、细胞凋亡等;下调的差异基因富集出3个重要的信号通路(校正后的P < 0.05),为谷氨酸能突触、轴突导引、ABC转运蛋白,见图7B。 与模型对照组相比,给药组上调的基因中富集出一条信号通路,即Fanconi anemia pathway(校正后的P < 0.05),见图7C;下调的差异基因富集出4个重要的信号通路(校正后的P < 0.05),主要为白细胞跨内皮迁移、缺氧诱导因子1信号通路和焦点粘连,见图7D。"
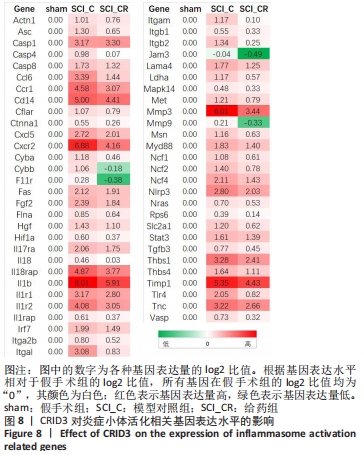
2.9 CRID3对炎症小体活化相关基因表达水平的影响 进一步对差异基因及其相关信号通路梳理,可以发现与炎症小体活化相关的信号通路主要包括肿瘤坏死因子、Toll样受体、核因子κB、PI3K-Akt、缺氧诱导因子1、MAPK和NOD-样受体信号通路。另外,炎症小体活化后诱发的信号通路包括细胞焦亡、白细胞跨内皮迁移、细胞因子与其受体相互作用等。这些信号通路中富集的基因在模型对照组的表达均较假手术组呈升高趋势。当给予药物干预后缺氧诱导因子1信号通路和白细胞跨内皮迁移明显被抑制。根据基因表达量(counts)的log2比值,又对上述信号通路相关的一些代表性基因表达水平进行了比较,整理出了57个在脊髓损伤后显著升高,而在给药后又明显降低的基因。根据基因表达水平相对于假手术组的log2比值,在假手术组所有的基因表达水平均为0,脊髓损伤后这些基因表达水平显著上调,当给药后其基因表达水平也相应降低。其中对CRID3最敏感的基因包括Asc、Casp4、Cyba、Cybb、F11r、Hif1a、Il18、Il1b、Itgal、Itgam、Itgb2、Jam3、Mmp3、Mmp9、Tlr4等,见图8。"
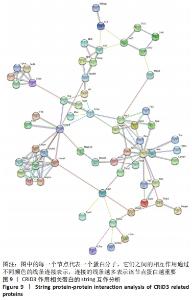
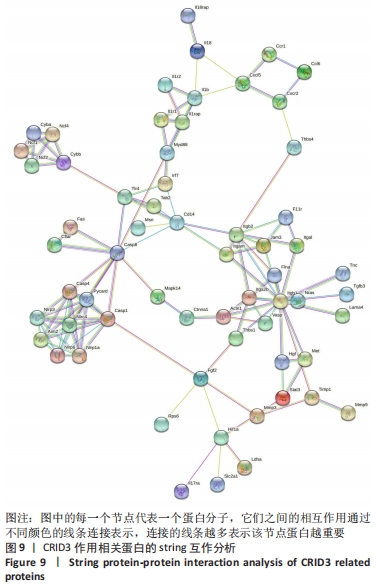
2.10 String蛋白互作分析结果 为了进一步探讨炎症小体组分与CRID3抑制的基因在蛋白水平的相互作用,对炎症小体组分与CRID3抑制的基因表达蛋白,在STRING数据库进行蛋白间相互作用分析,结果见图9,炎症小体组成成分Nlrp1、Nlrp3、Nlrp6、Nlrc4、Aim2、ASC(此数据库命名为Pycard)、Csap1和Csap4相互作用密切,并且它们又通过Csap8与白细胞介素1β、白细胞介素18形成完整的链接。此外,还有一些重要的节点蛋白,如Actn1、Cxcl5、Cd14、Cyba、Cybb、Fgf2、Hif1a、F11r、Itgal、Itga2b、Itgam、Itgb1、Jam3、Mmp3、Tlr4等被确定。"
| [1] GAO L, PENG Y, XU W, et al. Progress in Stem Cell Therapy for Spinal Cord Injury. Stem Cells Int. 2020;2020:2853650. [2] YUAN S, SHI Z, CAO F, et al. Epidemiological Features of Spinal Cord Injury in China: A Systematic Review. Front Neurol. 2018;9:683. [3] 程菡, 胡雪慧, 韩蕾, 等. 感恩对脊髓损伤患者心理资本和生活质量的影响[J]. 华南国防医学杂志,2020,34(11):805-809. [4] 汪晶, 李伦兰, 丁杨, 等. 脊髓损伤患者住院医疗费用及其影响因素分析[J]. 中国病案,2021,22(4):50-54. [5] GALEIRAS VR, FERREIRO VM, MOURELO FM, et al. Update on traumatic acute spinal cord injury. Part 1. Med Intensiva. 2017;41(4):237-247. [6] AHMED A, PATIL AA, AGRAWAL DK. Immunobiology of spinal cord injuries and potential therapeutic approaches. Mol Cell Biochem. 2018; 441(1-2):181-189. [7] 李鹏飞, 高枫. 脊髓损伤后神经修复与再生研究进展[J]. 延安大学学报(医学科学版),2021,19(1):92-95. [8] 王赛男, 胡建国, 吕合作. 炎症小体活化对脊髓损伤局部免疫微环境的影响[J]. 中国免疫学杂志,2018,34(5):765-768. [9] 何腾, 李何阳子, 王琳琳. 脊髓损伤后炎症小体在脊髓功能恢复中作用的研究进展[J]. 中华创伤杂志,2019,35(10):948-952. [10] 高书涛, 荀传辉, 盛伟斌. NLRP3炎症小体在创伤性脊髓损伤中作用的研究进展[J]. 中国脊柱脊髓杂志,2019,29(11):1042-1045. [11] MARTINON F, BURNS K, TSCHOPP J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell. 2002;10(2):417-426. [12] CHRISTGEN S, PLACE DE, KANNEGANTI TD. Toward targeting inflammasomes: insights into their regulation and activation. Cell Res. 2020;30(4):315-327. [13] CHEN YQ, WANG SN, SHI YJ, et al. CRID3, a blocker of apoptosis associated speck like protein containing a card, ameliorates murine spinal cord injury by improving local immune microenvironment. J Neuroinflammation. 2020;17(1):255. [14] ITO H, KANBE A, SAKAI H, et al. Activation of NLRP3 signalling accelerates skin wound healing. Exp Dermatol, 2018;27(1):80-86. [15] 魏荣志, 王玉玞, 闫景龙. 大鼠脊髓损伤模型研究进展概述[J]. 神经损伤与功能重建,2020,15(5):274-277. [16] 黄勇, 周英杰. 大鼠脊髓损伤模型研究进展[J]. 中华实验外科杂志, 2020,37(3):594-596. [17] WENG J, LI DD, JIANG BG, et al. Temporal changes in the spinal cord transcriptome after peripheral nerve injury. Neural Regen Res. 2020; 15(7):1360-1367. [18] ISMAEL S, NASOOHI S, ISHRAT T. MCC950, the Selective Inhibitor of Nucleotide Oligomerization Domain-Like Receptor Protein-3 Inflammasome, Protects Mice against Traumatic Brain Injury. J Neurotrauma. 2018;35(11):1294-1303. [19] COLL RC, ROBERTSON AA, CHAE JJ, et al. A small-molecule inhibitor of the NLRP3 inflammasome for the treatment of inflammatory diseases. Nat Med. 2015;21(3):248-255. [20] ISMAEL S, ZHAO L, NASOOHI S, et al. Inhibition of the NLRP3-inflammasome as a potential approach for neuroprotection after stroke. Sci Rep. 2018;8(1):5971. [21] COCKRAM PE, KIST M, PRAKASH S, et al. Ubiquitination in the regulation of inflammatory cell death and cancer. Cell Death Differ. 2021;28(2):591-605. [22] ZHANG W, JIA L, ZHAO B, et al. Quercetin reverses TNFalpha induced osteogenic damage to human periodontal ligament stem cells by suppressing the NFkappaB/NLRP3 inflammasome pathway. Int J Mol Med. 2021;47(4):39. [23] MANES NP, NITA-LAZAR A. Molecular Mechanisms of the Toll-Like Receptor, STING, MAVS, Inflammasome, and Interferon Pathways. mSystems. 2021:e33621. [24] SIVASINPRASASN S, WIKAN N, TOCHARUS J, et al. Pelargonic acid vanillylamide and rosuvastatin protect against oxidized low-density lipoprotein-induced endothelial dysfunction by inhibiting the NF-kappaB/NLRP3 pathway and improving cell-cell junctions. Chem Biol Interact. 2021:109572. [25] LIU Z, LI J, LIN S, et al. PI3K regulates the activation of NLRP3 inflammasome in atherosclerosis through part-dependent AKT signaling pathway. Exp Anim. 2021;70(4):488-497. [26] LI X, MEI W, HUANG Z, et al. Casticin suppresses monoiodoacetic acid-induced knee osteoarthritis through inhibiting HIF-1alpha/NLRP3 inflammasome signaling. Int Immunopharmacol. 2020;86:106745. [27] FANG F, XIE Z, QUAN J, et al. Baicalin suppresses Propionibacterium acnes-induced skin inflammation by downregulating the NF-kappaB/MAPK signaling pathway and inhibiting activation of NLRP3 inflammasome. Braz J Med Biol Res. 2020;53(12):e9949. [28] HEIM VJ, STAFFORD CA, NACHBUR U. NOD Signaling and Cell Death. Front Cell Dev Biol. 2019;7:208. [29] ZHANG WJ, CHEN SJ, ZHOU SC, et al. Inflammasomes and Fibrosis. Front Immunol. 2021;12:643149. [30] LISSNER D, SCHUMANN M, BATRA A, et al. Monocyte and M1 Macrophage-induced Barrier Defect Contributes to Chronic Intestinal Inflammation in IBD. Inflamm Bowel Dis. 2015;21(6):1297-1305. [31] ZHANG Y, SHAO L, ZHOU N, et al. Transcriptome Analysis of Porcine PBMCs Reveals the Immune Cascade Response and Gene Ontology Terms Related to Cell Death and Fibrosis in the Progression of Liver Failure. Can J Gastroenterol Hepatol. 2018;2018:2101906. [32] CHEN K, DENG S, LU H, et al. RNA-seq characterization of spinal cord injury transcriptome in acute/subacute phases: a resource for understanding the pathology at the systems level. PLoS One. 2013;8(8): e72567. [33] SHI LL, ZHANG N, XIE XM, et al. Transcriptome profile of rat genes in injured spinal cord at different stages by RNA-sequencing. BMC Genomics. 2017;18(1):173. [34] CHEN H, LI J, LIANG S, et al. Effect of hypoxia-inducible factor-1/vascular endothelial growth factor signaling pathway on spinal cord injury in rats. Exp Ther Med. 2017;13(3):861-866. [35] XIONG Y, XIA Y, DENG J, et al. Direct Peritoneal Resuscitation with Pyruvate Protects the Spinal Cord and Induces Autophagy via Regulating PHD2 in a Rat Model of Spinal Cord Ischemia-Reperfusion Injury. Oxid Med Cell Longev. 2020;2020:4909103. [36] LI Y, HAN W, WU Y, et al. Stabilization of Hypoxia Inducible Factor-1alpha by Dimethyloxalylglycine Promotes Recovery from Acute Spinal Cord Injury by Inhibiting Neural Apoptosis and Enhancing Axon Regeneration. J Neurotrauma. 2019;36(24):3394-3409. [37] LI JH, SHI ZJ, LI Y, et al. Bioinformatic identification of key candidate genes and pathways in axon regeneration after spinal cord injury in zebrafish. Neural Regen Res. 2020;15(1):103-111. [38] GRAHAM ZA, QIN W, HARLOW LC, et al. Focal adhesion kinase signaling is decreased 56 days following spinal cord injury in rat gastrocnemius. Spinal Cord. 2016;54(7):502-509. [39] NOURSHARGH S, ALON R. Leukocyte migration into inflamed tissues. Immunity. 2014;41(5):694-707. [40] ALON R, SHULMAN Z. Chemokine triggered integrin activation and actin remodeling events guiding lymphocyte migration across vascular barriers. Exp Cell Res. 2011;317(5):632-641. [41] DEHNE N, FUHRMANN D, BRUNE B. Hypoxia-inducible factor (HIF) in hormone signaling during health and disease. Cardiovasc Hematol Agents Med Chem. 2013;11(2):125-135. [42] RISBUD MV, SHAPIRO IM. Notochordal cells in the adult intervertebral disc: new perspective on an old question. Crit Rev Eukaryot Gene Expr. 2011;21(1):29-41. [43] KIM DH, WIRTZ D. Focal adhesion size uniquely predicts cell migration. FASEB J. 2013;27(4):1351-1361. [44] PRAGER-KHOUTORSKY M, LICHTENSTEIN A, KRISHNAN R, et al. Fibroblast polarization is a matrix-rigidity-dependent process controlled by focal adhesion mechanosensing. Nat Cell Biol. 2011;13(12):1457-1465. [45] GEIGER B, SPATZ JP, BERSHADSKY AD. Environmental sensing through focal adhesions. Nat Rev Mol Cell Biol. 2009;10(1):21-33. [46] PATLA I, VOLBERG T, ELAD N, et al. Dissecting the molecular architecture of integrin adhesion sites by cryo-electron tomography. Nat Cell Biol. 2010;12(9):909-915. [47] CHEN J, CHEN YQ, SHI YJ, et al. VX-765 reduces neuroinflammation after spinal cord injury in mice. Neural Regen Res. 2021;16(9):1836-1847. [48] BAKRI FG, MOLLIN M, BEAUMEL S, et al. Second Report of Chronic Granulomatous Disease in Jordan: Clinical and Genetic Description of 31 Patients From 21 Different Families, Including Families From Lybia and Iraq. Front Immunol. 2021;12:639226. [49] WU Z, LU Z, OU J, et al. Inflammatory response and oxidative stress attenuated by sulfiredoxin1 in neuronlike cells depends on nuclear factor erythroid2related factor 2. Mol Med Rep. 2020;22(6): 4734-4742. [50] BEDNAREK R, SELMI A, WOJKOWSKA D, et al. Functional inhibition of F11 receptor (F11R/junctional adhesion molecule-A/JAM-A) activity by a F11R-derived peptide in breast cancer and its microenvironment. Breast Cancer Res Treat. 2020,179(2):325-335. [51] ELZAKRA N, KIM Y. HIF-1alpha Metabolic Pathways in Human Cancer. Adv Exp Med Biol. 2021;1280:243-260. [52] ZHENG J, CHEN P, ZHONG J, et al. HIF1alpha in myocardial ischemiareperfusion injury (Review). Mol Med Rep. 2021;23(5):352. [53] JU Y, HE M, MAO B, et al. [Sequential changes of hypoxia-inducible factor 1 alpha in experimental spinal cord injury and its significance]. Hua Xi Yi Ke Da Xue Xue Bao. 2002;33(1):43-45. [54] SIDDIQ A, AMINOVA LR, TROY CM, et al. Selective inhibition of hypoxia-inducible factor (HIF) prolyl-hydroxylase 1 mediates neuroprotection against normoxic oxidative death via HIF- and CREB-independent pathways. J Neurosci. 2009;29(27):8828-8838. [55] WANG X, MA J, FU Q, et al. Role of hypoxiainducible factor1alpha in autophagic cell death in microglial cells induced by hypoxia. Mol Med Rep. 2017;15(4):2097-2105. [56] LEFORT C T, LEY K. Neutrophil arrest by LFA-1 activation. Front Immunol. 2012;3:157. [57] BLIGHT BJ, GILL AS, SUMSION JS, et al. Cell Adhesion Molecules are Upregulated and May Drive Inflammation in Chronic Rhinosinusitis with Nasal Polyposis. J Asthma Allergy. 2021;14:585-593. [58] CHAVAKIS T, PREISSNER KT, SANTOSO S. Leukocyte trans-endothelial migration: JAMs add new pieces to the puzzle. Thromb Haemost. 2003;89(1):13-17. [59] YANG Z, LV Q, WANG Z, et al. Identification of crucial genes associated with rat traumatic spinal cord injury. Mol Med Rep. 2017;15(4):1997-2006. [60] LI Y, CHEN Y, LI X, et al. RNA sequencing screening of differentially expressed genes after spinal cord injury. Neural Regen Res. 2019;14(9): 1583-1593. [61] WANG T, WU B, ZHANG X, et al. Identification of gene coexpression modules, hub genes, and pathways related to spinal cord injury using integrated bioinformatics methods. J Cell Biochem. 2019. doi: 10.1002/jcb.27908. [62] LI Z, SUN Y, HE M, et al. Differentially-expressed mRNAs, microRNAs and long noncoding RNAs in intervertebral disc degeneration identified by RNA-sequencing. Bioengineered. 2021;12(1):1026-1039. [63] SHI Z, NING G, ZHANG B, et al. Signatures of altered long noncoding RNAs and messenger RNAs expression in the early acute phase of spinal cord injury. J Cell Physiol. 2019;234(6):8918-8927. [64] BASSIOUNI W, ALI M, SCHULZ R. Multifunctional intracellular matrix metalloproteinases: implications in disease. FEBS J. 2021. doi: 10.1111/febs.15701. [65] DE CASTRO R J, BURNS C L, MCADOO DJ, et al. Metalloproteinase increases in the injured rat spinal cord. Neuroreport. 2000;11(16): 3551-3554. [66] LEE JY, CHOI HY, AHN HJ, et al. Matrix metalloproteinase-3 promotes early blood-spinal cord barrier disruption and hemorrhage and impairs long-term neurological recovery after spinal cord injury. Am J Pathol, 2014;184(11):2985-3000. [67] LEE JY, NA WH, CHOI HY, et al. Jmjd3 mediates blood-spinal cord barrier disruption after spinal cord injury by regulating MMP-3 and MMP-9 expressions. Neurobiol Dis. 2016;95:66-81. [68] PALSSON-MCDERMOTT EM, O’NEILL LA. Signal transduction by the lipopolysaccharide receptor, Toll-like receptor-4. Immunology. 2004; 113(2):153-162. [69] XIA H, WANG D, GUO X, et al. Catalpol Protects Against Spinal Cord Injury in Mice Through Regulating MicroRNA-142-Mediated HMGB1/TLR4/NF-kappaB Signaling Pathway. Front Pharmacol. 2020;11:630222. [70] CHEN D, PAN D, TANG S, et al. Administration of chlorogenic acid alleviates spinal cord injury via TLR4/NFkappaB and p38 signaling pathway antiinflammatory activity. Mol Med Rep. 2018;17(1):1340-1346. [71] NI H, JIN W, ZHU T, et al. Curcumin modulates TLR4/NF-kappaB inflammatory signaling pathway following traumatic spinal cord injury in rats. J Spinal Cord Med, 2015;38(2):199-206. [72] JEVNIKAR Z, OBERMAJER N, KOS J. LFA-1 fine-tuning by cathepsin X. IUBMB Life. 2011;63(9):686-693. [73] ZHANG W, WANG H, SUN M, et al. CXCL5/CXCR2 axis in tumor microenvironment as potential diagnostic biomarker and therapeutic target. Cancer Commun (Lond). 2020;40(2-3):69-80. [74] WANG LY, TU YF, LIN YC, et al. CXCL5 signaling is a shared pathway of neuroinflammation and blood-brain barrier injury contributing to white matter injury in the immature brain. J Neuroinflammation. 2016;13:6. [75] LI BH, GARSTKA MA, LI ZF. Chemokines and their receptors promoting the recruitment of myeloid-derived suppressor cells into the tumor. Mol Immunol. 2020;117:201-215. [76] ZITO G, BUSCETTA M, CIMINO M, et al. Cellular Models and Assays to Study NLRP3 Inflammasome Biology. Int J Mol Sci. 2020;21(12):4294. [77] SAMPATH P, MOIDEEN K, RANGANATHAN UD, et al. Monocyte Subsets: Phenotypes and Function in Tuberculosis Infection. Front Immunol. 2018;9:1726. [78] DETILLIEUX KA, SHEIKH F, KARDAMI E, et al. Biological activities of fibroblast growth factor-2 in the adult myocardium. Cardiovasc Res. 2003;57(1):8-19. |
| [1] | Kan Houming, Fan Lijun, Chen Xuetai, Shen Wen. Application of platelet-rich plasma in neuropathic pain [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(8): 1286-1292. |
| [2] | Hu Wei, Xie Xingqi, Tu Guanjun. Exosomes derived from bone marrow mesenchymal stem cells improve the integrity of the blood-spinal cord barrier after spinal cord injury [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 992-998. |
| [3] | Fan Yiming, Liu Fangyu, Zhang Hongyu, Li Shuai, Wang Yansong. Serial questions about endogenous neural stem cell response in the ependymal zone after spinal cord injury [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1137-1142. |
| [4] | Lin Xuchen, Zhu Hainian, Wang Zengshun, Qi Tengmin, Liu Limin, Suonan Angxiu. Effect of xanthohumol on inflammatory factors and articular cartilage in a mouse mode of osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(5): 676-681. |
| [5] | Shi Yao, Han Shufeng, Yuan Yitong, Du Ruochen, Jing Zhijie, Zhao Bichun, Zhang Ruxin, Zhang Yujuan, Wang Chunfang. Efficacy and safety of human umbilical cord mesenchymal stem cells in the treatment of spinal cord injury: a meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(25): 4093-4100. |
| [6] | Jiang Jie, Zhao Baixiao, Chen Libin, Wen Li, Zhang Shanshan, Ma Jie, Zhao Hua. Effect of moxibustion pretreatment on autophagy and NLRP3 inflammasome expression in cerebral ischemia-reperfusion model rats [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(23): 3615-3619. |
| [7] | Chen Pingbo, Wang Jing, Sun Yong, Xu Xiaofeng, Chen Qian, Zhang Zhijian. Genipin crosslinked sonic hedgehog composite fibrin scaffolds for the repair of spinal cord injury in rats [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(21): 3345-3350. |
| [8] | Li Jianfeng, Zhang Shulian, Yan Jinyu, Li jiayi, Jin Zhao, Deng Qi. Vimentin silenced by small interfering RNA inhibits glial scar formation in a rat model of acute spinal cord injury [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(20): 3190-3195. |
| [9] | Liu Yulu, Jia Weiwei, Dai Yaling, Xu Wenshan, Ding Yanyi, Liang Shengxiang, Liu Weilin, Chen Lidian. Effects of time-specific AMP-activated protein kinase alpha1/2 gene knockout on hippocampal energy metabolism and synaptic plasticity in mice [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(20): 3230-3235. |
| [10] | Wang Jiajia, Liu Jie, Wang Min. Establishing a murine model of experimental apical periodontitis induced by Fusobacterium nucleatum [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(2): 176-181. |
| [11] | Geng Yuanwen, Lin Qinqin, Li Ruoming, Tang Shaokai, Wang Baihui, Tian Zhenjun. A single bout of exhaustive exercise induces renal NOD-like receptor protein 3 inflammasome expression in rats [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(2): 190-196. |
| [12] | Huo Hua, Liu Guanjuan, Song Na, Zhou Qian, Cheng Yuting, Luo Shanshan, Liao Jian. Effects of zoledronic acid on alveolar bone metabolism and nucleotide-binding oligomerization domain-like receptor protein 3 inflammasome in the alveolar bone of ovariectomized osteoporosis rats [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(17): 2660-2666. |
| [13] | Yang Qin, Wang Min. Constructing a mouse model of periodontitis with occlusal trauma [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(17): 2667-2672. |
| [14] | Zhu Yaping, Li Xiang, Chen Zhiwen, Liu Ying, Zhao Wenzhuo, Ma Ketao, Gu Qiang. Calcium-sensitive receptors influence pulmonary vascular remodeling in neonatal mice with persistent pulmonary hypertension [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(17): 2708-2712. |
| [15] | Yin Liang, Zhang Mingxue, Li Jianan, Jiang Feng. Inducing macrophage polarization to M2 anti-inflammatory type reduces oxidative damage in diabetic retinopathy mice [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(17): 2685-2689. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||