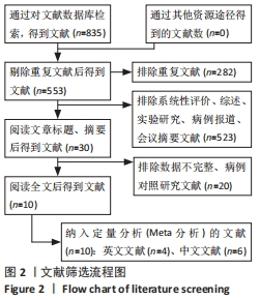
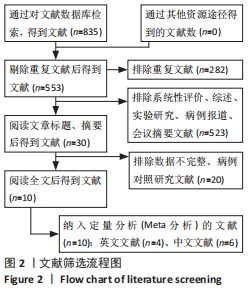
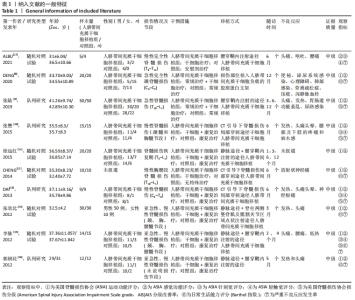
Chinese Journal of Tissue Engineering Research ›› 2022, Vol. 26 ›› Issue (25): 4093-4100.doi: 10.12307/2022.419
Efficacy and safety of human umbilical cord mesenchymal stem cells in the treatment of spinal cord injury: a meta-analysis
Shi Yao1, Han Shufeng2, Yuan Yitong3, Du Ruochen3, Jing Zhijie3, Zhao Bichun3, Zhang Ruxin3, Zhang Yujuan3, Wang Chunfang3
- 1Shanxi Medical University, Taiyuan 030001, Shanxi Province, China; 2First Hospital of Shanxi Medical University, Taiyuan 030001, Shanxi Province, China; 3Experimental Animal Center of Shanxi Medical University, Taiyuan 030001, Shanxi Province, China
-
Received:2021-03-29Accepted:2021-05-08Online:2022-09-08Published:2022-01-26 -
Contact:Han Shufeng, Master, Professor, Chief physician, First Hospital of Shanxi Medical University, Taiyuan 030001, Shanxi Province, China Wang Chunfang, PhD, Professor, Experimental Animal Center of Shanxi Medical University, Taiyuan 030001, Shanxi Province, China -
About author:Shi Yao, Master candidate, Shanxi Medical University, Taiyuan 030001, Shanxi Province, China -
Supported by:the National Natural Science Youth Foundation, No. 82001326 (to YYT); Natural Science Foundation of Shanxi Province, No. 201801D121212 (to WCF), No. 201901D111384 (to WCF, YYT), No. 201901D211319 (to YYT); Shanxi Provincial Key Social Development Project, No. 201803D31068 (to WCF); Science and Technology Innovation Project of Colleges and Universities in Shanxi Province, No. 2019L0445 (to YYT), No. 2019L0418 (to DRC)
CLC Number:
Cite this article
Shi Yao, Han Shufeng, Yuan Yitong, Du Ruochen, Jing Zhijie, Zhao Bichun, Zhang Ruxin, Zhang Yujuan, Wang Chunfang. Efficacy and safety of human umbilical cord mesenchymal stem cells in the treatment of spinal cord injury: a meta-analysis[J]. Chinese Journal of Tissue Engineering Research, 2022, 26(25): 4093-4100.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
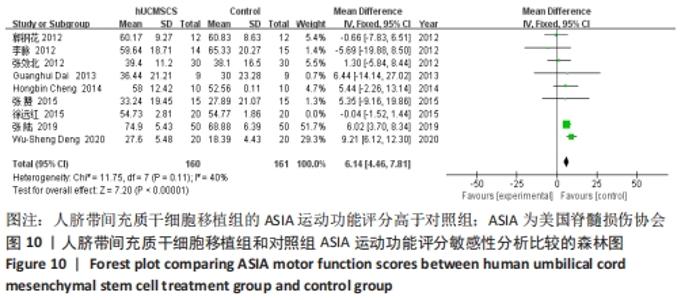
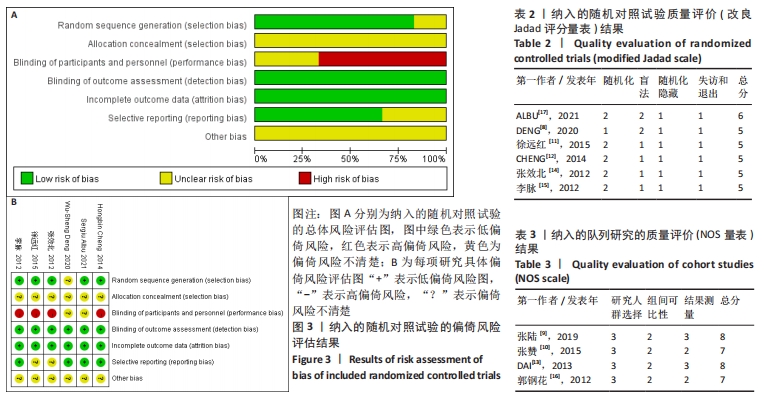
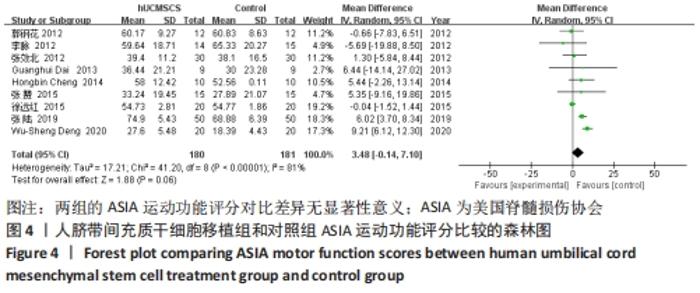
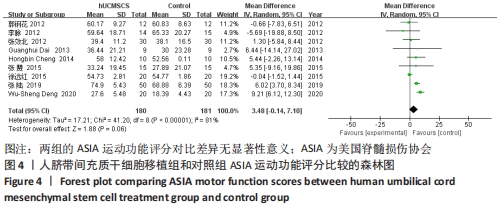
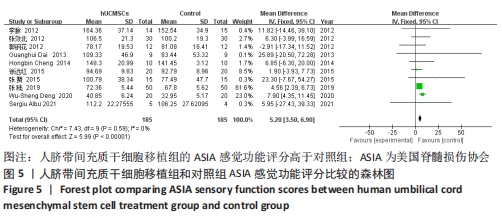
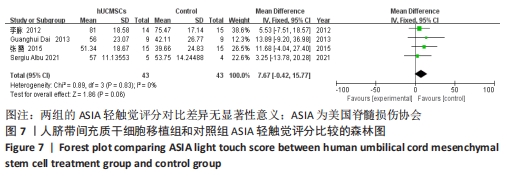
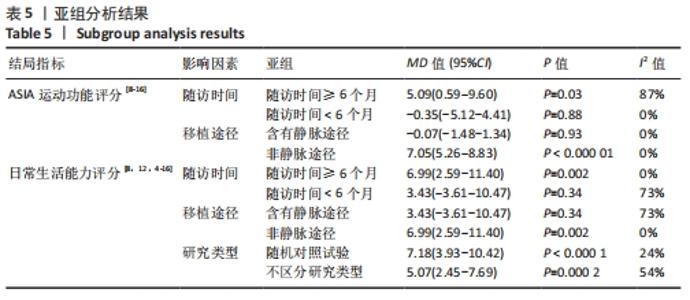
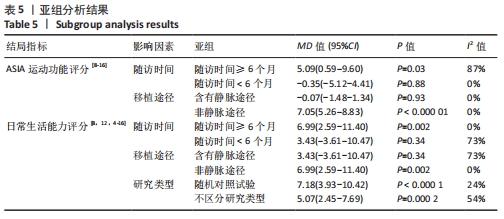
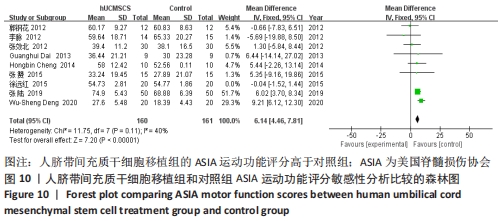
2.5 敏感性分析结果 在ASIA运动功能评分的Meta分析结果中,有9篇文献比较了人脐带间充质干细胞移植组与对照组的ASIA运动功能评分[8-16],异质性检验提示纳入的研究之间异质性较大(P < 0.000 01,I2=81%),采用随机效应模型进行Meta分析,结果显示人脐带间充质干细胞移植组的ASIA运动功能评分与对照组相比,差异无显著性意义(MD=3.48,95%CI:-0.14-7.10,P=0.06)。因此,需要对这项指标进行敏感性分析,经过逐一剔除纳入文献后,发现徐远红等[11]的研究结果对异质性的影响较大,剔除后发现,异质性明显降低(P=0.11,I2=40%),采用固定效应模型进行Meta分析,结果显示人脐带间充质干细胞移植组的ASIA运动功能评分高于对照组(MD=6.14,95%CI:4.46-7.81,P < 0.000 01),见图10。因此,对于人脐带间充质干细胞移植组的ASIA运动功能评分与对照组相比差异无显著性意义的这一结论要谨慎对待。"
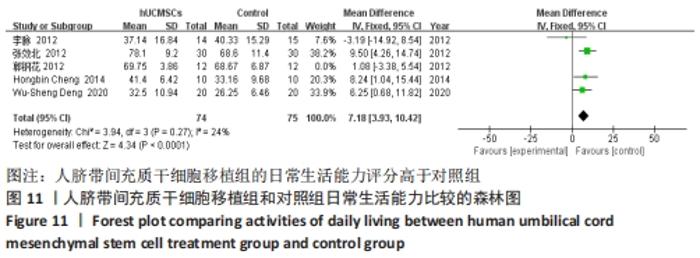
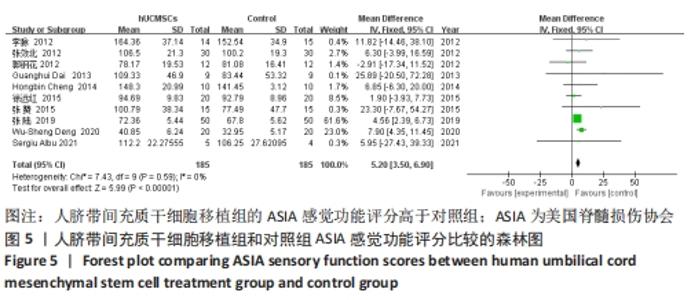
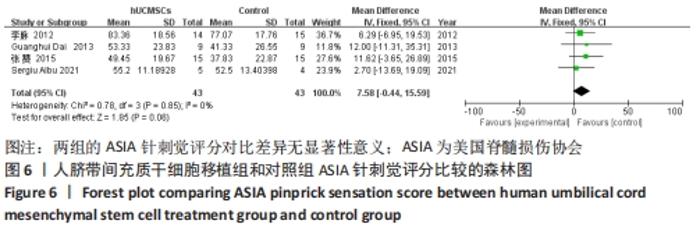
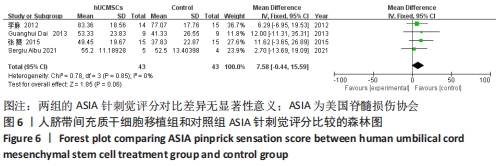
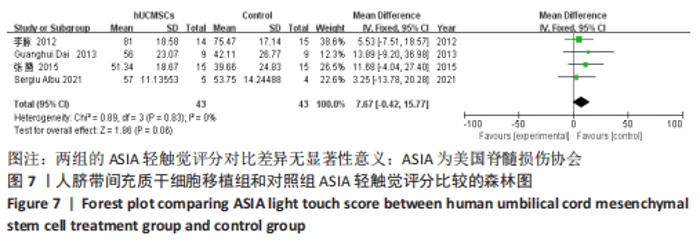
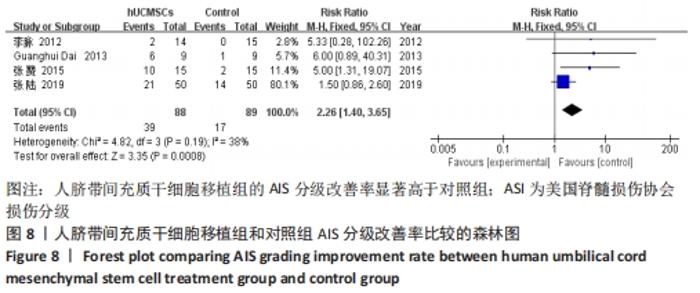
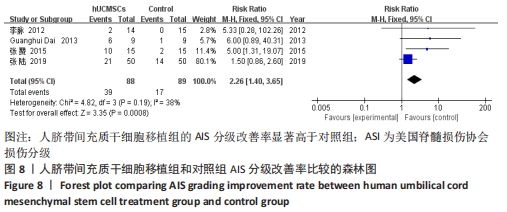
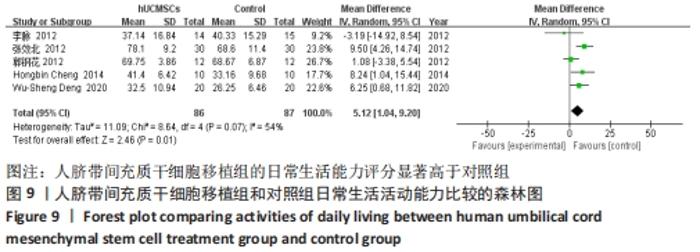
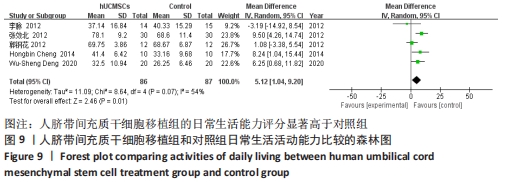
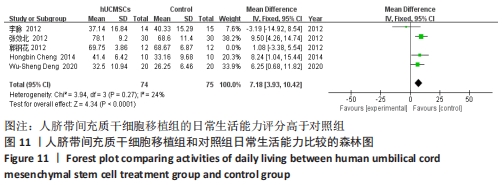
在日常生活能力评分(Barthel指数量表)的Meta分析结果中,有5篇文献比较了人脐带间充质干细胞移植后与对照组的日常生活能力评分[8,12,14-16],异质性检验提示纳入的研究之间异质性较大(P=0.07,I2=54%),采用随机效应模型进行Meta分析,结果显示人脐带间充质干细胞移植组的日常生活活动能力评定高于对照组,差异有显著性意义(MD=5.12,95%CI:1.04-9.20,P=0.01)。因此,需对这项指标进行敏感性分析,经过逐一剔除纳入文献后,发现郭钢花等[16]的研究结果对异质性的影响较大,剔除此研究后发现异质性明显降低(P=0.27,I2=24%),采用固定效应模型进行Meta分析,结果显示人脐带间充质干细胞移植组的日常生活能力评分依旧高于对照组,且差异有显著性意义(MD=7.18,95%CI:3.93-10.42,P < 0.000 1),见图11。说明人脐带间充质干细胞移植组的日常生活能力评分高于对照组这一结论稳健可靠。"
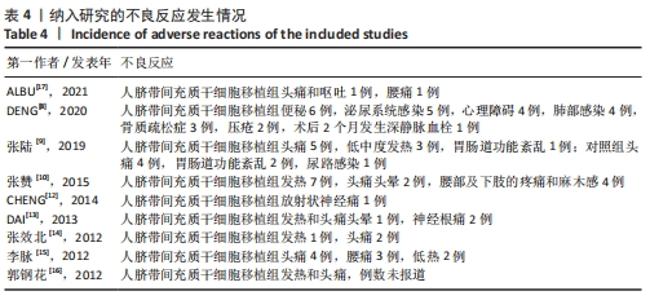
| [1] 陈星月,陈栋,陈春慧,等.中国创伤性脊髓损伤流行病学和疾病经济负担的系统评价[J].中国循证医学杂志,2018,18(2):143-150. [2] 樊保佑,冯世庆,陈琳,等.脊髓损伤神经修复临床治疗指南(IANR/CANR 2019年版)[J].西部医学,2020,32(6):790-802. [3] LI T, XIA M, GAO Y, et al. Human umbilical cord mesenchymal stem cells: an overview of their potential in cell-based therapy. Expert Opin Biol Ther. 2015;15(9):1293-1306. [4] WANG H, QIU X, NI P, et al. Immunological characteristics of human umbilical cord mesenchymal stem cells and the therapeutic effects of their transplantion on hyperglycemia in diabetic rats. Int J Mol Med. 2014;33(2):263-270. [5] JADAD A, MOORE R, CARROLL D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17(1):1-12. [6] 曾宪涛,刘慧,陈曦,冷卫东.Meta分析系列之四:观察性研究的质量评价工具[J].中国循证心血管医学杂志,2012,4(4):297-299. [7] 曾宪涛,冷卫东,李胜,等.如何正确理解及使用GRADE系统[J].中国循证医学杂志,2011,11(9):985-990. [8] DENG W, MA K, LIANG B, et al. Collagen scaffold combined with human umbilical cord-mesenchymal stem cells transplantation for acute complete spinal cord injury. Neural Regen Res. 2020;15(9):1686-1700. [9] 张陆,刘志昂,姜岩,等.脐带间充质干细胞移植治疗急性脊髓损伤的临床价值[J].中国脊柱脊髓杂志, 2019,29(3):254-260. [10] 张赞,代广辉,刘学彬,等.脐带间充质干细胞移植治疗脊髓损伤疗效观察[J].中华实用诊断与治疗杂志,2015,29(5):478-480. [11] 徐远红,王俊华,李海峰,等.脐带间充质干细胞移植联合综合康复训练对脊髓损伤患者神经功能及功能独立性的影响[J].中国康复医学杂志,2015,30(8):828-830. [12] CHENG H, LIU X, HUA R, et al. Clinical observation of umbilical cord mesenchymal stem cell transplantation in treatment for sequelae of thoracolumbar spinal cord injury. J Transl Med. 2014;12:253. [13] DAI G, LIU X, ZHANG Z, et al. Comparative analysis of curative effect of CT-guided stem cell transplantation and open surgical transplantation for sequelae of spinal cord injury. J Transl Med. 2013;11:315. [14] 张效北,李江涛,赵和泰,等.间质干细胞治疗脊髓损伤的临床分析[J].亚太传统医药,2012,8(3):116-117. [15] 李脉.干细胞移植治疗脊髓损伤的临床研究[D].昆明:昆明医科大学,2012. [16] 郭钢花,申利坊,李哲.脐血间充质干细胞治疗脊髓损伤临床研究[J].中国实用医刊,2012,39(10):58-60. [17] ALBU S, KUMRU H, COLL R, et al. Clinical effects of intrathecal administration of expanded Wharton jelly mesenchymal stromal cells in patients with chronic complete spinal cord injury: a randomized controlled study. Cytotherapy. 2021;23(2):146-156. [18] KUMAR R, LIM J, MEKARY R, et al. Traumatic spinal injury: global epidemiology and worldwide volume. World Neurosurg. 2018; 113:e345-e363. [19] JAZAYERI S, BEYGI S, SHOKRANEH F, et al. Incidence of traumatic spinal cord injury worldwide: a systematic review. Eur Spine J. 2015;24(5):905-918. [20] O’SHEA T, BURDA J, SOFRONIEW M. Cell biology of spinal cord injury and repair. J Clin Invest. 2017;127(9):3259-3270. [21] 樊洪,郝定均.急性脊髓损伤治疗的研究进展[J].中华创伤杂志,2019,35(4):340-347. [22] MUKHAMEDSHINA Y, GRACHEVA O, MUKHUTDINOVA D, et al. Mesenchymal stem cells and the neuronal microenvironment in the area of spinal cord injury. Neural Regen Res. 2019;14(2):227-237. [23] 巫丹,尹宇.人脐带间充质干细胞治疗不同疾病的研究进展[J]. 中国比较医学杂志, 2020,30(8):125-130. [24] CORRAO S, LA ROCCA G, LO IACONO M, et al. Umbilical cord revisited: from Wharton’s jelly myofibroblasts to mesenchymal stem cells. Histol Histopathol. 2013;28(10):1235-1244. [25] LI C, ZHENG Z, SU X, et al. Activation of the extracellular signal-regulated kinase signaling is critical for human umbilical cord mesenchymal stem cell osteogenic differentiation. Biomed Res Int. 2016;2016:3764372. [26] DE LA FUENTE A, MATEOS J, LESENDE-RODRÍGUEZ I, et al. Proteome analysis during chondrocyte differentiation in a new chondrogenesis model using human umbilical cord stroma mesenchymal stem cells. Mol Cell Proteomics. 2012;11(2):M111.010496. [27] SABEN J, THAKALI K, LINDSEY F, et al. Distinct adipogenic differentiation phenotypes of human umbilical cord mesenchymal cells dependent on adipogenic conditions. Exp Biol Med (Maywood). 2014;239(10):1340-1351. [28] JIANG L, WANG Y, PAN F, et al. Synergistic effect of bioactive lipid and condition medium on cardiac differentiation of human mesenchymal stem cells from different tissues. Cell Biochem Funct. 2016;34(3):163-172. [29] NAN C, SHI Y, ZHAO Z, et al. Monosialoteterahexosyl ganglioside induces the differentiation of human umbilical cord-derived mesenchymal stem cells into neuron-like cells. Int J Mol Med. 2015;36(4):1057-1062. [30] YANG Y, CAO TT, TIAN ZM, et al. Subarachnoid transplantation of human umbilical cord mesenchymal stem cell in rodent model with subacute incomplete spinal cord injury: Preclinical safety and efficacy study. Exp Cell Res. 2020;395(2):112184. [31] LI C, CHEN X, QIAO S, et al. Effects of Wharton’s jelly cells of the human umbilical cord on acute spinal cord injury in rats, and expression of interleukin-1β and nerve growth factor in spinal cord tissues. Artif Cells Nanomed Biotechnol. 2016;44(5):1254-1258. [32] 张勇,马迅,孙麟,等.人脐带间充质干细胞来源外泌体减轻大鼠脊髓星形胶质细胞氧糖剥夺/复氧损伤所致的水肿[J].中国组织工程研究,2019,23(25):4011-4017. [33] XIAO Z, TANG F, ZHAO Y, et al. Significant improvement of acute complete spinal cord injury patients diagnosed by a combined criteria implanted with neuroregen scaffolds and mesenchymal stem cells. Cell Transplant. 2018;27(6):907-915. [34] LIU J, HAN D, WANG Z, et al. Clinical analysis of the treatment of spinal cord injury with umbilical cord mesenchymal stem cells. Cytotherapy. 2013;15(2):185-191. [35] 王培申,刘学彬,伊龙,等.脐带间充质干细胞鞘内移植治疗不完全性颈髓损伤的疗效和安全性[J].武警医学,2015,26(3):282-285. |
| [1] | Jing Jinpeng, Zhang Yue, Liu Xiaomin, Liu Yi. Traditional Chinese medicine injection for promoting blood circulation in prevention of deep vein thrombosis after orthopedic surgery: network meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(9): 1467-1476. |
| [2] | Wang Jing, Xiong Shan, Cao Jin, Feng Linwei, Wang Xin. Role and mechanism of interleukin-3 in bone metabolism [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(8): 1260-1265. |
| [3] | Xiao Hao, Liu Jing, Zhou Jun. Research progress of pulsed electromagnetic field in the treatment of postmenopausal osteoporosis [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(8): 1266-1271. |
| [4] | Kan Houming, Fan Lijun, Chen Xuetai, Shen Wen. Application of platelet-rich plasma in neuropathic pain [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(8): 1286-1292. |
| [5] | Liu Gang, Ma Chao, Wang Le, Zeng Jie, Jiao Yong, Zhao Yi, Ren Jingpei, Hu Chuanyu, Xu Lin, Mu Xiaohong. Ankle-foot orthoses improve motor function of children with cerebral palsy: a Meta-analysis based on 12 randomized controlled trials [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(8): 1299-1304. |
| [6] | Wu Min, Zhang Yeting, Wang Lu, Wang Junwei, Jin Yu, Shan Jixin, Bai Bingyi, Yuan Qiongjia. Effect of concurrent training sequences on body composition and hormone response: a Meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(8): 1305-1312. |
| [7] | Tian Chuan, Zhu Xiangqing, Yang Zailing, Yan Donghai, Li Ye, Wang Yanying, Yang Yukun, He Jie, Lü Guanke, Cai Xuemin, Shu Liping, He Zhixu, Pan Xinghua. Bone marrow mesenchymal stem cells regulate ovarian aging in macaques [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 985-991. |
| [8] | Hu Wei, Xie Xingqi, Tu Guanjun. Exosomes derived from bone marrow mesenchymal stem cells improve the integrity of the blood-spinal cord barrier after spinal cord injury [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 992-998. |
| [9] | Hou Jingying, Guo Tianzhu, Yu Menglei, Long Huibao, Wu Hao. Hypoxia preconditioning targets and downregulates miR-195 and promotes bone marrow mesenchymal stem cell survival and pro-angiogenic potential by activating MALAT1 [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1005-1011. |
| [10] | Liang Xuezhen, Yang Xi, Li Jiacheng, Luo Di, Xu Bo, Li Gang. Bushen Huoxue capsule regulates osteogenic and adipogenic differentiation of rat bone marrow mesenchymal stem cells via Hedgehog signaling pathway [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1020-1026. |
| [11] | Wen Dandan, Li Qiang, Shen Caiqi, Ji Zhe, Jin Peisheng. Nocardia rubra cell wall skeleton for extemal use improves the viability of adipogenic mesenchymal stem cells and promotes diabetes wound repair [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1038-1044. |
| [12] | Zhu Bingbing, Deng Jianghua, Chen Jingjing, Mu Xiaoling. Interleukin-8 receptor enhances the migration and adhesion of umbilical cord mesenchymal stem cells to injured endothelium [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1045-1050. |
| [13] | Fang Xiaolei, Leng Jun, Zhang Chen, Liu Huimin, Guo Wen. Systematic evaluation of different therapeutic effects of mesenchymal stem cell transplantation in the treatment of ischemic stroke [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1085-1092. |
| [14] | Guo Jia, Ding Qionghua, Liu Ze, Lü Siyi, Zhou Quancheng, Gao Yuhua, Bai Chunyu. Biological characteristics and immunoregulation of exosomes derived from mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1093-1101. |
| [15] | Fan Yiming, Liu Fangyu, Zhang Hongyu, Li Shuai, Wang Yansong. Serial questions about endogenous neural stem cell response in the ependymal zone after spinal cord injury [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1137-1142. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||