Chinese Journal of Tissue Engineering Research ›› 2022, Vol. 26 ›› Issue (25): 4016-4021.doi: 10.12307/2022.408
Previous Articles Next Articles
Key problems of preparation and quality control of human amniotic mesenchymal stem cells pharmaceutics
Wang Yuying1, 2, Yu Limei1, 2
- 1Key Laboratory of Cell Engineering of Guizhou Province, 2Zunyi City Engineering Research Center of Stem Cell and Regenerative Medicine, Affiliated Hospital of Zunyi Medical University, Zunyi 563003, Guizhou Province, China
-
Received:2020-11-16Accepted:2021-05-07Online:2022-09-08Published:2022-01-26 -
Contact:Yu Limei, MD, Professor, Master’s supervisor, Key Laboratory of Cell Engineering of Guizhou Province, and Zunyi City Engineering Research Center of Stem Cell and Regenerative Medicine, Affiliated Hospital of Zunyi Medical University, Zunyi 563003, Guizhou Province, China -
About author:Wang Yuying, Master, Associate professor, Key Laboratory of Cell Engineering of Guizhou Province, and Zunyi City Engineering Research Center of Stem Cell and Regenerative Medicine, Affiliated Hospital of Zunyi Medical University, Zunyi 563003, Guizhou Province, China -
Supported by:the National Natural Science Foundation of China, No. 82060270 (to YLM); the Talent Base Project of Guizhou Province, No. [2013]15 (to YLM); the Special Project of Academic New Seedling Cultivation and Innovation Exploration of Zunyi Medical University, No. [2017]5733-023 (to WYY)
CLC Number:
Cite this article
Wang Yuying, Yu Limei. Key problems of preparation and quality control of human amniotic mesenchymal stem cells pharmaceutics[J]. Chinese Journal of Tissue Engineering Research, 2022, 26(25): 4016-4021.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
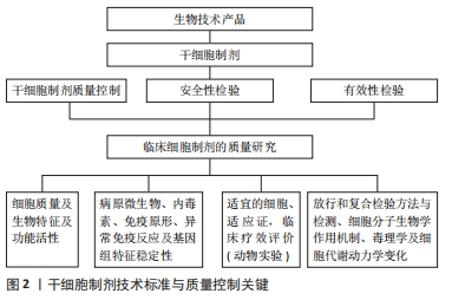
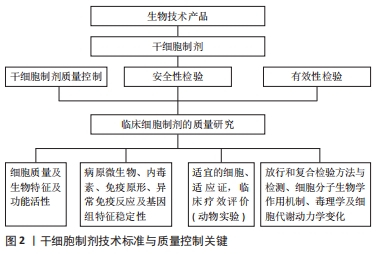
2.1 人羊膜间充质干细胞的主要生物学特征与功能 人羊膜间充质干细胞与骨髓间充质干细胞具有相似的生物学特征和标志,为梭形细胞贴壁生长,阳性表达CD44,CD73,CD90和CD105,不表达CD34,CD45,CD11b,CD19和HLA-DR,表达波形蛋白和干性标志物Oct3/4,SSEA-4,Nanoge,Sox2和整合素家族CD29,CD49b,CD49c,CD49e和CD49d[14-15];人羊膜间充质干细胞可在体内外诱导分化为3个胚层的细胞[16]。在动物实验中,人羊膜间充质干细胞对神经元退行性疾病、心肌梗死、糖尿病、重症肝病、肾病、卵巢早衰及类风湿性关节炎等多种自身免疫性疾病具有良好的治疗作用,作用机制除涉及多向分化替代特定组织功能细胞外,还与其强大的抗炎、免疫调节及分泌功能密切相关,可明显降低致炎因子水平,提高抗炎因子水平,上调T细胞中Treg/Th17和CD4/CD8比值,恢复免疫平衡,改善微环境;通过释放外泌体、胰岛素生长因子和转化生长因子等促再生细胞因子及相关信号分子的miRNA,减轻炎症损伤,促进血管及损伤组织再生修复[17-19]。 2.2 人羊膜间充质干细胞制备工艺及质量鉴定 2.2.1 羊膜的采集 羊膜作为人羊膜间充质干细胞制备的原料,供者的科学选择,采集方法的稳定、可靠与规范是人羊膜间充质干细胞制剂质量保证的第一关。羊膜的采集需符合伦理要求,除了需提供家属或法定监护人签署的知情同意外,还需要对供者进行健康筛查,包括既往史和家族史,并需要进行传染性病原微生物感染和严重缺陷遗传性疾病的检测,如艾滋病毒、乙肝病毒、丙肝病毒、巨细胞病毒、人类疱疹病毒及梅毒螺旋体感染的检查,以排除感染性风险。采集过程也应在无菌条件下,使用灭菌的实验器具,防止耗材共用带来的交叉污染。机械剥离所采集到的羊膜放入含抗生素的营养液中,迅速转移至符合cGMP标准的细胞制备实验室进行后续实验操作。 2.2.2 人羊膜间充质干细胞的制备 细胞制备的每一个步骤都,决定着最终细胞的安全性和有效性,因此全过程标准化操作和质量保证至关重要。实验室的环境、仪器、试剂和操作人员均相对固定,操作方法应严格遵照技术操作规程。培养过程中注意观察细胞形态及微生物污染情况,避免出现细胞变大、多形性、皱缩与生长缓慢等衰老的形态改变。课题组通过反复实验,优化了一套成熟、简单、稳定的标准化人羊膜间充质干细胞制备技术,收集冻存一两代细胞作为种子细胞,每张羊膜可获得原始数量高达1×109个细胞[20]。 2.2.3 从有血清到无血清的培养 早期的人羊膜间充质干细胞培养均采用含体积分数10%胎牛血清的LD-DMEM培养基及后续添加丙酮酸及非必需氨基酸等[21]。随着临床级间充质干细胞制备发展的需求,胎牛血清完全培养基应用的安全性问题日益突出:①胎牛血清存在多种动物源性微生物感染的风险,可能会传播动物源性疾病;②引发免疫反应,刺激相应抗体产生;③传代中易发生人羊膜间充质干细胞衰老、分化、甚至转化;④血清成分复杂,易引发异常免疫反应等,与人胚胎干细胞培养一样,人羊膜间充质干细胞培养也由有胎牛血清进入到无血清、无动物源性成分添加物的新培养体系时代[22-23];⑤严重影响人羊膜间充质干细胞外泌体等衍生物分泌。 有研究利用激素代替胎牛血清诱导分化是干细胞无血清培养的开端,在基础培养基中补充维持细胞增殖活性的血清替代物和其他添加物,如血小板裂解物、激素、非必需氨基酸、碱性成纤维细胞生长因子、转铁蛋白和胰岛素等生长因子和促贴壁因子纤粘蛋白或胶原等[24-25],是目前无血清培养的主要方案。无血清培养基具有以下优点:①避免血清不同批次间的质量差异和胎牛血清中不明确成分导致的毒副反应和动物源微生物污染;②其成分明确,含量已知,避免了血清组分不同对干细胞制剂质量的影响;③其组分稳定,适用于大批量生产;④不含有丝分裂原抑制剂,促进细胞增殖明显;⑤能良好地保持人羊膜间充质干细胞的生物学特征与功能。自体库人羊膜间充质干细胞制备中,自体血清及血浆培养体系也可以作为一种工艺策略,但随着干细胞产业的规模化发展及异基因干细胞移植的增加,Life Technologies及Biological Industries等国外公司生产的脐带间充质干细胞血清替代物等,已成功应用于人羊膜间充质干细胞的无血清体外扩增。 目前国内外研究者利用无血清培养的骨髓、脐带和脂肪来源间充质干细胞已成功应用于临床研究。研究证明,用含β-巯基乙醇、丁化羟基苯甲醚的无血清培养基培养脐血间充质干细胞,扩增能力保持较好,可被诱导分化为神经元特异性烯醇化酶、神经丝蛋白、神经胶质元纤维酸性蛋白和神经元细胞,表达大量油红O染色阳性的脂滴,或茜素红染色阳性的骨结节、阿利新蓝染色阳性的软骨细胞等[26-27]。与含胎牛血清培养基相比,无血清培养基培养的脐带间充质干细胞的扩增能力和免疫抑制功能均明显较高[28]。无血清培养基培养的人羊膜间充质干细胞扩增代次增加,三胚层细胞分化能力保持良好,细胞衰老延缓,明显优于胎牛血清[29]。用体积分数5%人卵泡液替代体积分数10%胎牛血清的无血清培养基所培养人羊膜间充质干细胞,其形态、增殖、核型、基因和表面标志蛋白表达都无明显改变,也能被诱导分化为神经元和胰岛细胞[30]。较胎牛血清培养而言,自体脐血血清培养传代的人羊膜间充质干细胞形态和分化特性明显更优。而第5-7代人羊膜间充质干细胞表面标志分子表达相似[31]。此外无血清冻存也已很好地应用于人羊膜间充质干细胞的长期深低温存储。 2.3 人羊膜间充质干细胞表型标志与功能评价 为确保干细胞制剂的有效性与安全性,除对人羊膜间充质干细胞的基本生物学特性,如细胞活性与状态、纯度和均一性、细胞数量、干性基因表达、免疫表型、分化潜能等进行检测,及分泌、免疫调节等功能评价外,还需考虑适应证的发病特点和机制,从而进行更为精准的质量控制。有研究发现,细胞传代10-15代内,流式细胞分析人羊膜间充质干细胞和骨髓、脐带间充质干细胞的免疫表型类似[20-21]。人羊膜间充质干细胞与异基因正常人,低白细胞介素6分泌型人羊膜间充质干细胞与类风湿性关节炎患者外周血单个核细胞共培养后,人羊膜间充质干细胞均可抑制淋巴细胞增殖,诱导CD4+Foxp3+、CD4+CD25+CD127(low/-)的Treg细胞比例增高,减少Th1、Th17、Tc1细胞比例[32]。免疫原性评价既是功能评价指标,也是重要的安全性指标。人羊膜间充质干细胞的低免疫原性和诱导免疫耐受功能,为异基因个体使用人羊膜间充质干细胞奠定了基础[32]。经静脉或局部途径注射人羊膜间充质干细胞治疗急性心肌梗死、肝纤维化和免疫佐剂诱导的骨关节炎等大量动物实验也显示,受损的心脏、肝脏和关节的功能和组织结构均得到显著改善,而少见相应组织功能细胞分化,移植后2-12周在损伤局部组织内仍有大量人羊膜间充质干细胞存活而不被排斥[32-34],均证明了人羊膜间充质干细胞的低免疫原性,较好的免疫豁免特性或诱导免疫耐受特性,有利于同种异体移植后较长时间的疗效维持及避免某些免疫相关不良反应的发生。 2.4 人羊膜间充质干细胞的致瘤性 目前上市的干细胞产品涉及的细胞类型为干性较强的各类组织干细胞和干性相对较弱的组织前体细胞或祖细胞两大类。美国Geron公司开展了全球首例利用人的胚胎干细胞制剂GRNOPCI治疗脊髓损伤的临床试验[35]。此后,胚胎干细胞制剂也被批准用于老年人视网膜黄斑病变,试验结果取得了安全性验证和满意疗效[36]。然而不可否认的是胚胎干细胞的致瘤性,一直是其临床转化应用的争议焦点,尽管间充质干细胞目前尚未见致瘤性的报道,但连续体外扩增过程中,间充质干细胞的生物功能也受多种因子及表观遗传调控,衰老的人羊膜间充质干细胞存在多种基因mRNA和microRNA表达谱的改变[20],移植后人羊膜间充质干细胞在体内的非特异分化或嵌合体形成,是否存在肿瘤发生的风险,仍然有待长期随访。众所周知,细胞衰老的结局无外乎导致细胞凋亡、死亡或转化,衰老细胞的恶性转化具有导致肿瘤的高风险。因此,人羊膜间充质干细胞体外制备过程中,避免间充质干细胞衰老的发生及不使用衰老的间充质干细胞,也可能是规避风险不可或缺的质控关键。人羊膜间充质干细胞的细胞群体倍增时间减慢,形态改变和面积变大,高表达半乳糖苷酶,CD90阳性表达降低及成骨或成软骨细胞分化能力下降,成脂肪细胞分化增多等,都是值得关注的人羊膜间充质干细胞衰老质控指标[20]。 致瘤性除了取决于干细胞的来源、分化状态、多能性或培养条件外,高代次细胞发生DNA遗传或表观遗传学改变导致致瘤风险增高已是较为明确的共识。干细胞植入体内后,除了在靶向组织发挥作用外,也可能迁移到其他组织进行非预期分化[37-39]。因此,人羊膜间充质干细胞在体内长期示踪技术的突破则更加有利于长期随访,除致瘤性外,还需高度重视间充质干细胞促瘤性的风险,对供受者早期肿瘤的筛查也不容忽视。 2.5 人羊膜间充质干细胞制备质量控制关键环节 2.5.1 制备实验室条件 人羊膜间充质干细胞制剂与其他临床级干细胞制备实验室要求的中试条件相似,须符合《医药工业洁净厂房设计标准》,并充分保障各制备环境符合《药品生产质量管理规范》规定[13]:实验室洁净度,恒温恒湿条件,过滤除菌新风流入,低噪度和压力差等。实验室加强日常运行管理,定期做好室内环境检测,在“人、机、料、法、环”各环节的有效管理,都是明显影响人羊膜间充质干细胞制剂质量控制的关键。此外,综合质量检定中,除配备常规形态观察、微生物检测等必须基础设备外,需拥有可完成细胞因子检测、细胞表型鉴定的仪器外,还需增加检测干性基因、多向分化标志基因和DNA遗传标记的仪器设备,这便于发现遗传缺陷和人羊膜间充质干细胞供者与受者的个体识别信息等。按照质量标准、针对制备工艺,制定和有效地执行上述实验室与设备使用、物料质控及细胞制剂制备流程的标准操作手册,及时、准确地做好实验记录,是人羊膜间充质干细胞制剂制备管理的最核心的软实力。 2.5.2 制剂安全性评价 无微生物污染或交叉污染是实验室安全和制剂安全性质量控制的基本要求。收集胎盘的同时,采集少量脐血,进行血清免疫学检测是评价供者感染情况的重要方法。制剂的制备、环境监测、放行检验和复核检验中,通过显微镜观察细胞形态,培养和涂片染色检菌,定量PCR检测病原体及内毒素测定都是必不可少的安全性监测。 临床前安全性评价中间充质干细胞给药局部和人体的毒性反应、非预期分化和异常免疫学反应,同样与制剂工艺有关,尤其是间充质干细胞的异质性高或细胞活力降低,可能会明显影响人羊膜间充质干细胞治疗的有效性和安全性。因此,获得高纯度(标志分子>85%)的一致性细胞,采用恰当的细胞保存液,避免细胞聚集和破坏(存活率>90%)[40],采用合理的移植途径,减少非靶器官或靶组织的存留,都是保证安全性和有效性不可忽略的环节。 2.5.3 放行检验与复核检验 放行检验应按照标准操作规程,需逐项查对材料来源及人羊膜间充质干细胞的分离、培养、存储及复苏、运送及交接过程,还需留样、长期保存备查。制定切实可行、科学合理、及时有效的复核检验操作程序和质控标准也成为临床移植前的最后一道安全、有效性控制防线,见图2,表1。远程运输的时空条件监测,制剂包装完整性和外观,细胞形态、数量、活力、胎牛血清残留量、内毒素、细菌涂片染色、人羊膜间充质干细胞免疫表型、病原微生物的定量PCR检测及酶联免疫吸附法或多重免疫荧光微球法检测多种细胞因子 [40-41],都可以准确快速地评价人羊膜间充质干细胞的多种特征与功能活性及制剂安全性。采用法医物证的短串联重复序列分型技术,可对供者人羊膜间充质干细胞进行个体身份核实,确保所用批次、批号准确无误,避免人源细胞交叉污染[40],STR分型也可应用于受者体内嵌合体形成的监测。"
| [1] 毛开云,范月蕾,王跃,等.间充质干细胞治疗产品开发现状与趋势[J].中国生物工程杂志,2017,37(10):126-134. [2] LISINI D, NAVA S, POGLIANI S, et al. Adipose tissue-derived mesenchymal stromal cells for clinical application: an efficient isolation approach. Curr Res Transl Med. 2018;10(18):2452-3186. [3] ANGELOPOULOS I, BRIZUELA C, KHOURY M. Gingival mesenchymal stem cells outperform haploidentical dental pulp-derived mesenchymal stem cells in proliferation rate, migration ability, and angiogenic potential. Cell Transplant. 2018;27(6):967-978. [4] GASIŪNIENĖ M, PETKUS G, MATUZEVIČIUS D, et al. Angiotensin II and TGF-β1 Induce Alterations in Human Amniotic Fluid-Derived Mesenchymal Stem Cells Leading to Cardiomyogenic Differentiation Initiation. Int J Stem Cells. 2019;12(2):251-264. [5] GASIŪNIEN M, VALATKAIT E, NAVAKAUSKIEN R. Long‐term cultivation of human amniotic fluid stem cells: the impact on proliferative capacity and differentiation potential. J Cell Biochem. 2020;121(7):3491-3501. [6] TOPOLUK N, HAWKINS R, TOKISH J, et al. Amniotic mesenchymal stromal cells exhibit preferential osteogenic and chondrogenic differentiation and enhanced matrix production compared with adipose mesenchymal stromal cells. Am J Sports Med. 2017;45(11):2637-2646. [7] YANG L, ZHU S, LI Y, et al. Overexpression of Pygo2 Increases Differentiation of Human Umbilical Cord Mesenchymal Stem Cells into Cardiomyocyte-like Cells. Curr Mol Med. 2020;20(4):318-324. [8] 冯国纹,余丽梅.人羊膜应用的研究进展[J].生物医学工程学杂志,2014, 31(4):930- 934. [9] ALONSO-CARPIO M, SÁNCHEZ-GARCÍA A, TRAPERO A, et al. Use of amniotic membrane as a biological dressing for the treatment of torpid venous ulcers: a case report. use of amniotic membrane as a biological dressing for the treatment of torpid venous ulcers: a case report. Plast Surg Nurs. 2020;40(3):135-137. [10] PHERMTHAI T, THONGBOPIT S, POKATHIKORN P, et al. Carcinogenicity, efficiency and biosafety analysis in xeno-free human amniotic stem cells for regenerative medical therapies. Cytotherapy. 2017;19(8):990-1001. [11] Adamowicz J, Pasternak I, Kloskowski T, et al. Development of a conductive biocomposite combining graphene and amniotic membrane for replacement of the neuronal network of tissue-engineered urinary bladder. Sci Rep. 2020;10(1):5824. [12] 卢世璧,吴祖泽,付小兵,等.中国细胞技术类再生医学创新型技术产业发展战略研究[J].中国工程科学,2017,19(2):95-99. [13] 吴祖泽.致细胞库质量管理规范制定和公示[J].中国医药生物技术, 2017,12(6):483. [14] KEHL D, GENERALI M, GORTZ S, et al. Amniotic fluid cells show higher pluripotency-related gene expression than allantoic fluid cells. Stem Cells. 2017;26(19):1424-1437. [15] EMANUEL K, KATARZYNA K, HALINA KK, et al. Increased immunomodulatory capacity of human amniotic cells after activation by pro-inflammatory chemokines. Eur J Pharmacol. 2019;859:172545. [16] SPRINGER SCIENCE+BUSINESS MEDIA, LLC. Retraction note: can two-way direct communication protocols be considered secure? Nanoscale Res Lett. 2019;14(1):242. [17] FAN X, LI L, YE Z, et al. Regulation of osteogenesis of human amniotic mesenchymal stem cells by sodium butyrate. Cell Biol Int. 2018;42(4): 457-469. [18] FOUAD H, SABRY D, ELSETOHY K, et al. Therapeutic efficacy of amniotic membrane stem cells and adipose tissue stem cells in rats with chemically induced ovarian failure. J Adv Res. 2016;7(2):233-241. [19] CHAI HH, CHEN MB, CHEN GZ, et al. Inhibitory effect of TGF-β gene modified human amniotic mesenchymal stem cells on rejection after xenotransplantation of peripheral nerves. Eur Rev Med Pharmacol Sci. 2019; 23:3198-3205. [20] 齐斌,余丽梅.人羊膜间充质干细胞衰老改变及miRNA和mRNA差异表达分析[D].遵义:遵义医学院,2015. [21] 王钰莹,粟旭,刘波,等.人羊膜间充质干细胞原位移植治疗大鼠脑梗死[J].中国组织工程研究,2017,21(9):1414-1419. [22] 张哲,曾宪卓,鲁飞,等.无血清培养人胚胎干细胞的体系研究与进展[J].中国组织工程研究,2015,41(19):6711-6717. [23] TSAI HH, YANG KC, WU MH, et al. The effects of different dynamic culture systems on cell proliferation and osteogenic differentiation in human mesenchymal stem cells. Int J Mol Sci. 2019;20(16):4024. [24] HAYASHI Y, FURUE MK. Biological effects of culture substrates on human pluripotent stem cells. Stem Cells Int. 2016;2016:5380560. [25] SATO K, ITOH T, KATO T, et al. Serum-free isolation and culture system to enhance the proliferation and bone regeneration of adipose tissue-derived mesenchymal stem cells. In Vitro Cell Dev Biol Anim. 2015;51(5):515-529. [26] 韩颢.脐带间充质干细胞无血清培养方案的优化[D].天津:天津医科大学,2019. [27] 屈玟,宋磊,赵瑶,等.人脐带间充质干细胞不同培养方案的比较与优化[J].海南医学院学报,2017,23(8):1009-1013. [28] Hoang VT, Trinh QM, Phuong DTM, et al. Standardized xeno- and serum-free culture platform enables large-scale expansion of high-quality mesenchymal stem/stromal cells from perinatal and adult tissue sources. Cytotherapy. 2021;23(1):88-99. [29] CARMELO JG, FERNANDES-PLATZGUMMER A, DIOGO MM, et al. A xeno-free microcarrier-based stirred culture system for the scalable expansion of human mesenchymal stem/stromal cells isolated from bone marrow and adipose tissue. Biotechnol J. 2015;10(8):1235-1247. [30] BUI HTH, NGUYEN LT, THAN UTT, et al. Influences of xeno-free media on mesenchymal stem cell expansion for clinical application. Tissue Eng Regen Med. 2021;18(1):15-23. [31] 余丽梅.一种完全培养基培养羊膜间充质干细胞的方法[P].中国专利: 201110080968.6, 2013-07-24. [32] 穆思婕,于泓.人羊膜间充质干细胞体外上调类风湿关节炎患者外周血Treg细胞比例[D].遵义:遵义医学院,2015. [33] Wang AT, Zhang QF, Wang NX, et al. Cocktail of hyaluronic acid and human amniotic mesenchymal cells effectively repairs cartilage injuries in sodium iodoacetate-induced osteoarthritis rats. Front Bioeng Biotechnol. 2020;6(8):87. [34] Giada P, Anabel F, Mariangela P, et al. Human amniotic stem cells improve hepatic microvascular dysfunction and portal hypertension in cirrhotic rats. Liver Int. 2020;40(10):2500-2514. [35] 平崎诚司.美国Geron公司实施胚胎干细胞治疗脊髓损伤的临床试验[J].生物产业技术,2011(1):6. [36] DA CRUZ L, FYNES K, GEORGIADIS O, KERBY J, et al. Phase 1 clinical study of an embryonic stem cell-derived retinal pigment epithelium patch in age-related macular degeneration. Nat Biotechnol. 2018;36(4):328-337. [37] CARDOSO1 MT, PINHEIRO1 AO, VIDANE AS, et al. Characterizaton of teratogenic potental and gene expressionin canine and feline amniotc membrane-derived stem cells. Origin alarticle. 2017;52(2):58-64. [38] WANG JY, WU PK, CHEN PC, et al. Generation of Osteosarcomas From a Combination of Rb Silencing and c-Myc Overexpression in Human Mesenchymal Stem Cells. Stem Cells Transl. 2017;6(2):512-526. [39] GAGGI G, DI CREDICO A, IZZICUPO P, et al. Epigenetic features of human perinatal stem cells redefine their stemness potential. Cells. 2020;9(5):1304. [40] 张可华,纳涛,刘静,等.建立间充质干细胞制剂质量控制评价体系[C].2013中国生物制品年会暨第十三次全国生物制品学术研讨会论文集.2013:317-318. [41] 孙丽娟.脐带间充质干细胞制剂制备及质量控制[J].沈阳药科大学学报, 2020,289(2):79-83. [42] 中国医药生物技术协会.干细胞制剂制备质量管理自律规范[J].中国医药生物技术,2016,11(6):481-490. [43] Liu H, Li R, Liu T, et al. Immunomodulatory effects of mesenchymal stem cells and mesenchymal stem cell-derived extracellular vesicles in rheumatoid arthritis. Front Immunol. 2020;11:1912. [44] PIEROZAN P, CATTANI D, KARLSSON O. Hippocampal neural stem cells are more susceptible to the neurotoxin BMAA than primary neurons: effects on apoptosis, cellular differentiation, neurite outgrowth, and DNA methylation .Cell Death Dis. 2020;11(10):910. [45] 王立生,吴祖泽.精准医学时代的细胞治疗[J].齐鲁医学杂志,2018, 33(2):95-97. [46] THOMPSON AA, WALTERS MC, KWIATKOWSKI J, et al. Gene therapy in patients with transfusion-dependent β-thalassemia. N Engl J Med. 2018;378 (16):1479-1493. [47] STIRNADEL-FARRANT H, KUDARI M, GARMAN N, et al. Gene therapy in rare diseases: the benefits and challenges of developing a patient-centric registry for Strimvelis in ADA-SCID. Orphanet J Rare Dis. 2018;13(1):49-59. |
| [1] | Wang Jing, Xiong Shan, Cao Jin, Feng Linwei, Wang Xin. Role and mechanism of interleukin-3 in bone metabolism [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(8): 1260-1265. |
| [2] | Xiao Hao, Liu Jing, Zhou Jun. Research progress of pulsed electromagnetic field in the treatment of postmenopausal osteoporosis [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(8): 1266-1271. |
| [3] | Wen Dandan, Li Qiang, Shen Caiqi, Ji Zhe, Jin Peisheng. Nocardia rubra cell wall skeleton for extemal use improves the viability of adipogenic mesenchymal stem cells and promotes diabetes wound repair [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1038-1044. |
| [4] | Zhu Bingbing, Deng Jianghua, Chen Jingjing, Mu Xiaoling. Interleukin-8 receptor enhances the migration and adhesion of umbilical cord mesenchymal stem cells to injured endothelium [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1045-1050. |
| [5] | Luo Xiaoling, Zhang Li, Yang Maohua, Xu Jie, Xu Xiaomei. Effect of naringenin on osteogenic differentiation of human periodontal ligament stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1051-1056. |
| [6] | Wang Xinmin, Liu Fei, Xu Jie, Bai Yuxi, Lü Jian. Core decompression combined with dental pulp stem cells in the treatment of steroid-associated femoral head necrosis in rabbits [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1074-1079. |
| [7] | Fang Xiaolei, Leng Jun, Zhang Chen, Liu Huimin, Guo Wen. Systematic evaluation of different therapeutic effects of mesenchymal stem cell transplantation in the treatment of ischemic stroke [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1085-1092. |
| [8] | Guo Jia, Ding Qionghua, Liu Ze, Lü Siyi, Zhou Quancheng, Gao Yuhua, Bai Chunyu. Biological characteristics and immunoregulation of exosomes derived from mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1093-1101. |
| [9] | Huang Chenwei, Fei Yankang, Zhu Mengmei, Li Penghao, Yu Bing. Important role of glutathione in stemness and regulation of stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1119-1124. |
| [10] | Hui Xiaoshan, Bai Jing, Zhou Siyuan, Wang Jie, Zhang Jinsheng, He Qingyong, Meng Peipei. Theoretical mechanism of traditional Chinese medicine theory on stem cell induced differentiation [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1125-1129. |
| [11] | An Weizheng, He Xiao, Ren Shuai, Liu Jianyu. Potential of muscle-derived stem cells in peripheral nerve regeneration [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1130-1136. |
| [12] | Fan Yiming, Liu Fangyu, Zhang Hongyu, Li Shuai, Wang Yansong. Serial questions about endogenous neural stem cell response in the ependymal zone after spinal cord injury [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1137-1142. |
| [13] | Tian Chuan, Zhu Xiangqing, Yang Zailing, Yan Donghai, Li Ye, Wang Yanying, Yang Yukun, He Jie, Lü Guanke, Cai Xuemin, Shu Liping, He Zhixu, Pan Xinghua. Bone marrow mesenchymal stem cells regulate ovarian aging in macaques [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 985-991. |
| [14] | Hou Jingying, Guo Tianzhu, Yu Menglei, Long Huibao, Wu Hao. Hypoxia preconditioning targets and downregulates miR-195 and promotes bone marrow mesenchymal stem cell survival and pro-angiogenic potential by activating MALAT1 [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1005-1011. |
| [15] | Zhou Ying, Zhang Huan, Liao Song, Hu Fanqi, Yi Jing, Liu Yubin, Jin Jide. Immunomodulatory effects of deferoxamine and interferon gamma on human dental pulp stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1012-1019. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||

