中国组织工程研究 ›› 2026, Vol. 30 ›› Issue (13): 3359-3369.doi: 10.12307/2026.339
• 干细胞综述 stem cell review • 上一篇 下一篇
改善间充质干细胞体外培养效率的策略分析
杨羽茜1,徐 丹1,刘忠山2
- 1贵州医科大学,贵州省贵阳市 550004;2贵州医科大学附属医院,贵州省贵阳市 550004
-
接受日期:2025-08-20出版日期:2026-05-08发布日期:2025-12-26 -
通讯作者:刘忠山,博士,主任医师,硕士研究生导师,贵州医科大学附属医院,贵州省贵阳市 550004 -
作者简介:杨羽茜,女,1998年生,贵州省凯里市人,布依族,贵州医科大学在读硕士,主要从事干细胞基础研究。 -
基金资助:贵州省国家自然科学基金地区科学基金科学项目(82460450),项目负责人:刘忠山
Analysis of strategies to improve efficiency of in vitro culture of mesenchymal stem cells
Yang Yuxi1, Xu Dan1, Liu Zhongshan2
- 1Guizhou Medical University, Guiyang 550004, Guizhou Province, China; 2Affiliated Hospital of Guizhou Medical University, Guiyang 550004, Guizhou Province, China
-
Accepted:2025-08-20Online:2026-05-08Published:2025-12-26 -
Contact:Liu Zhongshan, MD, Chief physician, Master’s supervisor, Affiliated Hospital of Guizhou Medical University, Guiyang 550004, Guizhou Province, China -
About author:Yang Yuxi, Master candidate, Guizhou Medical University, Guiyang 550004, Guizhou Province, China -
Supported by:Guizhou Provincial National Natural Science Foundation (Regional Science Fund Science Project), No. 82460450 (to LZS)
摘要:
文题释义:
间充质干细胞:是一类具有自我更新能力和多向分化潜能的成体干细胞,主要存在于骨髓、脂肪、脐带、胎盘等结缔组织和间充质中,在一定条件下可以分化成多种功能细胞。间充质干细胞在组织工程、再生医学、自身免疫性疾病治疗及抗炎疗法中具有潜力。三维培养:将细胞在三维空间环境中培养,使其形成类似体内组织的立体结构,以更好地模拟细胞在真实组织或器官中的微环境。三维培养能提供更接近生理条件的细胞-细胞和细胞-基质相互作用微环境,广泛应用于组织工程、药物筛选、肿瘤研究和再生医学等领域。
摘要
背景:随着传代次数的增加,间充质干细胞在体外培养过程中表现出明显的功能衰退现象,这一局限性严重制约了其在临床治疗中的应用效果。近年来,得益于生物技术的突破性进展和生物工程材料的显著改良,已提出多种优化培养方案,但目前仍无规范化干细胞生产标准。
目的:总结间充质干细胞在体外培养过程中出现的问题,简述间充质干细胞体外培养的优化方案。
方法:以“间充质干细胞,体外培养,细胞培养,培养条件,预处理,细胞衰老”为中文检索词,以“mesenchymal stem cells,cell culture,in vitro,culture conditions,preconditioning,cell senescence”为英文检索词,检索中国知网、PubMed数据库于2025年1月之前发表的文献,排除与主题相关性较差、年代久远及重复的文章,最后纳入98篇文献进行综述。
结果与结论:①总结了间充质干细胞体外培养时出现细胞形态学改变、增殖分化能力下降、迁移归巢能力下降、细胞代谢障碍、分泌衰老相关表型的问题;②梳理了间充质干细胞体外传代衰老的可能机制如遗传物质损伤、蛋白质稳态丧失、细胞内信号通路和转录因子表达改变;③总结了提高间充质干细胞体外培养效率的策略:基因工程修饰干细胞、药理学方法干预细胞增殖分化、优化干细胞体外培养环境、低氧预处理、调节细胞因子等。上述研究为提高间充质干细胞临床应用疗效提供了理论依据。
中图分类号:
引用本文
杨羽茜, 徐 丹, 刘忠山. 改善间充质干细胞体外培养效率的策略分析[J]. 中国组织工程研究, 2026, 30(13): 3359-3369.
Yang Yuxi, Xu Dan, Liu Zhongshan. Analysis of strategies to improve efficiency of in vitro culture of mesenchymal stem cells[J]. Chinese Journal of Tissue Engineering Research, 2026, 30(13): 3359-3369.
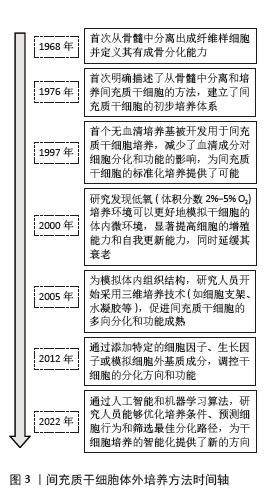
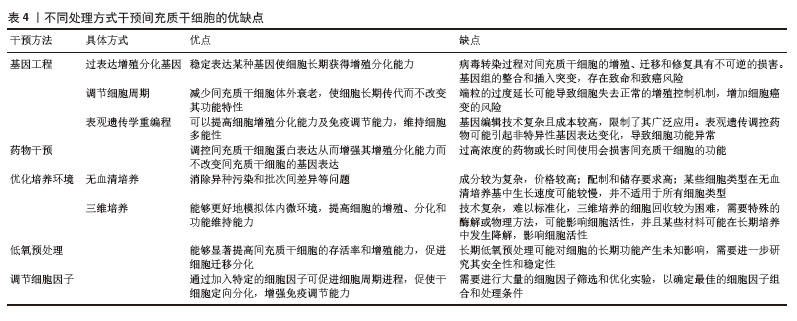
不同来源人间充质干细胞在体外培养中的传代能力存在显著差异,主要受组织来源、供者年龄、培养条件等因素影响。在同种条件下培养3种不同来源的间充质干细胞:人脐带来源间充质干细胞(human umbilical cord mesenchymal stem cells,hUC-MSCs)、骨髓来源间充质干细胞(human bone marrow mesenchymal stem cells,hBM-MSCs)及脂肪来源间充质干细胞(adipose tissue mesenchymal stem cells,AT-MSCs),同一代次下,人脐带来源间充质干细胞的增殖能力较骨髓来源间充质干细胞更强,体外传代次数更多,人脐带来源间充质干细胞可达37代,而骨髓来源间充质干细胞只能传至17代就失去干细胞特性[11]。不同来源的干细胞分化潜能也各不相同,脂肪来源间充质干细胞更倾向于向脂肪细胞分化,骨髓来源间充质干细胞在成骨分化上明显优于脂肪来源间充质干细胞[12]。而过度传代后细胞出现不可逆改变,包括细胞形态学改变、增殖活性降低、免疫原性改变、致瘤性增加、迁移和归巢能力下降等,这些改变会使人间充质干细胞的功能特性减退并诱发细胞不可逆改变甚至癌变,从而影响间充质干细胞在临床使用中的疗效(图4)。
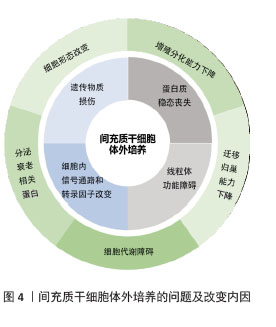
2.1.1 细胞形态学改变 人间充质干细胞的典型形态是贴壁生长的长梭形细胞,随着培养时间的延长和传代次数的增加,人间充质干细胞的形态逐渐出现不均一性,细胞从细纺锤形转变为扁平肥大,边界模糊[13],并逐渐失去贴壁能力,细胞核缩小、呈现颗粒状细胞质,肌动蛋白纤维增加,迁移能力减弱[14],细胞核中异染色质结构呈点状聚集,称为“衰老相关异染色质灶”[15]。以上细胞形态改变出现均表示细胞逐渐进入衰老阶段。
2.1.2 增殖分化能力下降 人间充质干细胞拥有极高的自我更新能力,理论上可以自我复制到任何符合实验目的的细胞量。但是,在实际体外传代培养过程中人间充质干细胞却无法达到理论上的细胞体量,其原因就在于体外传代过程中人间充质干细胞发生衰老而逐渐失去增殖能力。多项研究表明人间充质干细胞的寿命范围为30-40个体外群体倍增[16],不同组织来源及不同年龄供者来源的人间充质干细胞传代次数也不同。在体外的生长曲线一般呈“滞缓-对数生长-平台”模式,平台期过后细胞增殖能力减退,与传代次数呈反比,S期细胞明显减少,G1期细胞阻滞[17]。
人间充质干细胞具有显著的成骨、成软骨和成脂潜力,在传代后期其分化潜能也逐渐减退,且存在分化偏移现象,即衰老人间充质干细胞的成骨分化能力下降程度远大于成脂分化能力,部分衰老细胞仍保有一定的成脂能力。传代后期间充质干细胞中的成骨标志物,如碱性磷酸酶活性和骨钙素表达下降[17],参与细胞激活成骨分化的转录因子Runt相关转录因子2和Osterix表达也下降;同时,参与调节成脂分化的CCAAT增强子结合蛋白和过氧化物酶体增殖物激活受体γ也出现表达失衡,并表现出成脂分化潜能降低[18]。由以上研究可知长期培养会使间充质干细胞逐渐丧失祖细胞特征,其在体外发挥疾病治疗的功能也随之减退。
2.1.3 迁移和归巢能力下降 人间充质干细胞的组织修复作用很大程度上依赖于移植后细胞的迁移和归巢能力,人间充质干细胞迁移至受损部位通过分化及旁分泌发挥治疗作用[19]。在体外培养时发现,传代次数较多的间充质干细胞的迁移和侵袭能力逐渐减弱。细胞形态上,老化的间充质干细胞中局部细胞骨架改变、伪足形成减少[20],细胞迁移运动能力减弱;基因层面上,编码细胞骨架和肌动蛋白的特定基因亚群(如整合素、α-辅肌动蛋白等)的mRNA表达下降;同时,参与定向迁移的趋化因子mRNA下调,如基质细胞衍生因子1(stromal cell-derived factor 1,SDF-1)及其受体(CXC chemokine receptor 4,CXCR4),导致人间充质干细胞受趋化因子作用而发生迁移的能力也减弱[21]。人间充质干细胞的迁移和归巢能力降低,导致细胞不能迁移至受损组织部位,从而难以发挥治疗作用。
2.1.4 细胞代谢障碍 线粒体作为细胞的能量中心,其功能变化与间充质干细胞的命运紧密相连。在体外培养期间,线粒体体积随细胞衰老肥大而增大,内膜皱褶减少,导致电子传递链复合体空间排列紊乱。传代后期间充质干细胞内ATP含量明显低于原代间充质干细胞,而活性氧水平较原代细胞增加,表明长期培养后细胞线粒体功能受损导致氧化应激水平升高。与年轻干细胞相比,多次传代后间充质干细胞的基础有氧呼吸能力、最大有氧呼吸能力、有氧呼吸储备和ATP产生量均明显降低,活性氧等有害物质沉积引起DNA损伤导致细胞衰老及死亡。
2.1.5 分泌衰老相关分泌表型 间充质干细胞在长期培养后细胞分泌表型发生改变,高产出某些分泌物,包括白细胞介素1α、白细胞介素1β、白细胞介素6、白细胞介素8、转化生长因子β、表皮生长因子、成纤维细胞生长因子2、肝细胞生长因子、胰岛素样生长因子结合蛋白7,趋化因子CCL1/3和基质金属蛋白酶等,统称为衰老相关分泌表型因子。这类因子通过与细胞表面受体结合,激活信号转导通路,从而影响细胞的生长、分化和功能[22]。阻断衰老相关分泌表型相关的反馈环路,例如,耗尽白细胞介素6受体、白细胞介素8受体或胰岛素样生长因子结合蛋白7可以延缓衰老,甚至阻止衰老进程[23]。除了自我调节之外,衰老相关分泌表型因子还倾向于通过旁分泌机制影响邻近细胞以加速衰老。例如,衰老人间充质干细胞分泌的衰老相关分泌表型因子可以通过限制邻近年轻造血干细胞的增殖速度来诱导提前衰老[24]。
2.2 长期体外传代导致人间充质干细胞改变的可能机制 在长期传代过程中,受外界环境物理化学因素的影响,细胞内部发生多种改变,如基因组学改变、蛋白表达差异、细胞信号通路改变及线粒体功能失调等,以上细胞内复杂的改变共同影响人间充质干细胞的寿命及功能。
2.2.1 遗传物质损伤 在间充质干细胞体外培养过程中,外界物理和化学环境的刺激会导致细胞内DNA损伤,随着受损遗传物质的不断积累,细胞逐渐走向衰老及死亡。此外,随着细胞的每一次复制分裂,染色体末端的端粒逐渐缩短,每传2代端粒缩短约为100 bp[15],直至端粒缩短到临界长度,细胞周期停滞并停止分裂。此外,在表观遗传学方面,长期传代的人间充质干细胞也出现不可逆改变,DNA甲基化促进细胞和组织中的转录组改变并影响组蛋白修饰模式,以调节衰老过程中的基因表达。组蛋白甲基化和组蛋白乙酰化也在表观遗传改变中起着至关重要的作用。衰老间充质干细胞在特定CpG位点显示出高度一致的衰老相关修饰,甲基化水平整体升高。促衰老miRNA(如miR-34a、miR-145)上调,靶向抑制细胞周期调控相关蛋白,导致细胞周期阻滞[25]。
2.2.2 蛋白质稳态丧失 蛋白质是构成细胞生物体的基础,维持蛋白质系统的相对稳定对细胞发挥正常功能至关重要。一旦蛋白质稳态失衡,过量无活性或者活性异常的蛋白质在细胞中堆积,从而导致细胞无法发挥正常功能。其中,自噬是一种经过严格调控的非特异性降解途径,通过降解蛋白质大分子和功能失调的细胞器(如线粒体和溶酶体)来维持细胞稳态。长期培养后间充质干细胞的自噬功能下降,导致细胞内大量异常及损伤的细胞器沉积、氧化应激平衡失调,最终引发细胞衰老和凋亡[26]。
2.2.3 细胞内信号通路和转录因子表达改变 当人间充质干细胞留存在体内时,细胞长时间处于静止期,只有通过适当信号激活细胞才开始发挥再生和修复功能,这种静止状态能保护干细胞免受氧化应激造成的损伤。而在间充质干细胞体外培养时,外界刺激导致多种分子途径激活,如肿瘤抑制剂p53信号通路相关基因在间充质干细胞长期培养后表达上调,导致细胞衰老及凋亡[27]。体外长期培养诱导p21WAF1/CIP1和p16INK4A表达增加,引起细胞周期阻滞,细胞逐渐走向衰老[28],信号转导和转录活化因子3表达也异常升高,导致神经干细胞终末分化为神经胶质细胞,而不是神经元[29]。以上这些信号通路的改变影响细胞的增殖、分化及衰老[30-31]。
2.2.4 线粒体功能障碍引起细胞代谢紊乱 线粒体是细胞的“能量工厂”和代谢调控中心,其功能稳定对维持细胞活力、组织稳态及机体健康至关重要。人间充质干细胞在体外传代培养过程中可以观察到随传代次数的增加线粒体融合增加、分裂减少,同时细胞内ATP产生减少、活性氧堆积,导致细胞代谢紊乱,抗氧化能力降低。堆积的活性氧会损伤细胞内的蛋白质、脂质和DNA,进而激活p53和pRb信号通路,导致细胞周期停滞,引起间充质干细胞体外衰老[32],主要与衰老标志物的表达增加有关,包括β-半乳糖苷酶、p16INK4a和p21[33]。线粒体功能障碍还会导致细胞慢性炎症,激活Wnt/β-连环蛋白通路以抑制线粒体自噬,从而使大量受损的线粒体在间充质干细胞中积累[34],以上因素形成一个正反馈回路加剧线粒体损伤和细胞衰老。此外,线粒体功能障碍与多种线粒体损伤引起的代谢紊乱还会使NAD+/NADH比率失衡,影响细胞的能量状态,导致生物系统功能障碍,从而使体外培养的间充质干细胞无法达到预期的治疗效果[35-36]。
2.3 逆转干细胞衰老的手段 间充质干细胞在体外培养时发生的形态和功能改变均会影响它在实际临床治疗中的作用效果,因此为提高间充质干细胞的培养效率,增强干细胞的治疗效果,需进一步探索优化干细胞的体外培养方案。目前主要从培养基、培养条件、基因与表观遗传调控、质量控制等方面提出改善间充质干细胞体外存活的方案,以期使间充质干细胞在体外获得更强的生存能力并获得更好的治疗效果。
2.3.1 细胞基因工程 细胞功能改变的原因很大一部分归因于遗传物质改变,通过对细胞的基因进行改造,在分子水平上调节间充质干细胞,是改善干细胞功能退化的重要方法。按不同干预层面可分为两大方向:其一是通过基因编辑技术将某类基因插入或从原有序列中敲除,从而使间充质干细胞获得更强的增殖分化能力及免疫调节能力;其二是通过表观遗传学间接操控细胞遗传学表达,通过DNA甲基化及组蛋白修饰等方式对间充质干细胞的非编码序列进行微观调控。基因工程能使细胞获得稳定的基因表达,从而在长期传代中保持长久恒定的生物特性。
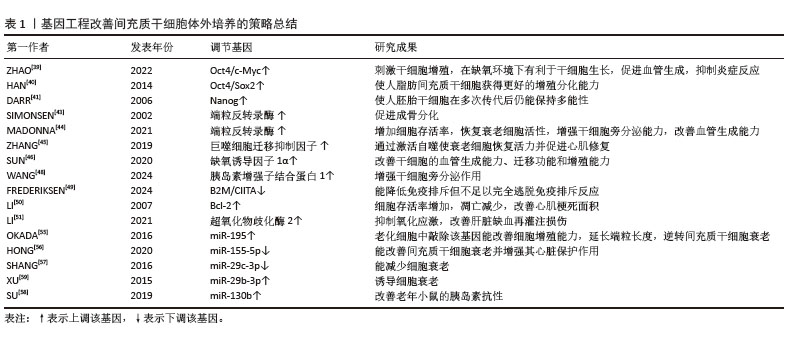
基因修饰根据干预目的又可大致分为3个方向,即提高细胞增殖分化相关基因、调整细胞周期相关基因以及为使细胞获得某项特定分化能力导入特定基因从而靶向治疗相关疾病。在2006年,由日本科学家山中弥生团队提出了一项开创性研究,即通过引入4个特定的转录因子Oct4、Sox2、Klf4和c-Myc,即Yamanaka因子,将已终末分化的体细胞重编程为诱导多能干细胞。这一研究是干细胞领域的里程碑,推动了再生医学的发展[37]。后续研究又从诱导多能干细胞中诱导出功能性间充质干细胞,相比传统骨髓、脂肪来源间充质干细胞拥有更强的增殖能力、更高水平的再生基因表达及更低的免疫原性,在促进骨再生、多发性硬化症等治疗中获得较好的治疗效果[38]。这一研究解决了干细胞在人体内数量有限的难题,使得干细胞在再生医学领域中拥有更广阔的发展潜力。进一步研究发现转录因子c-Myc通过激活多种细胞周期相关基因,如特异性周期蛋白D1和细胞周期蛋白依赖性激酶4来促进细胞周期的进程,特别是从G1期到S期的转换。而Oct4是维持干细胞多能性的关键转录因子,能够激活多种多能性相关基因,同时抑制分化相关基因的表达。利用慢病毒将过表达Oct4/c-Myc的基因整合入间充质干细胞中可促进细胞存活和新生血管形成,改善其治疗心肌缺血的效果[39]。过表达Oct4/Sox2的脂肪来源间充质干细胞在传代晚期拥有更显著的代谢活性及增殖能力,并且细胞的干性维持较好[40]。在人胚胎干细胞中过表达多能细胞特异性基因Nanog可以使细胞在多次传代后仍保持较强的多能分化潜力[41]。通过上述增强增殖分化基因表达的方法,细胞可以在体外维持更长久的增殖分化能力,但这种方法效率低并且技术难度要求较高,可用细胞数量有限,实验室和临床治疗之间仍然有很大的差距。另一种途径是通过调整细胞周期相关基因延缓细胞衰老,其中端粒缩短是细胞衰老的主要内在因素,而端粒反转录酶是负责延长端粒的一种核蛋白反转录酶,可向真核细胞染色体末端添加TTAGGG序重复列从而延长端粒。然而,随着年龄的增长,端粒酶活性趋于下降,端粒酶功能丧失加速了衰老的进展,并增加了癌症发病率[42]。因此,可以通过过表达端粒反转录酶基因从而延长端粒,延缓细胞衰老[43]。在动物模型中,过表达端粒反转录酶的间充质干细胞在心肌缺血再灌注中能更好地保护心脏基质细胞的活力并促进其向心血管表型发展,并且能促进细胞成骨分化[44]。此外,过表达巨噬细胞迁移抑制因子可使来自老年供者的间充质干细胞恢复活力[45]。过表达缺氧诱导因子1α可提高缺氧损伤干细胞的血管生成能力、迁移功能和增殖能力,同时在急性心肌梗死模型中通过促进新血管形成和抑制纤维化来改善心脏功能[46]。胰岛素增强子结合蛋白1是一种LIM同源结构域转录因子,在骨髓来源间充质干细胞中过表达胰岛素增强子结合蛋白1可以改善急性心肌梗死大鼠的心脏功能并促进血管生成[47],改善肾缺血再灌注损伤大鼠的肾功能,抑制了肾小管细胞凋亡、炎症和氧化应激[48]。利用CRISPR-Cas9技术敲除组织相容性复合物Ⅰ和Ⅱ编码CIITA基因,能够显著降低人胚胎干细胞在异体移植中的免疫原性,逃避免疫排斥反应,从而提高干细胞在体内移植后的存活率[49-50]。Bcl-2作为一种癌基因,有明显抑制细胞凋亡的作用。过表达Bcl-2可使间充质干细胞凋亡减少32%,血管内皮生长因子分泌增加60%以上,在急性心肌缺血动物模型中,与单独使用间充质干细胞治疗相比,移植过表达Bcl-2间充质干细胞后心肌梗死面积小17%,功能恢复显著。氧化应激是引起细胞体外衰老的重要因素,提高细胞抗氧化能力对维持细胞体外活性也十分重要,因此,移植过表达超氧化物歧化酶2的骨髓来源间充质干细胞增强了超氧化物歧化酶和谷胱甘肽过氧化物酶活性,抑制了丙二醛和活性氧的产生,改善肝缺血再灌注损伤[51]。
表观遗传重编程在恢复衰老干细胞活性方面也有重要作用,即通过改变非编码物质的表达,包括DNA甲基化、组蛋白修饰及非编码RNA介导的遗传修饰从而改变细胞命运。间充质干细胞的成骨、成脂、成软骨分化受DNA甲基化和去甲基化的调节,高甲基化会维持细胞多能性基因的表达,使用DNA甲基转移酶抑制剂地西他滨能够降低p16INK4a 启动子的甲基化水平,可以显著提高间充质干细胞的增殖能力和克隆形成能力,降低衰老标志物的表达,同时刺激干细胞分化[52]。组蛋白乙酰化是一种重要的表观遗传修饰,通过在组蛋白的赖氨酸残基上添加乙酰基团来调控基因表达。抑制组蛋白去乙酰化酶就可以增强间充质干细胞的神经分化效率[53]。miRNAs是细胞衰老相关基因表达程序中的关键调节因子[54]。其中miR-195直接靶向端粒反转录酶基因的3 '-非翻译区,负向调控细胞的生存分化能力,敲低miR-195能显著增加衰老间充质干细胞中端粒反转录酶的表达,诱导端粒延长,逆转间充质干细胞衰老[55]。miR-155-5p通过抑制骨髓来源间充质干细胞中的线粒体裂变,增加线粒体融合,导致间充质干细胞衰老。敲除miR-155-5p可使衰老的间充质干细胞恢复活力并增强急性心肌梗死后的心肌细胞保护能力[56]。miR-29c-3p、miR-29b-3p和miR-130b通过靶向不同的下游途径来恢复间充质干细胞衰老[57-59](表1)。
通过上述基因工程,可在细胞基因及表观遗传学上改造间充质干细胞,使其获得更强的增殖分化及抗凋亡能力。但在安全性方面,病毒载体介导的基因转染可能会导致插入突变,影响细胞的基因稳定性和功能,基因编辑技术(如CRISPR/Cas9)可能会产生脱靶效应,导致非目标基因的意外突变。过度的基因操作可能会导致间充质干细胞的多能性下降,影响细胞的分化能力。因此需要进一步优化基因工程技术,提高其安全性和有效性,以推动基因工程间充质干细胞在临床治疗中的广泛应用。
2.3.2 药理学方法 近年来,药物干预作为一种有效手段,逐渐被应用于间充质干细胞的体外培养。通过添加特定的药物或小分子化合物,可以调节间充质干细胞的增殖、分化、凋亡和免疫特性[60]。雷帕霉素可通过激活蛋白激酶B/哺乳动物雷帕霉素靶蛋白信号通路增加细胞克隆率,抵抗氧化应激,减少线粒体功能障碍,延缓细胞衰老[61-62]。5-甲氧基色氨酸和褪黑激素可通过保护线粒体完整性和功能并减少活性氧产生来逆转间充质干细胞衰老[63]。白藜芦醇是一种天然的植物多酚,可激活沉默信息调节因子1,抑制p53和p16的表达,从而发挥抗衰老的作用[64]。维生素C促进衰老小鼠骨髓间充质干细胞的体外增殖,延缓复制性衰老[65]。使用达沙替尼处理衰老间充质干细胞,能使细胞获得更好的成脂分化能力,并清除衰老细胞[66]。槲皮素干预能显著增强骨髓间充质干细胞的增殖能力,提高碱性磷酸酶活性,促进细胞外基质产生、矿化及成骨分化[67]。在二甲双胍处理的脂肪间充质干细胞中,衰老相关标志物和活性氧减少,增殖潜能增加,成骨分化潜能降低,成脂分化潜能增加,提高了细胞在慢性肾病中的治疗效果[68]。去铁胺可通过增加缺氧诱导因子1α蛋白的稳定性来促进祖细胞的动员和归巢[69]。2,4-二硝基苯酚预处理的间充质干细胞可通过促进血管生成改善心脏功能并减少心脏重构[70]。干扰素γ预处理能增强间充质干细胞的免疫抑制,减轻移植物抗宿主病,提高了间充质干细胞移植后的存活率[71](表2)。

尽管某些药物对间充质干细胞的体外培养有改善作用,但过高浓度的药物或长时间与干细胞共培养会损害间充质干细胞的功能。因此,在使用药物干预间充质干细胞时需注意不同来源的间充质干细胞对药物敏感性不同,并且长期使用表观遗传药物(如5-氮杂胞苷)可能增加基因突变风险,因此需要对不同药物进行预实验确定最佳浓度和作用时长,对其进行剂量及时间优化。
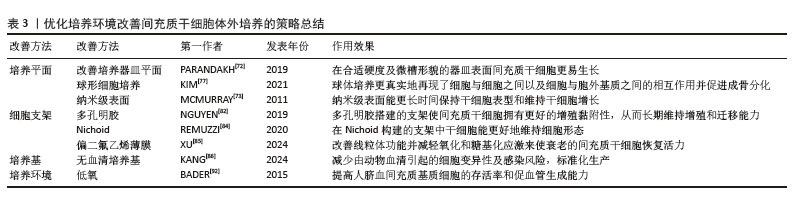
2.3.3 优化体外培养环境 细胞培养时的温度、氧浓度、pH值、渗透压、气压、生物力学和电磁都紧密关联着细胞的命运。选择合适的物理化学环境对于干细胞长时间体外培养至关重要,更决定了间充质干细胞规模化量产的执行标准。首先,在培养器皿方面,间充质干细胞的分离和扩增通常在具有一定硬度的塑料器皿上进行,尽管有着优异的细胞黏附性能,但在长期培养后仍会出现细胞增殖能力和分化潜能丧失。调节基质刚度和底物形貌能影响细胞增殖、表型维持和分化能力[72],在具有相对刚性及适当尺寸的微槽中能更好地维持间充质干细胞的未分化状态和多能性[73]。然而二维培养难以模拟自然器官系统的“细胞-细胞”和“细胞-细胞外基质”之间基本的信号通讯,以及细胞对动态机械刺激的反应,因此提出构建三维支架或形成类器官环境进行三维培养。与传统贴壁培养相比,三维培养能更好地模拟细胞在体内的生长环境,提供更接近生理条件的细胞行为和研究模型,具有培养效率更高的优势[74]。脂肪间充质干细胞三维培养后干性相关基因、抗衰老相关基因和端粒酶表达发生明显变化[75],细胞形态、增殖、分化能力和能量代谢较二维培养效果更好,表现出比二维培养更强的再生能力[76]。三维球体培养过程可减少细胞间隙、增加细胞间黏附和细胞外基质分泌[77],诱导成骨整合素和钙黏蛋白表达增加,提高间充质干细胞的成骨能力[78-79]。
除了球体培养增加细胞间通讯功能外,运用天然和合成生物材料构建细胞支架可以更好地模拟间充质干细胞在体内的环境。通常采用的生物材料都是高度可调、均质和无细胞的材料,并且具有高的批次间一致性,可使间充质干细胞的生产更加规范。常用的支架材料有琼脂糖、胶原、纤连蛋白、明胶、层粘连蛋白和玻连蛋白,特定类型的基质或支架适合于对培养细胞引发特定类型的形态学和生理学改变[80]。Ⅰ型胶原是三维培养系统中常用的基质,具有易于处理、低成本和活细胞操作灵活性等优势[81]。多孔明胶材料为干细胞在悬浮培养中提供黏附位点,从而促进细胞增殖和分化[82-83]。有研究团队开发出一种名为Nichoid的微结构支架,能够使间充质干细胞保持更高水平的干性,而无需添加外源性因子[84]。使用偏二氟乙烯薄膜培养物对间充质干细胞进行干预,可以在保持干性的同时进行高效、直接的抗衰老扩增,在大规模生产间充质干细胞方面具有巨大潜力[85]。
培养基的选择也至关重要,不同的血清类型及浓度也可影响体外间充质干细胞的分离、扩增和分化。传统的间充质干细胞培养方案依赖于体积分数10%胎牛血清促进细胞黏附并提供必需的营养和生长因子,然而,使用动物血清可能会带来异种污染、病毒、朊病毒、细菌感染的风险并引起批次间差异[86]。因此,近年针对人间充质干细胞扩增的培养基配方通常采用无血清培养基,通过在培养基中额外添加细胞因子促使间充质干细胞体外生长[87],此方法旨在减少变异性,增强可追溯性。另有研究表示含有血清的培养基能为间充质干细胞提供与体内更相似的微环境[88],并指出含有原始组织的培养基能更好地保持间充质干细胞的干性[89],但想要规范化生产人间充质干细胞,未来的方向仍然是无血清培养。
2.3.4 低氧预处理 在体外培养中,间充质干细胞通常在含体积分数21%氧气的培养箱中培养,实测该氧气体积分数显然高于间充质干细胞在体内微环境中的氧气体积分数。过量的氧气会增加氧化应激和相关信号通路的激活,最终导致细胞衰老。间充质干细胞的低氧预处理限制了氧化应激、DNA损伤、端粒缩短和染色体畸变的关键生理过程[90]。测序分析低氧(3%)和常氧(21%)条件下培养间充质干细胞的RNA表达谱,发现二者参与细胞周期调控的遗传物质不同,在低氧环境下分化能力下降,但增殖能力明显提升[91];缺氧和复氧过程促进间充质干细胞中促生存基因和各种营养因子分泌,从而提高了细胞增殖能力[92-93](表3)。
2.3.5 调节细胞因子 构建适合干细胞生长的微环境是维持干细胞功能特性最重要的一环,不同的微环境条件决定细胞的命运。通过调控细胞因子的表达或外源性添加特定细胞因子,可以增强人间充质干细胞的增殖、分化、迁移和归巢能力,延缓细胞衰老,从而提高间充质干细胞在再生医学和临床治疗中的应用效果。基质细胞衍生因子1预处理通过增强细胞增殖、迁移和存活保护间充质干细胞,抑制过氧化氢诱导的细胞凋亡,同时能上调碱性成纤维细胞生长因子、血管内皮生长因子以及蛋白激酶B和细胞外调节蛋白激酶的表达[94]。将转化生长因子β添加到脂肪形成培养基中能促进人间充质干细胞从成脂分化转变成骨分化[95]。选择的生长因子和培养基补充剂可以有效维持人间充质干细胞自我更新和分化潜能,补充细胞因子的培养基均提高了增殖速率并显著增加了在达到衰老之前的细胞倍增数,为人间充质干细胞在再生医学和临床治疗中的应用提供了重要支持[96]。
间充质干细胞衰老是一个复杂而综合的问题,大多数细胞调节过程不是独立的事件,因此需要多种不同的方法来缓解或预防衰老。不同的干预方式均存在一定的优缺点(表4),各种方式间可发挥协同作用共同改善间充质干细胞的体外培养效率,DNA甲基转移酶抑制剂5-氮杂胞苷/缺氧模拟剂去铁胺预处理促进了人骨髓间充质干细胞增殖、组蛋白乙酰化和低甲基化,细胞外基质胶原蛋白产生增加,细胞成骨分化能力增强,并且二者预处理骨髓间充质干细胞来源细胞外囊泡也促进了人骨髓间充质干细胞的成骨分化和矿化,增强了促骨骼修复的治疗效果[93]。尽管干扰素γ预处理通常用于在移植前激发间充质干细胞的免疫调节活性,但预处理的瞬时效应可能会限制间充质干细胞有效调节免疫反应的潜力。通过在三维间充质干细胞培养体系中持续呈递干扰素γ可精确调节和诱导免疫调节活性,从而提高间充质干细胞的疗效[97]。此外,应用生物材料联合药理学方案,控制药物释放,从而延长细胞暴露于小分子的持续时间,保证药物干预浓度,精准调控干细胞受干预的时间和剂量,如使用明胶包封微粒装载间充质干细胞可以提高持续作用时间,可根据其组成、聚合物分子质量、载药量和释放能力进行调整[98]。
| 1] XIE Z, YU W, YE G, et al. Single-cell RNA sequencing analysis of human bone-marrow-derived mesenchymal stem cells and functional subpopulation identification. Exp Mol Med. 2022;54(4):483-492. [2] HAN X, YANG B, ZOU F, et al. Clinical therapeutic efficacy of mesenchymal stem cells derived from adipose or bone marrow for knee osteoarthritis: a meta-analysis of randomized controlled trials. J Comp Eff Res. 2020;9(5):361-374. [3] ZENG CW. Multipotent Mesenchymal Stem Cell-Based Therapies for Spinal Cord Injury: Current Progress and Future Prospects. Biology (Basel). 2023;12(5):653. [4] YAN W, XIA Y, ZHAO H, et al. Stem cell-based therapy in cardiac repair after myocardial infarction: Promise, challenges, and future directions. J Mol Cell Cardiol. 2024;188:1-14. [5] FARABI B, ROSTER K, HIRANI R, et al. The Efficacy of Stem Cells in Wound Healing: A Systematic Review. Int J Mol Sci. 2024; 25(5):3006. [6] MUNDRA V, GERLING IC, MAHATO RI. Mesenchymal stem cell-based therapy. Mol Pharm. 2013;10(1):77-89. [7] SHI L, WANG L, XU R, et al. Mesenchymal stem cell therapy for severe COVID-19. Signal Transduct Target Ther. 2021;6(1):339. [8] MAZINE A, RUSHANI D, YAU TM. Clinical mesenchymal stem cell therapy in ischemic cardiomyopathy. JTCVS Open. 2021;8: 135-141. [9] 陈思铭,胡加伟,李丽丽,等.两种方法提取人脐带间充质干细胞三维培养生物学特性的比较[J].中国组织工程研究, 2022,26(19):2997-3003. [10] HWANG ES. Senescence suppressors: their practical importance in replicative lifespan extension in stem cells. Cell Mol Life Sci. 2014;71(21):4207-4219. [11] 白金萍.三种不同来源间充质干细胞增殖能力的比较研究[D].长春:吉林大学,2014. [12] HAYASHI O, KATSUBE Y, HIROSE M, et al. Comparison of osteogenic ability of rat mesenchymal stem cells from bone marrow, periosteum, and adipose tissue. Calcif Tissue Int. 2008;82(3):238-247. [13] OJA S, KOMULAINEN P, PENTTILÄ A, et al. Automated image analysis detects aging in clinical-grade mesenchymal stromal cell cultures. Stem Cell Res Ther 2018; 9(1): 6. [14] YANG YK, OGANDO CR, WANG SEE C, et al. Changes in phenotype and differentiation potential of human mesenchymal stem cells aging in vitro. Stem Cell Res Ther. 2018;9(1):131. [15] LI Y, WU Q, WANG Y, et al. Senescence of mesenchymal stem cells (Review). Int J Mol Med. 2017;39(4):775-782. [16] BONAB MM, ALIMOGHADDAM K, TALEBIAN F, et al. Aging of mesenchymal stem cell in vitro. BMC Cell Biol. 2006;7:14. [17] 赵文静,刘百坤,李秋莲,等.长期传代培养对骨髓间充质干细胞生物学特性的影响[J].中国组织工程研究,2024, 28(31):4926-4930. [18] TJEMPAKASARI A, SUROTO H, SANTOSO D. Mesenchymal Stem Cell Senescence and Osteogenesis. Medicina (Kaunas). 2021;58(1):61. [19] LIN W, XU L, ZWINGENBERGER S, et al. Mesenchymal stem cells homing to improve bone healing. J Orthop Translat. 2017;9: 19-27. [20] FU X, LIU G, HALIM A, et al. Mesenchymal Stem Cell Migration and Tissue Repair. Cells. 2019;8(8):784. [21] GEISSLER S, TEXTOR M, KÜHNISCH J, et al. Functional comparison of chronological and in vitro aging: differential role of the cytoskeleton and mitochondria in mesenchymal stromal cells. PLoS One. 2012;7(12):e52700. [22] BIRCH J, GIL J. Senescence and the SASP: many therapeutic avenues. Genes Dev. 2020;34(23-24):1565-1576. [23] SIRAJ Y, APRILE D, ALESSIO N, et al. IGFBP7 is a key component of the senescence-associated secretory phenotype (SASP) that induces senescence in healthy cells by modulating the insulin, IGF, and activin A pathways. Cell Commun Signal. 2024;22(1):540. [24] LEE BC, YU KR. Impact of mesenchymal stem cell senescence on inflammaging. BMB Rep. 2020;53(2):65-73. [25] SMITH N, SHIRAZI S, CAKOUROS D, et al. Impact of Environmental and Epigenetic Changes on Mesenchymal Stem Cells during Aging. Int J Mol Sci. 2023;24(7):6499. [26] REVUELTA M, MATHEU A. Autophagy in stem cell aging. Aging Cell. 2017;16(5):912-915. [27] MULLER M. Cellular senescence: molecular mechanisms, in vivo significance, and redox considerations. Antioxid Redox Signal. 2009;11(1):59-98. [28] SHIBATA KR, AOYAMA T, SHIMA Y, et al. Expression of the p16INK4A gene is associated closely with senescence of human mesenchymal stem cells and is potentially silenced by DNA methylation during in vitro expansion. Stem Cells. 2007;25(9):2371-2382. [29] KIM DY, LEE J, KANG D, et al. Multipotent neurogenic fate of mesenchymal stem cell is determined by Cdk4-mediated hypophosphorylation of Smad-STAT3. Cell Cycle. 2016;15(13):1787-1795. [30] BRUNET A, GOODELL MA, RANDO TA. Ageing and rejuvenation of tissue stem cells and their niches. Nat Rev Mol Cell Biol. 2023;24(1):45-62. [31] JIANG X, LI W, GE L, et al. Mesenchymal Stem Cell Senescence during Aging:From Mechanisms to Rejuvenation Strategies. Aging Dis. 2023;14(5):1651-1676. [32] VONO R, JOVER GARCIA E, SPINETTI G, et al. Oxidative Stress in Mesenchymal Stem Cell Senescence: Regulation by Coding and Noncoding RNAs. Antioxid Redox Signal. 2018;29(9):864-879. [33] GUO J, HUANG X, DOU L, et al. Aging and aging-related diseases: from molecular mechanisms to interventions and treatments. Signal Transduct Target Ther. 2022;7(1):391. [34] YE G, XIE Z, ZENG H, et al. Oxidative stress-mediated mitochondrial dysfunction facilitates mesenchymal stem cell senescence in ankylosing spondylitis. Cell Death Dis. 2020;11(9):775. [35] WANG Y, LIU Y, CHEN E, et al. The role of mitochondrial dysfunction in mesenchymal stem cell senescence. Cell Tissue Res. 2020; 382(3):457-462. [36] MIWA S, KASHYAP S, CHINI E, et al. Mitochondrial dysfunction in cell senescence and aging. J Clin Invest. 2022; 132(13):e158447. [37] KARAGIANNIS P, TAKAHASHI K, SAITO M, et al. Induced Pluripotent Stem Cells and Their Use in Human Models of Disease and Development. Physiol Rev. 2019;99(1):79-114. [38] JUNGBLUTH P, SPITZHORN LS, GRASSMANN J, et al. Human iPSC-derived iMSCs improve bone regeneration in mini-pigs. Bone Res. 2019;7:32. [39] ZHAO L, WANG J, WANG P, et al. Oct4 cooperates with c-Myc to improve mesenchymal-to-endothelial transition and myocardial repair of cardiac-resident mesenchymal stem cells. Stem Cell Res Ther. 2022;13(1):445. [40] HAN SM, HAN SH, COH YR, et al. Enhanced proliferation and differentiation of Oct4- and Sox2-overexpressing human adipose tissue mesenchymal stem cells. Exp Mol Med. 2014;46(6):e101. [41] DARR H, MAYSHAR Y, BENVENISTY N. Overexpression of NANOG in human ES cells enables feeder-free growth while inducing primitive ectoderm features. Development. 2006;133(6):1193-1201. [42] ROAKE CM, ARTANDI SE. Regulation of human telomerase in homeostasis and disease. Nat Rev Mol Cell Biol. 2020;21(7): 384-397. [43] SIMONSEN JL, ROSADA C, SERAKINCI N, et al. Telomerase expression extends the proliferative life-span and maintains the osteogenic potential of human bone marrow stromal cells. Nat Biotechnol. 2002; 20(6):592-596. [44] MADONNA R, GUARNIERI S, KOVÁCSHÁZI C, et al. Telomerase/myocardin expressing mesenchymal cells induce survival and cardiovascular markers in cardiac stromal cells undergoing ischaemia/reperfusion. J Cell Mol Med. 2021;25(12):5381-5390. [45] ZHANG Y, ZHU W, HE H, et al. Macrophage migration inhibitory factor rejuvenates aged human mesenchymal stem cells and improves myocardial repair. Aging (Albany NY). 2019;11(24):12641-12660. [46] SUN J, SHEN H, SHAO L, et al. HIF-1α overexpression in mesenchymal stem cell-derived exosomes mediates cardioprotection in myocardial infarction by enhanced angiogenesis. Stem Cell Res Ther. 2020;11(1):373. [47] XIANG Q, LIAO Y, CHAO H, et al. ISL1 overexpression enhances the survival of transplanted human mesenchymal stem cells in a murine myocardial infarction model. Stem Cell Res Ther. 2018;9(1):51. [48] WANG J, WANG J, LU C, et al. ISL1-overexpressing BMSCs attenuate renal ischemia-reperfusion injury by suppressing apoptosis and oxidative stress through the paracrine action. Cell Mol Life Sci. 2024; 81(1):312. [49] FREDERIKSEN HR, GLANTZ A, VØLS KK, et al. CRISPR-Cas9 immune-evasive hESCs are rejected following transplantation into immunocompetent mice. Front Genome Ed. 2024;6:1403395. [50] LI W, MA N, ONG LL, et al. Bcl-2 engineered MSCs inhibited apoptosis and improved heart function. Stem Cells. 2007;25(8): 2118-2127. [51] LI Q, ZHANG W, XIAO E. SOD2 overexpression in bone marrow‑derived mesenchymal stem cells ameliorates hepatic ischemia/reperfusion injury. Mol Med Rep. 2021;24(3):671. [52] ZHOU Y, HU Z. Genome-wide demethylation by 5-aza-2’-deoxycytidine alters the cell fate of stem/progenitor cells. Stem Cell Rev Rep. 2015;11(1):87-95. [53] HUANG M, XIAO X, JI G, et al. Histone modifications in neurodifferentiation of embryonic stem cells. Heliyon. 2021;8(1): e08664. [54] KOCH L. microRNAs as systemic regulators of ageing. Nat Rev Genet. 2023;24(7):415. [55] OKADA M, KIM HW, MATSU-URA K, et al. Abrogation of Age-Induced MicroRNA-195 Rejuvenates the Senescent Mesenchymal Stem Cells by Reactivating Telomerase. Stem Cells. 2016;34(1):148-159. [56] HONG Y, HE H, JIANG G, et al. miR-155-5p inhibition rejuvenates aged mesenchymal stem cells and enhances cardioprotection following infarction. Aging Cell. 2020;19(4): e13128. [57] SHANG J, YAO Y, FAN X, et al. miR-29c-3p promotes senescence of human mesenchymal stem cells by targeting CNOT6 through p53-p21 and p16-pRB pathways. Biochim Biophys Acta. 2016;1863(4):520-532. [58] SU T, XIAO Y, XIAO Y, et al. Bone Marrow Mesenchymal Stem Cells-Derived Exosomal MiR-29b-3p Regulates Aging-Associated Insulin Resistance. ACS Nano. 2019;13(2):2450-2462. [59] XU J, HUANG Z, LIN L, et al. miRNA-130b is required for the ERK/FOXM1 pathway activation-mediated protective effects of isosorbide dinitrate against mesenchymal stem cell senescence induced by high glucose. Int J Mol Med. 2015;35(1):59-71. [60] MADL CM, HEILSHORN SC, BLAU HM. Bioengineering strategies to accelerate stem cell therapeutics. Nature. 2018; 557(7705):335-342. [61] GHARIBI B, FARZADI S, GHUMAN M, et al. Inhibition of Akt/mTOR attenuates age-related changes in mesenchymal stem cells. Stem Cells. 2014;32(8):2256-2266. [62] MOTA-MARTORELL N, JOVÉ M, PAMPLONA R. mTOR Complex 1 Content and Regulation Is Adapted to Animal Longevity. Int J Mol Sci. 2022;23(15):8747. [63] CHANG TC, HSU MF, SHIH CY, et al. 5-methoxytryptophan protects MSCs from stress induced premature senescence by upregulating FoxO3a and mTOR. Sci Rep. 2017;7(1):11133. [64] DAI Z, LI Y, QUARLES LD, et al. Resveratrol enhances proliferation and osteoblastic differentiation in human mesenchymal stem cells via ER-dependent ERK1/2 activation. Phytomedicine. 2007;14(12):806-814. [65] ZHENG C, SUI B, HU C, et al. Vitamin C promotes in vitro proliferation of bone marrow mesenchymal stem cells derived from aging mice. Nan Fang Yi Ke Da Xue Xue Bao. 2015;35(12):1689-1693. [66] HEINRICHS DP, MALDONADO VV, ARDANA IKK, et al. Assessing the Effects of Dasatinib on Mesenchymal Stem/Stromal Cells. Cell Mol Bioeng. 2024;17(6):609-618. [67] PANG XG, CONG Y, BAO NR, et al. Quercetin Stimulates Bone Marrow Mesenchymal Stem Cell Differentiation through an Estrogen Receptor-Mediated Pathway. Biomed Res Int. 2018;2018:4178021. [68] KIM H, YU MR, LEE H, et al. Metformin inhibits chronic kidney disease-induced DNA damage and senescence of mesenchymal stem cells. Aging Cell. 2021;20(2):e13317. [69] NAJAFI R, SHARIFI AM. Deferoxamine preconditioning potentiates mesenchymal stem cell homing in vitro and in streptozotocin-diabetic rats. Expert Opin Biol Ther. 2013;13(7):959-972. [70] KHAN I, ALI A, AKHTER MA, et al. Preconditioning of mesenchymal stem cells with 2,4-dinitrophenol improves cardiac function in infarcted rats. Life Sci. 2016;162:60-69. [71] KIM DS, JANG IK, LEE MW, et al. Enhanced Immunosuppressive Properties of Human Mesenchymal Stem Cells Primed by Interferon-γ. EBioMedicine. 2018;28: 261-273. [72] PARANDAKH A, ANBARLOU A, TAFAZZOLI-SHADPOUR M, et al. Substrate topography interacts with substrate stiffness and culture time to regulate mechanical properties and smooth muscle differentiation of mesenchymal stem cells. Colloids Surf B Biointerfaces. 2019;173:194-201. [73] MCMURRAY RJ, GADEGAARD N, TSIMBOURI PM, et al. Nanoscale surfaces for the long-term maintenance of mesenchymal stem cell phenotype and multipotency. Nat Mater. 2011;10(8):637-644. [74] HUANG X, HUANG Z, GAO W, et al. Current Advances in 3D Dynamic Cell Culture Systems. Gels. 2022;8(12):829. [75] GUO L, ZHOU Y, WANG S, et al. Epigenetic changes of mesenchymal stem cells in three-dimensional (3D) spheroids. J Cell Mol Med. 2014;18(10):2009-2019. [76] CHENG NC, WANG S, YOUNG TH. The influence of spheroid formation of human adipose-derived stem cells on chitosan films on stemness and differentiation capabilities. Biomaterials. 2012;33(6):1748-1758. [77] KIM J, ADACHI T. Cell-fate decision of mesenchymal stem cells toward osteocyte differentiation is committed by spheroid culture. Sci Rep. 2021;11(1):13204. [78] GRIFFIN FE, SCHIAVI J, MCDEVITT TC, et al. The role of adhesion junctions in the biomechanical behaviour and osteogenic differentiation of 3D mesenchymal stem cell spheroids. J Biomech. 2017;59:71-79. [79] YAMAGUCHI Y, OHNO J, SATO A, et al. Mesenchymal stem cell spheroids exhibit enhanced in-vitro and in-vivo osteoregenerative potential. BMC Biotechnol. 2014;14:105. [80] RAVI M, PARAMESH V, KAVIYA SR, et al. 3D cell culture systems: advantages and applications. J Cell Physiol. 2015;230(1): 16-26. [81] SULTANA N, COLE A, STRACHAN F. Biocomposite Scaffolds for Tissue Engineering: Materials, Fabrication Techniques and Future Directions. Materials (Basel). 2024;17(22):5577. [82] NGUYEN L, BANG S, NOH I. Tissue Regeneration of Human Mesenchymal Stem Cells on Porous Gelatin Micro-Carriers by Long-Term Dynamic In Vitro Culture. Tissue Eng Regen Med. 2019;16(1):19-28. [83] BHUPTANI RS, PATRAVALE VB. Porous microscaffolds for 3D culture of dental pulp mesenchymal stem cells. Int J Pharm. 2016;515(1-2):555-564. [84] REMUZZI A, BONANDRINI B, TIRONI M, et al. Effect of the 3D Artificial Nichoid on the Morphology and Mechanobiological Response of Mesenchymal Stem Cells Cultured In Vitro. Cells. 2020;9(8):1873. [85] XU L, REN W, LONG Y, et al. Antisenescence Expansion of Mesenchymal Stem Cells Using Piezoelectric β-Poly(vinylidene fluoride) Film-Based Culture. ACS Appl Mater Interfaces. 2024;16(46):63207-63224. [86] KANG M, YANG Y, ZHANG H, et al. Comparative Analysis of Serum and Serum-Free Medium Cultured Mesenchymal Stromal Cells for Cartilage Repair. Int J Mol Sci. 2024;25(19):10627. [87] NG F, BOUCHER S, KOH S, et al. PDGF, TGF-beta, and FGF signaling is important for differentiation and growth of mesenchymal stem cells (MSCs): transcriptional profiling can identify markers and signaling pathways important in differentiation of MSCs into adipogenic, chondrogenic, and osteogenic lineages. Blood. 2008;112(2):295-307. [88] SHAFAEI H, ESMAEILI A, MARDANI M, et al. Effects of human placental serum on proliferation and morphology of human adipose tissue-derived stem cells. Bone Marrow Transplant. 2011;46(11):1464-1471. [89] OTTE A, BUCAN V, REIMERS K, et al. Mesenchymal stem cells maintain long-term in vitro stemness during explant culture. Tissue Eng Part C Methods. 2013; 19(12):937-948. [90] ZHANG B, YANG S, ZHANG Y, et al. Co-culture of mesenchymal stem cells with umbilical vein endothelial cells under hypoxic condition. J Huazhong Univ Sci Technolog Med Sci. 2012;32(2):173-180. [91] BOYETTE LB, CREASEY OA, GUZIK L, et al. Human bone marrow-derived mesenchymal stem cells display enhanced clonogenicity but impaired differentiation with hypoxic preconditioning. Stem Cells Transl Med. 2014;3(2):241-254. [92] BADER AM, KLOSE K, BIEBACK K, et al. Hypoxic Preconditioning Increases Survival and Pro-Angiogenic Capacity of Human Cord Blood Mesenchymal Stromal Cells In Vitro. PLoS One. 2015;10(9):e0138477. [93] MAN K, BRUNET MY, LEES R, et al. Epigenetic Reprogramming via Synergistic Hypomethylation and Hypoxia Enhances the Therapeutic Efficacy of Mesenchymal Stem Cell Extracellular Vesicles for Bone Repair. Int J Mol Sci. 2023;24(8):7564. [94] LIU X, DUAN B, CHENG Z, et al. SDF-1/CXCR4 axis modulates bone marrow mesenchymal stem cell apoptosis, migration and cytokine secretion. Protein Cell. 2011;2(10):845-854. [95] VAN ZOELEN EJ, DUARTE I, HENDRIKS JM, et al. TGFβ-induced switch from adipogenic to osteogenic differentiation of human mesenchymal stem cells: identification of drug targets for prevention of fat cell differentiation. Stem Cell Res Ther. 2016; 7(1):123. [96] TURINETTO V, VITALE E, GIACHINO C. Senescence in Human Mesenchymal Stem Cells: Functional Changes and Implications in Stem Cell-Based Therapy. Int J Mol Sci. 2016;17(7):1164. [97] ZIMMERMANN JA, HETTIARATCHI MH, MCDEVITT TC. Enhanced Immunosuppression of T Cells by Sustained Presentation of Bioactive Interferon-γ Within Three-Dimensional Mesenchymal Stem Cell Constructs. Stem Cells Transl Med. 2017;6(1):223-237. [98] TZOUANAS SN, EKENSEAIR AK, KASPER FK, et al. Mesenchymal stem cell and gelatin microparticle encapsulation in thermally and chemically gelling injectable hydrogels for tissue engineering. J Biomed Mater Res A. 2014;102(5):1222-1230. |
| [1] | 陈秋函, 杨 龙, 袁代柱, 吴展羽, 邹梓豪, 叶 川. 膝关节周围截骨治疗膝骨关节炎:治疗策略的优化[J]. 中国组织工程研究, 2026, 30(9): 2303-2312. |
| [2] | 赵非凡, 曹玉净. 股骨近端防旋髓内钉治疗股骨转子间骨折内固定失效的危险因素与应对策略[J]. 中国组织工程研究, 2026, 30(9): 2323-2333. |
| [3] | 蒋祥龙, 厉中山, 车同同. 低频脉冲电磁场在肌肉修复与增长中的应用效果和作用机制[J]. 中国组织工程研究, 2026, 30(9): 2350-2360. |
| [4] | 刘金龙, 阿卜杜吾普尔•海比尔, 白 臻, 苏丹阳, 苗 鑫, 李 菲, 杨晓鹏. 不同非手术方法治疗青少年特发性脊柱侧凸效果的系统综述与网状Meta分析[J]. 中国组织工程研究, 2026, 30(9): 2370-2379. |
| [5] | 吴妍廷, 李 宇, 廖金凤. 氧化镁纳米粒调控成骨与血管生成相关基因表达促进骨缺损愈合[J]. 中国组织工程研究, 2026, 30(8): 1885-1895. |
| [6] | 蒋星海, 宋玉林, 李德津, 邵建敏, 徐军志, 刘华凯, 吴应国, 沈岳辉, 冯思诚. 血管内皮生长因子165基因转染骨髓间充质干细胞构建血管化两亲性肽凝胶模块[J]. 中国组织工程研究, 2026, 30(8): 1903-1911. |
| [7] | 胡雄科, 刘少华, 谭 谦, 刘 昆, 朱光辉. 紫草素干预骨髓间充质干细胞改善老年小鼠股骨的微结构[J]. 中国组织工程研究, 2026, 30(7): 1609-1615. |
| [8] | 宋浦蓁, 马贺宾, 陈宏广, 章亚东. 骨髓间充质干细胞外泌体联合转化生长因子β1对巨噬细胞的作用[J]. 中国组织工程研究, 2026, 30(7): 1616-1623. |
| [9] | 蔡子鸣, 于庆贺, 马鹏飞, 张 鑫, 周龙千, 张崇阳, 林文平. 血红素氧合酶1减轻脂多糖诱导髓核间充质干细胞的炎症反应[J]. 中国组织工程研究, 2026, 30(7): 1624-1631. |
| [10] | 袁小霜, 杨 姁, 杨 波, 陈晓旭, 田 婷, 王飞清, 李艳菊, 刘 洋, 杨文秀. 弥漫性大B细胞淋巴瘤细胞条件培养液对人骨髓间充质干细胞增殖、凋亡的影响[J]. 中国组织工程研究, 2026, 30(7): 1632-1640. |
| [11] | 李镇宇, 张思明, 柏家祥, 朱 晨. 蛇床子素改善高糖环境下骨髓间充质干细胞的成骨分化功能[J]. 中国组织工程研究, 2026, 30(7): 1641-1648. |
| [12] | 韩念荣, 黄异飞, 艾克热木·吾斯曼, 刘岩路, 胡 炜. 高糖微环境中程序性细胞死亡受体1抑制大鼠骨髓间充质干细胞的成骨分化[J]. 中国组织工程研究, 2026, 30(7): 1649-1657. |
| [13] | 金东升, 赵张红, 朱子银, 张 森, 孙祖延, 邓 江. 淫羊藿苷缓释微球三维支架对兔骨髓间充质干细胞成骨分化的影响[J]. 中国组织工程研究, 2026, 30(7): 1658-1668. |
| [14] | 邹玉莲, 陈朝沛, 黄海霞, 兰玉燕, 刘 敏, 黄 婷. 白藜芦醇在炎症微环境下促进骨髓间充质干细胞的成骨分化[J]. 中国组织工程研究, 2026, 30(7): 1669-1678. |
| [15] | 王秋花, 杜孜玮, 王文双, 赵冬梅, 张晓晴. 雌雄大鼠脂肪间充质干细胞代谢、增殖、分化及向血管平滑肌细胞分化的差异性[J]. 中国组织工程研究, 2026, 30(7): 1687-1698. |
尽管人间充质干细胞在多种疾病治疗中展现出显著的临床应用前景,但在实际临床转化中,间充质干细胞获取及扩增面临诸多困难。由于间充质干细胞的特殊性,需要对它进行体外培养以获得足量细胞用于治疗,自干细胞研究至今仍没有统一的生产规范,使得许多研究出现参差不齐甚至相悖的结果,同时间充质干细胞在体外培养期间逐渐出现增殖分化能力下降、免疫原性改变、迁移和归巢能力下降、线粒体功能障碍等问题,这些改变导致间充质干细胞在体外难以长期保持功能特性,进而影响在实际临床应用中的效果。因此,近年来多项研究为改善间充质干细胞的体外培养衰老问题提出了改进方案,包括改善培养条件、优化培养方式、调控干细胞基因表达、在培养基中加入药物及生长因子等,以此维持干细胞生物功能特性,从而解决间充质干细胞的临床应用受限问题。此文章就提高间充质干细胞临床应用效果的方法策略作一综述,并对间充质干细胞在再生医学中的应用进行展望。
中国组织工程研究杂志出版内容重点:干细胞;骨髓干细胞;造血干细胞;脂肪干细胞;肿瘤干细胞;胚胎干细胞;脐带脐血干细胞;干细胞诱导;干细胞分化;组织工程
1.1.1 检索人及检索时间 由第一作者在2025年1月进行检索。
1.1.2 检索文献时限 2014年1月至2025年1月。
1.1.3 检索数据库 PubMed数据库和中国知网。
1.1.4 检索词 中文检索词为“间充质干细胞,体外培养,细胞培养,培养条件,预处理,细胞衰老”,英文检索词为“mesenchymal stem cells,cell culture,in vitro,culture conditions,preconditioning,cell senescence”。
1.1.5 检索文献类型 综述、研究原著及著作。
1.1.6 检索策略 中国知网和PubMed数据库检索策略见图1。
1.1.7 检索文献量 共检索到中文文献215篇,英文文献2 807篇。
1.2 纳入标准 ①各类间充质干细胞在体外培养时的问题及原因;②各类提高间充质干细胞体外培养效能的研究。
1.3 排除标准 ①研究内容或目的与文章主旨无关的文献;②重复性研究;③未能阐明具体作用机制的研究;④内容陈旧、质量不高的文献。
1.4 资料整合 由第一作者进行信息情报检索,通过剔除不符合纳入标准的文献,排除与研究目的相关性较低及内容重复、资料陈旧的文献,选取98篇相关文献进行归纳整理。文献筛选流程见图2。
中国组织工程研究杂志出版内容重点:干细胞;骨髓干细胞;造血干细胞;脂肪干细胞;肿瘤干细胞;胚胎干细胞;脐带脐血干细胞;干细胞诱导;干细胞分化;组织工程
中国组织工程研究杂志出版内容重点:干细胞;骨髓干细胞;造血干细胞;脂肪干细胞;肿瘤干细胞;胚胎干细胞;脐带脐血干细胞;干细胞诱导;干细胞分化;组织工程
文题释义:
间充质干细胞:是一类具有自我更新能力和多向分化潜能的成体干细胞,主要存在于骨髓、脂肪、脐带、胎盘等结缔组织和间充质中,在一定条件下可以分化成多种功能细胞。间充质干细胞在组织工程、再生医学、自身免疫性疾病治疗及抗炎疗法中具有潜力。
三维培养:将细胞在三维空间环境中培养,使其形成类似体内组织的立体结构,以更好地模拟细胞在真实组织或器官中的微环境。三维培养能提供更接近生理条件的细胞-细胞和细胞-基质相互作用微环境,广泛应用于组织工程、药物筛选、肿瘤研究和再生医学等领域。
#br#
近年来,随着生物材料、微环境调控和类器官技术的发展,干细胞培养体系不断优化,细胞体外培养技术正朝着更精准、更高效、更临床化的方向发展。但在实现规模化临床应用过程中,培养系统的批次稳定性差、细胞表型异质性高等关键瓶颈问题依然突出,严重制约了干细胞治疗产品的产业化进程。本文就干细胞在体外培养时所发生的改变及其可能原因作一综述,并重点评述了当前研究中最具应用前景的几类优化策略,旨在为建立规范化的间充质干细胞工业化生产体系提供理论依据和技术路线参考,为深入理解细胞体外培养命运的微环境调控规律提供新的研究思路,或将为突破现有规模化生产瓶颈提供创新性的解决路径。
#br#
中国组织工程研究杂志出版内容重点:干细胞;骨髓干细胞;造血干细胞;脂肪干细胞;肿瘤干细胞;胚胎干细胞;脐带脐血干细胞;干细胞诱导;干细胞分化;组织工程
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||