[1] DUMBUYA JS, CHEN L, WU JY, et al. The role of G-CSF neuroprotective effects in neonatal hypoxic-ischemic encephalopathy (HIE): current status. J Neuroinflammation. 2021;18(1):55.
[2] FINDER M, BOYLAN GB, TWOMEY D, et al. Two-Year Neurodevelopmental Outcomes After Mild Hypoxic Ischemic Encephalopathy in the Era of Therapeutic Hypothermia. JAMA Pediatr. 2020;174(1):48-55.
[3] DENG Z, OU H, REN F, et al. LncRNA SNHG14 promotes OGD/R-induced neuron injury by inducing excessive mitophagy via miR-182-5p/BINP3 axis in HT22 mouse hippocampal neuronal cells. Biol Res. 2020; 53(1):38.
[4] PARMENTIER CEJ, DE VRIES LS, GROENENDAAL F. Magnetic Resonance Imaging in (Near-)Term Infants with Hypoxic-Ischemic Encephalopathy. Diagnostics (Basel). 2022;12(3):645.
[5] RISTOVSKA S, STOMNAROSKA O, DANILOVSKI D. Hypoxic Ischemic Encephalopathy (HIE) in Term and Preterm Infants. Pril (Makedon Akad Nauk Umet Odd Med Nauki). 2022;43(1):77-84.
[6] RANJAN AK, GULATI A. Advances in Therapies to Treat Neonatal Hypoxic-Ischemic Encephalopathy. J Clin Med. 2023;12(20):6653.
[7] GLASS HC, WUSTHOFF CJ, COMSTOCK BA, et al. Risk of seizures in neonates with hypoxic-ischemic encephalopathy receiving hypothermia plus erythropoietin or placebo. Pediatr Res. 2023;94(1):252-259.
[8] PAN JJ, WU Y, LIU Y, et al. The effect of erythropoietin on neonatal hypoxic-ischemic encephalopathy: An updated meta-analysis of randomized control trials. Front Pediatr. 2023;10:1074287.
[9] PERRONE S, LEMBO C, GIRONI F, et al. Erythropoietin as a Neuroprotective Drug for Newborn Infants: Ten Years after the First Use. Antioxidants (Basel). 2022;11(4):652.
[10] XU Y, HUANG L, HAN J, et al. Effects of EPO combined with mild hypothermia on oxidative stress and neuroprotection in neonates with hypoxic-ischemic encephalopathy. Cell Mol Biol (Noisy-le-grand). 2022;68(4):36-45.
[11] BRIDGES MC, DAULAGALA AC, KOURTIDIS A. LNCcation: lncRNA localization and function. J Cell Biol. 2021;220(2):e202009045.
[12] QIU X, CHEN J. LSINCT5: A Novel lncRNA in Cancers. Curr Med Chem. 2023;30(39):4409-4420.
[13] YANG Z, JIANG S, SHANG J, et al. LncRNA: Shedding light on mechanisms and opportunities in fibrosis and aging. Ageing Res Rev. 2019;52:17-31.
[14] LI Y, ZHANG JK, YU ZT, et al. LncRNA XIST Exacerbates Oxygen-Glucose Deprivation/Reoxygenation-Induced Cerebral Injury Through the miR-25-3p/TRAF3 Axis. Mol Neurobiol. 2023;60(10):6109-6120.
[15] WANG C, DONG J, SUN J, et al. Silencing of lncRNA XIST impairs angiogenesis and exacerbates cerebral vascular injury after ischemic stroke. Mol Ther Nucleic Acids. 2021;26:148-160.
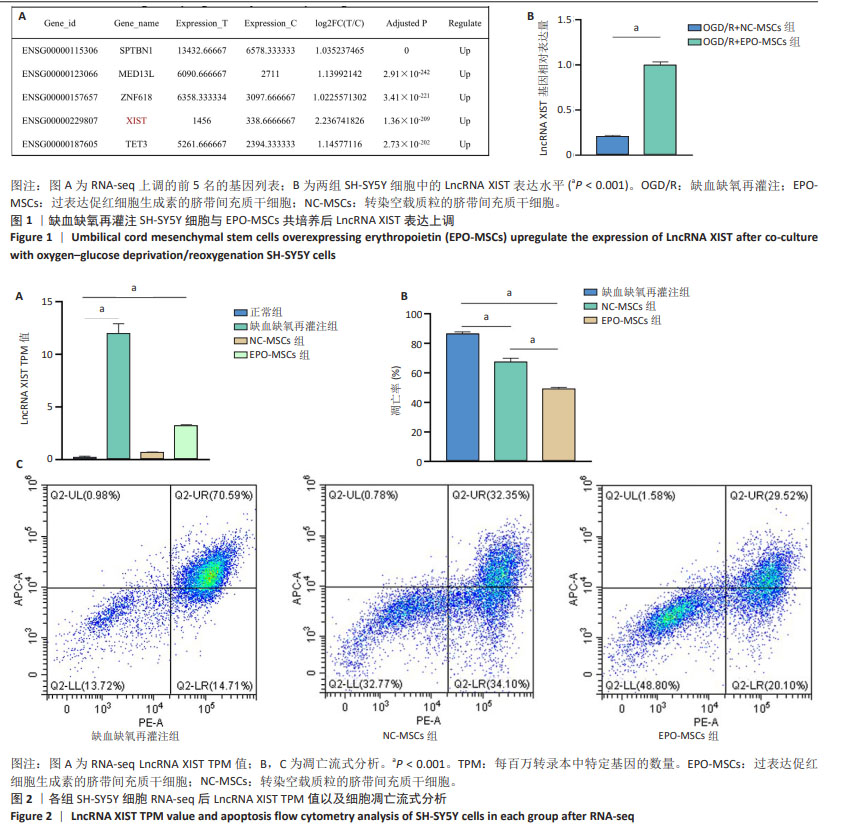
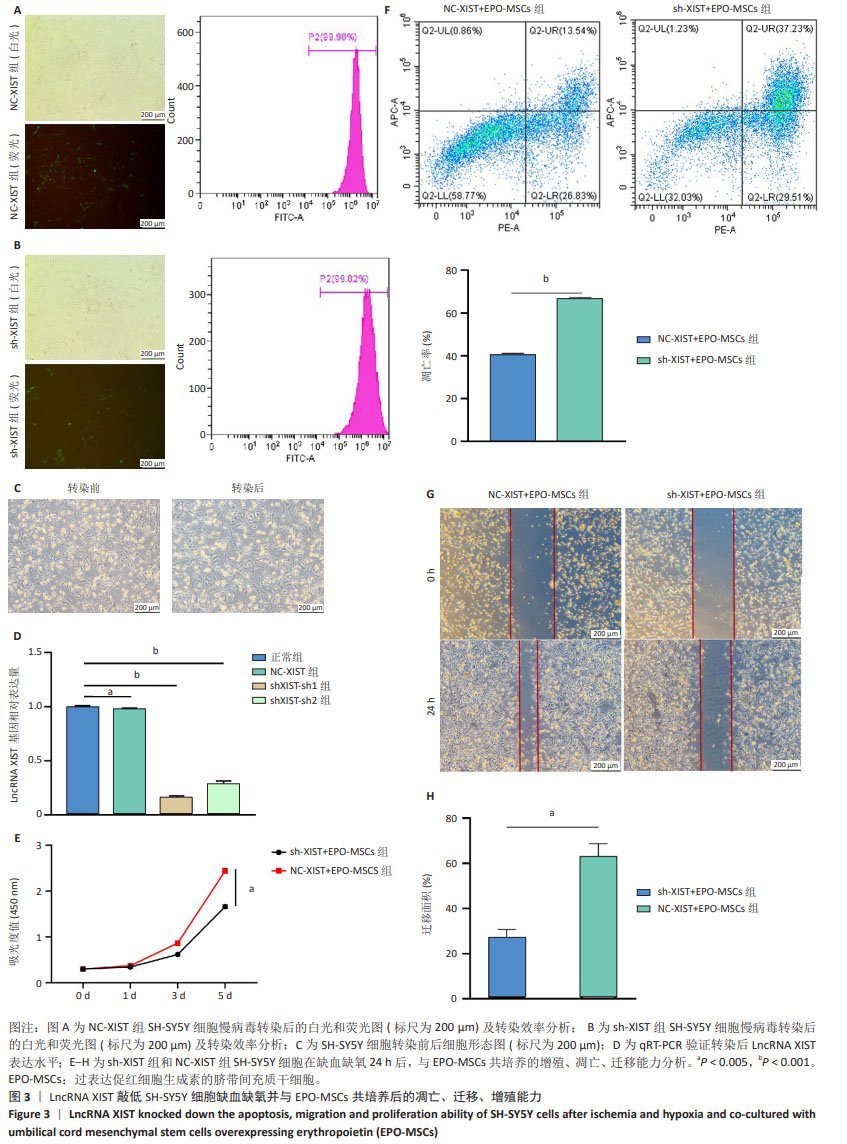
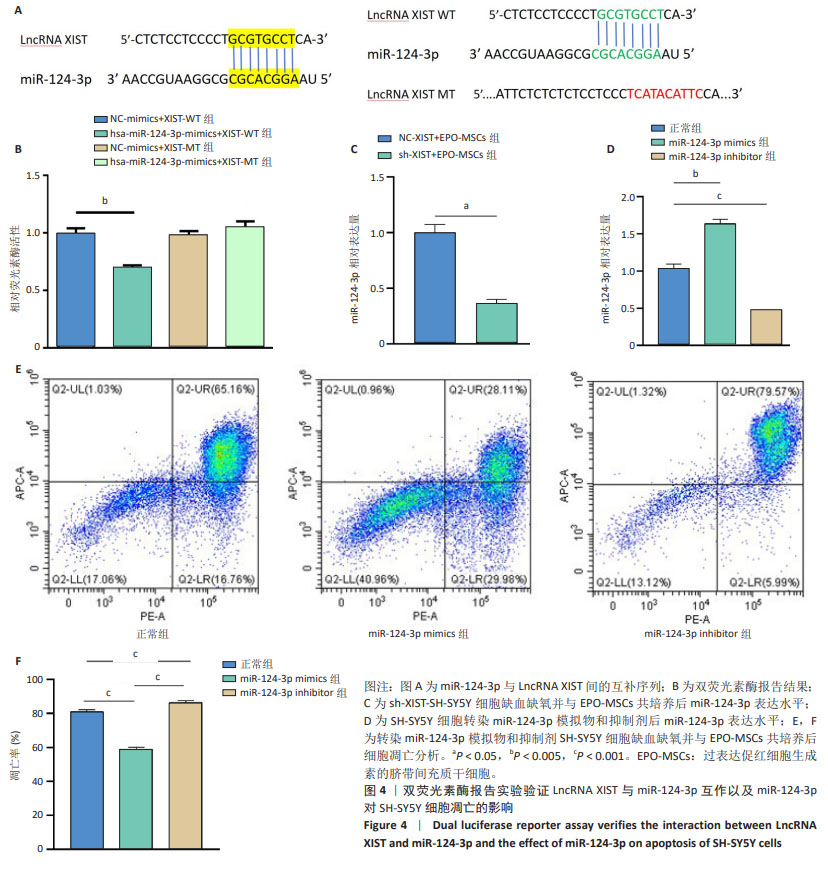
[16] 班跃耀,孙蕾,马保东,等.促红细胞生成素基因修饰的人脐带间充质干细胞对神经元周期和凋亡的调控作用及机制[J].新乡医学院学报,2023,40(10):909-916.
[17] 李瑞博,孔宁,孙蕾,等.过表达促红细胞生成素脐带间充质干细胞抑制缺血缺氧SH-SY5Y细胞凋亡及机制[J].中国组织工程研究, 2024,28(31):4937-4944.
[18] HU X, ZHANG H, ZHANG Q, et al. Emerging role of STING signalling in CNS injury: inflammation, autophagy, necroptosis, ferroptosis and pyroptosis. J Neuroinflammation. 2022;19(1):242.
[19] XUE L, DU R, BI N, et al. Transplantation of human placental chorionic plate-derived mesenchymal stem cells for repair of neurological damage in neonatal hypoxic-ischemic encephalopathy. Neural Regen Res. 2024;19(9):2027-2035.
[20] NABETANI M, MUKAI T, SHINTAKU H. Preventing Brain Damage from Hypoxic-Ischemic Encephalopathy in Neonates: Update on Mesenchymal Stromal Cells and Umbilical Cord Blood Cells. Am J Perinatol. 2022;39(16):1754-1763.
[21] ZHAO J, LE M, LI J, et al. LINC00938 alleviates hypoxia ischemia encephalopathy induced neonatal brain injury by regulating oxidative stress and inhibiting JNK/p38 MAPK signaling pathway. Exp Neurol. 2023;367:114449.
[22] FENG M, ZHU X, ZHUO C. H19/miR-130a-3p/DAPK1 axis regulates the pathophysiology of neonatal hypoxic-ischemia encephalopathy. Neurosci Res. 2021;163:52-62.
[23] MENG Q, YANG P, LU Y. MicroRNA-410 serves as a candidate biomarker in hypoxic-ischemic encephalopathy newborns and provides neuroprotection in oxygen-glucose deprivation-injured PC12 and SH-SY5Y cells. Brain Behav. 2021;11(8):e2293.
[24] BROCKDORFF N, BOWNESS JS, WEI G. Progress toward understanding chromosome silencing by Xist RNA. Genes Dev. 2020;34(11-12): 733-744.
[25] DOU DR, ZHAO Y, BELK JA, et al. Xist ribonucleoproteins promote female sex-biased autoimmunity. Cell. 2024;187(3):733-749.e16.
[26] RICHART L, PICOD-CHEDOTEL ML, WASSEF M, et al. XIST loss impairs mammary stem cell differentiation and increases tumorigenicity through Mediator hyperactivation. Cell. 2022;185(12):2164-2183.e25.
[27] LI J, MING Z, YANG L, et al. Long noncoding RNA XIST: Mechanisms for X chromosome inactivation, roles in sex-biased diseases, and therapeutic opportunities. Genes Dis. 2022;9(6):1478-1492.
[28] ELDESOUKI S, SAMARA KA, QADRI R, et al. XIST in Brain Cancer. Clin Chim Acta. 2022;531:283-290.
[29] HU C, BAI X, LIU C, et al. Long noncoding RNA XIST participates hypoxia-induced angiogenesis in human brain microvascular endothelial cells through regulating miR-485/SOX7 axis. Am J Transl Res. 2019; 11(10):6487-6497.
[30] XIAO M, LI J, LI W, et al. MicroRNAs activate gene transcription epigenetically as an enhancer trigger. RNA Biol. 2017;14(10): 1326-1334.
[31] ESTEVES M, ABREU R, FERNANDES H, et al. MicroRNA-124-3p-enriched small extracellular vesicles as a therapeutic approach for Parkinson’s disease. Mol Ther. 2022;30(10):3176-3192.
[32] MAVROUDIS I, BALMUS IM, CIOBICA A, et al. The Role of Microglial Exosomes and miR-124-3p in Neuroinflammation and Neuronal Repair after Traumatic Brain Injury. Life (Basel). 2023;13(9):1924.
[33] LIU F, QIU F, CHEN H. miR-124-3p Ameliorates Isoflurane-Induced Learning and Memory Impairment via Targeting STAT3 and Inhibiting Neuroinflammation. Neuroimmunomodulation. 2021;28(4):248-254.
[34] GE X, GUO M, HU T, et al. Increased Microglial Exosomal miR-124-3p Alleviates Neurodegeneration and Improves Cognitive Outcome after rmTBI. Mol Ther. 2020;28(2):503-522.
[35] LI L, LI M. Astrocyte-derived extracellular vesicles inhibit the abnormal activation of immune function in neonatal mice with hypoxic-ischemic brain damage by carrying miR-124-3p. Neurol Res. 2023;45(12): 1079-1090.
[36] YAO B, ZHANG Q, YANG Z, et al. CircEZH2/miR-133b/IGF2BP2 aggravates colorectal cancer progression via enhancing the stability of m6A-modified CREB1 mRNA. Mol Cancer. 2022;21(1):140.
[37] BLAKES AJM, ENGLISH J, BANKA S, et al. A homozygous GRIN1 null variant causes a more severe phenotype of early infantile epileptic encephalopathy. Am J Med Genet A. 2022;188(2):595-599.
[38] PLATZER K, LEMKE JR. GRIN1-Related Neurodevelopmental Disorder. In: Adam MP, Feldman J, Mirzaa GM, Pagon RA, Wallace SE, Amemiya A, editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993–2024.
[39] RAGNARSSON L, ZHANG Z, DAS SS, et al. GRIN1 variants associated with neurodevelopmental disorders reveal channel gating pathomechanisms. Epilepsia. 2023;64(12):3377-3388.
[40] XU Y, SONG R, CHEN W, et al. Recurrent seizure-related GRIN1 variant: Molecular mechanism and targeted therapy. Ann Clin Transl Neurol. 2021;8(7):1480-1494.
[41] ZHANG D, RUAN J, PENG S, et al. Synaptic-like transmission between neural axons and arteriolar smooth muscle cells drives cerebral neurovascular coupling. Nat Neurosci. 2024;27(2):232-248. |