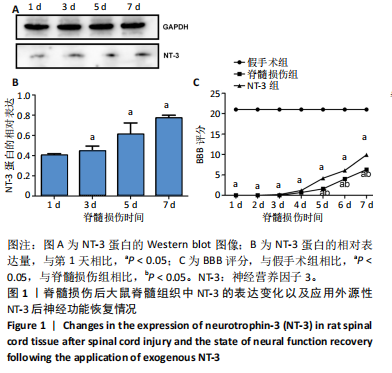
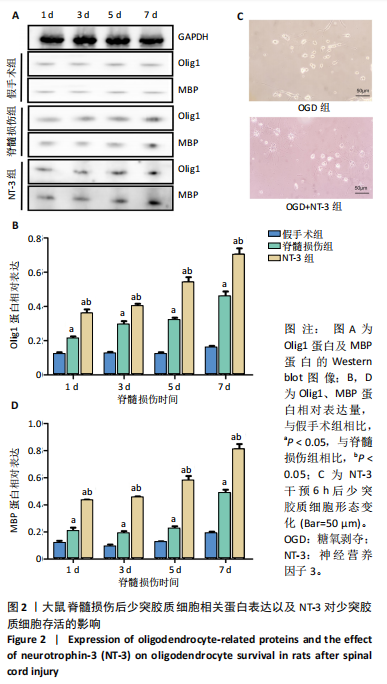
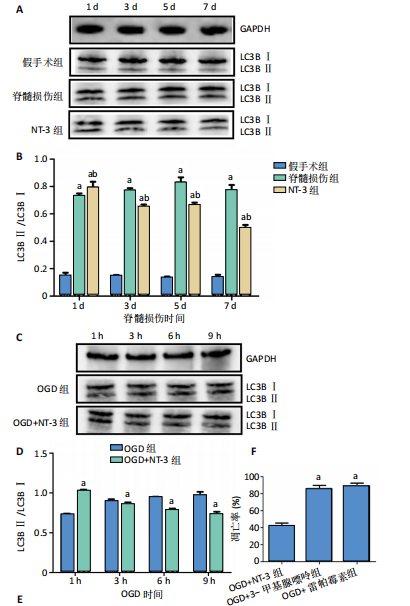
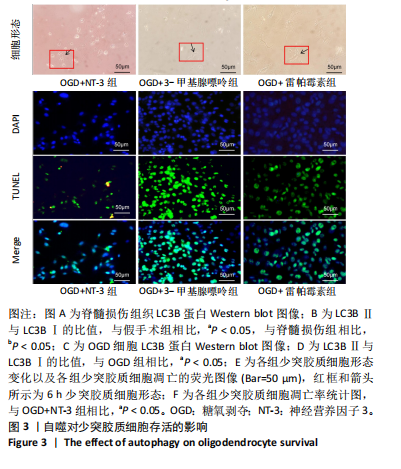
[1] DORRIAN RM, BERRYMAN CF, LAUTO A, et al. Electrical stimulation for the treatment of spinal cord injuries: A review of the cellular and molecular mechanisms that drive functional improvements. Front Cell Neurosci. 2023;17:1095259.
[2] PUKOS N, MARION CM, ARNOLD WD, et al. Chronic demyelination and myelin repair after spinal cord injury in mice: A potential link for glutamatergic axon activity. Glia. 2023;71(9):2096-2116.
[3] ALIZADEH A, KARIMI-ABDOLREZAEE S. Microenvironmental regulation of oligodendrocyte replacement and remyelination in spinal cord injury. J Physiol. 2016;594(13):3539-3552.
[4] FAN H, TANG HB, SHAN LQ, et al. Quercetin prevents necroptosis of oligodendrocytes by inhibiting macrophages/microglia polarization to M1 phenotype after spinal cord injury in rats. J Neuroinflammation. 2019;16(1):206.
[5] LI N, YAO M, LIU J, et al. Vitamin D Promotes Remyelination by Suppressing c-Myc and Inducing Oligodendrocyte Precursor Cell Differentiation after Traumatic Spinal Cord Injury. Int J Biol Sci. 2022; 18(14):5391-5404.
[6] KEEFE KM, SHEIKH IS, SMITH GM. Targeting Neurotrophins to Specific Populations of Neurons: NGF, BDNF, and NT-3 and Their Relevance for Treatment of Spinal Cord Injury. Int J Mol Sci. 2017;18(3):548.
[7] THOMAS AM, SEIDLITS SK, GOODMAN AG, et al. Sonic hedgehog and neurotrophin-3 increase oligodendrocyte numbers and myelination after spinal cord injury. Integr Biol (Camb). 2014;6(7):694-705.
[8] SMITH DR, DUMONT CM, PARK J, et al. Polycistronic Delivery of IL-10 and NT-3 Promotes Oligodendrocyte Myelination and Functional Recovery in a Mouse Spinal Cord Injury Model. Tissue Eng Part A. 2020;26(11-12):672-682.
[9] YAN H, WOOD PM. NT-3 weakly stimulates proliferation of adult rat O1(-)O4(+) oligodendrocyte-lineage cells and increases oligodendrocyte myelination in vitro. J Neurosci Res. 2000;62(3):329-335.
[10] KUMAR S, KAHN MA, DINH L, et al. NT-3-mediated TrkC receptor activation promotes proliferation and cell survival of rodent progenitor oligodendrocyte cells in vitro and in vivo. J Neurosci Res. 1998;54(6):754-765.
[11] AHUJA CS, MARTIN AR, FEHLINGS M. Recent advances in managing a spinal cord injury secondary to trauma. F1000Res. 2016;5:F1000 Faculty Rev-1017.
[12] FAN B, WEI Z, YAO X, et al. Microenvironment Imbalance of Spinal Cord Injury. Cell Transplant. 2018;27(6):853-866.
[13] RONG Y, LIU W, WANG J, et al. Neural stem cell-derived small extracellular vesicles attenuate apoptosis and neuroinflammation after traumatic spinal cord injury by activating autophagy. Cell Death Dis. 2019;10(5):340.
[14] LIU S, SARKAR C, DINIZO M, et al. Disrupted autophagy after spinal cord injury is associated with ER stress and neuronal cell death. Cell Death Dis. 2015;6(1):e1582.
[15] XU C, MAO L, TIAN H, et al. MICAL1 (molecule interacting with CasL 1) protects oligodendrocyte cells from oxidative injury through regulating apoptosis, autophagy in spinal cord injury. Neurosci Lett. 2021;750:135712.
[16] WALKER CL, WALKER MJ, LIU NK, et al. Systemic bisperoxovanadium activates Akt/mTOR, reduces autophagy, and enhances recovery following cervical spinal cord injury. PLoS One. 2012;7(1):e30012.
[17] LI W, YAO S, LI H, et al. Curcumin promotes functional recovery and inhibits neuronal apoptosis after spinal cord injury through the modulation of autophagy. J Spinal Cord Med. 2021;44(1):37-45.
[18] KAKOTY V, K C S, DUBEY SK, et al. Epigenetic regulation and autophagy modulation debilitates insulin resistance associated Alzheimer’s disease condition in rats. Metab Brain Dis. 2022;37(4):927-944.
[19] RICHNER M, ULRICHSEN M, ELMEGAARD SL, et al. Peripheral nerve injury modulates neurotrophin signaling in the peripheral and central nervous system. Mol Neurobiol. 2014;50(3):945-970.
[20] KIM MS, KIM GM, CHOI YJ, et al. TrkC promotes survival and growth of leukemia cells through Akt-mTOR-dependent up-regulation of PLK-1 and Twist-1. Mol Cells. 2013;36(2):177-184.
[21] LI P, SHI J, HE Q, et al. Streptococcus pneumoniae induces autophagy through the inhibition of the PI3K-I/Akt/mTOR pathway and ROS hypergeneration in A549 cells. PLoS One. 2015;10(3):e0122753.
[22] BARRETT GL. The p75 neurotrophin receptor and neuronal apoptosis. Prog Neurobiol. 2000;61(2):205-229.
[23] COSTANTINI C, ROSSI F, FORMAGGIO E, et al. Characterization of the signaling pathway downstream p75 neurotrophin receptor involved in beta-amyloid peptide-dependent cell death. J Mol Neurosci. 2005; 25(2):141-156.
[24] SUI X, KONG N, YE LI, et al. p38 and JNK MAPK pathways control the balance of apoptosis and autophagy in response to chemotherapeutic agents. Cancer letters. 2014;344(2):174-179.
[25] KARSY M, HAWRYLUK G. Modern Medical Management of Spinal Cord Injury. Curr Neurol Neurosci Rep. 2019;19(9):65.
[26] ALMAD A, SAHINKAYA FR, MCTIGUE DM. Oligodendrocyte fate after spinal cord injury. Neurotherapeutics. 2011;8(2):262-273.
[27] DUMONT RJ, OKONKWO DO, VERMA S, et al. Acute spinal cord injury, part I: pathophysiologic mechanisms. Clin Neuropharmacol. 2001;24(5):254-264.
[28] GONZALEZ A, MOYA-ALVARADO G, GONZALEZ-BILLAUT C, et al. Cellular and molecular mechanisms regulating neuronal growth by brain-derived neurotrophic factor. Cytoskeleton (Hoboken). 2016;73(10): 612-628.
[29] GORDON T. The physiology of neural injury and regeneration: The role of neurotrophic factors. J Commun Disord. 2010;43(4):265-273.
[30] BOYCE VS, MENDELL LM. Neurotrophic factors in spinal cord injury. Handb Exp Pharmacol. 2014;220:443-460.
[31] BRADBURY EJ, KING VR, SIMMONS LJ, et al. NT-3, but not BDNF, prevents atrophy and death of axotomized spinal cord projection neurons. Eur J Neurosci. 1998;10(10):3058-3068.
[32] DUNCAN GJ, MANESH SB, HILTON BJ, et al. The fate and function of oligodendrocyte progenitor cells after traumatic spinal cord injury. Glia. 2020;68(2):227-245.
[33] ZHANG C, QIU M, FU H. Oligodendrocytes in central nervous system diseases: the effect of cytokine regulation. Neural Regen Res. 2024; 19(10):2132-2143.
[34] MEKHAIL M, ALMAZAN G, TABRIZIAN M. Purine-crosslinked injectable chitosan sponges promote oligodendrocyte progenitor cells’ attachment and differentiation. Biomater Sci. 2015;3(2):279-287.
[35] KANNO H, OZAWA H, SEKIGUCHI A, et al. Induction of autophagy and autophagic cell death in damaged neural tissue after acute spinal cord injury in mice. Spine (Phila Pa 1976). 2011;36(22):E1427-1434.
[36] LI R, LI D, WU C, et al. Nerve growth factor activates autophagy in Schwann cells to enhance myelin debris clearance and to expedite nerve regeneration. Theranostics. 2020;10(4):1649-1677.
[37] XU B, FANG J, WANG J, et al. Inhibition of autophagy and RIP1/RIP3/MLKL-mediated necroptosis by edaravone attenuates blood spinal cord barrier disruption following spinal cord injury. Biomed Pharmacother. 2023;165:115165.
[38] TAVERNARAKIS N. Regulation and Roles of Autophagy in the Brain. Adv Exp Med Biol. 2020;1195:33.
[39] Mirzahosseini G, Ishrat T. Modulation of p75 neurotrophin receptor mitigates brain damage following ischemic stroke in mice. Neural Regen Res. 2024;19(10):2093-2094. |