中国组织工程研究 ›› 2025, Vol. 29 ›› Issue (1): 164-174.doi: 10.12307/2024.745
• 干细胞综述 stem cell review • 上一篇 下一篇
调整细胞培养条件提高间充质干细胞外泌体治疗骨关节炎的潜能
钱 燕,刘启颂
- 深圳国家感染性疾病临床医学研究中心,南方科技大学深圳市第三人民医院,广东省深圳市 518112
-
收稿日期:2023-10-30接受日期:2023-12-08出版日期:2025-01-08发布日期:2024-05-20 -
通讯作者:刘启颂,博士,深圳国家感染性疾病临床医学研究中心,南方科技大学深圳市第三人民医院,广东省深圳市 518112 -
作者简介:钱燕,女,1989年生,江苏省南京市人,汉族,2015年华中农业大学毕业,硕士,主要从事科研以及样本库管理工作。 -
基金资助:深圳市科创委基础研究专项(自然科学基金)(JCYJ20210324113401003),项目负责人: 刘启颂
Enhancing potential of mesenchymal stem cell exosomes for osteoarthritis by adjusting cell culture condition
Qian Yan, Liu Qisong
- National Clinical Research Center for Infectious Diseases, Shenzhen Third People’s Hospital, Southern University of Science and Technology, Shenzhen 518112, Guangdong Province, China
-
Received:2023-10-30Accepted:2023-12-08Online:2025-01-08Published:2024-05-20 -
Contact:Liu Qisong, MD, National Clinical Research Center for Infectious Diseases, Shenzhen Third People’s Hospital, Southern University of Science and Technology, Shenzhen 518112, Guangdong Province, China -
About author:Qian Yan, Master, National Clinical Research Center for Infectious Diseases, Shenzhen Third People’s Hospital, Southern University of Science and Technology, Shenzhen 518112, Guangdong Province, China -
Supported by:Shenzhen Science and Technology Plan Project, No. JCYJ20210324113401003 (to LQS)
摘要:
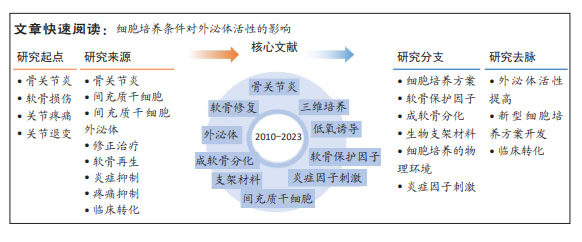
文题释义:
骨关节炎:是一种慢性关节疾病,主要病理特征是关节软骨破坏、骨质增生以及关节边缘的骨质增生(骨刺)。在全球范围内,骨关节炎是最常见的关节疾病之一,是关节疼痛和残疾的主要原因,然而现有临床策略仅能缓解骨关节炎的症状,不能实现疾病的修正治疗。
间充质干细胞外泌体:具有治疗骨关节炎的潜能,其能减轻炎症反应,促进软骨细胞的增殖和软骨基质的合成,抑制软骨细胞的凋亡和软骨基质的降解,实现软骨组织的修复和再生,具有临床转化潜能。
背景:间充质干细胞外泌体有望发展成为治疗骨关节炎的无细胞疗法,而通过调整细胞培养条件,可以进一步提高其治疗活性和临床转化潜能。
目的:综述分析细胞培养条件对外泌体活性的影响,分析其临床转化潜力。
方法:查阅国内外有关骨关节炎/软骨损伤修复与间充质干细胞来源外泌体的文献。检索词分别为“外泌体,间充质干细胞,骨关节炎,软骨修复”和“exosomes,extracellular vesicles,mesenchymal stem cells,osteoarthritis,cartilage repair”,检索数据库分别为PubMed和中国知网数据库,检索时限为2010-2023年,最后纳入100篇文献进行总结和分析。
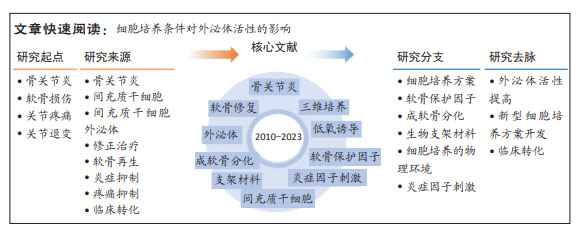
结果与结论:①在间充质干细胞的培养过程中,提供有利于软骨细胞生长的物理环境,或添加软骨保护小分子、成软骨诱导因子和炎症因子等刺激细胞,可以进一步提高间充质干细胞外泌体在维持软骨细胞表型、促进软骨再生和免疫抑制等方面的调控活性;因此,其他具有相似特征的物理或化学因素,也有可能提升间充质干细胞外泌体治疗骨关节炎的疗效。②低氧诱导、脉冲电磁场刺激、生物反应器及软骨保护分子(如人甲状旁腺素1-34)刺激等细胞培养方案具有安全、可规模化生产等优点,可用于制备临床用途的、疗效好的间充质干细胞外泌体。③间充质干细胞外泌体有望实现骨关节炎的修正治疗,通过调整细胞培养方案提升治疗活性,可进一步提高其临床转化的可行性。
https://orcid.org/0000-0003-3379-5308 (刘启颂)
中国组织工程研究杂志出版内容重点:干细胞;骨髓干细胞;造血干细胞;脂肪干细胞;肿瘤干细胞;胚胎干细胞;脐带脐血干细胞;干细胞诱导;干细胞分化;组织工程
中图分类号:
引用本文
钱 燕, 刘启颂. 调整细胞培养条件提高间充质干细胞外泌体治疗骨关节炎的潜能[J]. 中国组织工程研究, 2025, 29(1): 164-174.
Qian Yan, Liu Qisong. Enhancing potential of mesenchymal stem cell exosomes for osteoarthritis by adjusting cell culture condition[J]. Chinese Journal of Tissue Engineering Research, 2025, 29(1): 164-174.
引起骨关节炎的因素很多,包括衰老、肥胖、损伤及使用过度等,这些因素导致软骨损伤、软骨下骨重塑、滑膜炎症激活、关节周围组织(结缔组织和肌肉组织)和软组织(韧带、肌腱和半月板)退化,最终引起全关节损伤和功能障碍[11]。骨关节炎的发病分子机制涉及多种细胞和调控通路,如软骨细胞凋亡、软骨下骨的机械改变或旁分泌、炎性滑膜成纤维细胞和髌下脂肪垫分泌的细胞因子等均能造成软骨组织的退化,相关的调控分子如蛋白质水解/代谢酶、炎性细胞因子、转化生长因子β和miRNA等,都能促进骨关节炎的发展[11]。
软骨组织退化后,软骨细胞受限于细胞外基质,不能迁移到破损位置实现修复;同时软骨组织没有血供,导致软骨细胞的增殖能力也有限。因此一旦发生退化,软骨组织无法自行修复,这导致骨关节炎成为了一种难以治愈的疾病。目前临床上没有治愈骨关节炎的方法,主要通过全关节置换、透明质酸及非类固醇抗炎药等缓解疼痛等症状。随着细胞治疗的兴起,自体软骨细胞移植和基质辅助的自体细胞移植方法被用于软骨缺损等骨关节疾病的治疗,这些方法通过将体外扩增的自体软骨细胞植入关节腔内,以期通过补充软骨细胞来实现组织再生,但该方法存在软骨细胞来源有限、需二次手术、体外扩增软骨细胞易去分化等缺点,此外治疗后新生的组织是纤维软骨,不具备透明软骨的相应特征。因此,以软骨细胞为基础的治疗方案逐渐被间充质干细胞疗法所取代[12]。
2.2 间充质干细胞治疗骨关节炎概述 间充质干细胞可以定向分化为软骨细胞,具有增殖能力强及来源广泛等优点,研究证明关节腔注射间充质干细胞可以在缺损部位再生出透明软骨,因此间充质干细胞成为了治疗骨关节疾病的理想种子细胞。多种不同来源的间充质干细胞已成功进入治疗骨关节炎的临床试验阶段,随访结果显示间充质干细胞具有积极的骨关节炎治疗效果[13]。此外,2012年韩国开发了以脐血间充质干细胞为种子细胞的软骨修复产品Cartistem,该产品展现了良好的临床骨关节炎治疗效果[14-15]。
基础研究表明,间充质干细胞在体内外特定条件下被诱导分化为软骨细胞,可在软骨缺损部位发挥修复作用,并增殖分化为功能性软骨或软骨样组织,如XU等[16]通过使用外泌体靶向递送Kartogenin进入滑膜液间充质干细胞,促进其体内外成软骨分化,实现骨关节炎治疗。除分化潜能外,间充质干细胞的旁分泌调控功能也在促软骨修复中发挥着关键的作用,如JIA等[17]发现,未分化的间充质干细胞比体外预成软骨分化的间充质干细胞具有更好的透明软骨再生效果,关节腔注射的间充质干细胞通过向周围环境分泌可溶性因子和外泌体,可以调控免疫微环境,并促进细胞的增殖、迁移及合成代谢等,从而实现软骨组织的再生。
2.3 间充质干细胞分泌的外泌体治疗骨关节炎概述 间充质干细胞分泌的外泌体是间充质干细胞旁分泌调控中研究最为广泛的因子,与间充质干细胞相比,间充质干细胞分泌的外泌体更稳定,且易于制备、储存和运输,并有免疫原性低、非致瘤性、临床安全性高和伦理风险低等优势。无论是基础研究还是临床应用,间充质干细胞分泌的外泌体相对来说都更有优势。
在间充质干细胞分泌的外泌体的基础研究领域中,ZHANG等[6]于2016年首次发表了胚胎干细胞来源的间充质干细胞分泌的外泌体具有促进软骨缺损修复功能的研究结果,随后,大量研究表明多种不同组织来源的间充质干细胞分泌的外泌体均具有治疗骨关节炎或软骨缺损的潜能[18]。而一些相关的临床试验目前也在进行当中,如智利安第斯大学正在开展一项临床Ⅰ期研究,探讨关节腔注射同种异体来源间充质干细胞对轻度到中度骨关节炎患者的治疗效果。
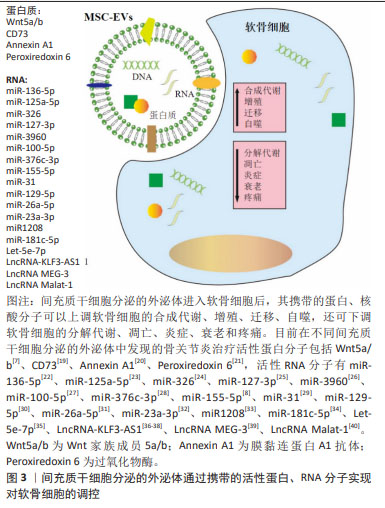
在关节腔中,间充质干细胞分泌的外泌体可以通过其携带的蛋白和核酸分子,实现对软骨细胞、巨噬细胞、滑膜细胞等的调控,见图3。总之,目前在不同间充质干细胞分泌的外泌体中发现的骨关节炎治疗活性蛋白分子包括Wnt5a/b[7]、CD73[19]、Annexin A1[20]、Peroxiredoxin 6[21],活性RNA分子有miR-136-5p[22]、miR-125a-5p[23]、miR-326[24]、miR-127-3p[25]、miR-3960[26]、miR-100-5p[27]、miR-376c-3p[28]、miR-155-5p[8]、miR-31[29]、miR-129-5p[30]、miR-26a-5p[31]、miR-23a-3p[32]、miR1208[33]、miR-181c-5p[34]、Let-5e-7p[35]、LncRNA-KLF3-AS1[36-38]、LncRNA MEG-3[39]、LncRNA Malat-1等[40],这些分子可以调控软骨细胞中的重要通路,如YAP[7]、AKT/ERK[19]、mTOR等[27],来提高软骨细胞增殖、合成、迁移及自噬水平,或降低软骨细胞凋亡、衰老及分解等,或调控巨噬细胞的炎症响应情况等,最终实现骨关节炎的抑制或逆转,除此以外,间充质干细胞分泌的外泌体也可通过调控周围感觉神经元过度兴奋来缓解骨关节炎的疼痛[41]。
尽管间充质干细胞分泌的外泌体在骨关节炎动物模型中广泛显示出了有效性,但也有部分研究数据表明有些间充质干细胞分泌的外泌体并无骨关节炎治疗效果,或者疗效不显著。如ARéVALO-TURRUBIARTE等[42]发现来源于马滑膜液间充质干细胞分泌的外泌体并不能有效抑制软骨细胞中由白细胞介素1β刺激产生的金属基质蛋白酶13。由于外泌体是细胞的分泌产物,其活性受细胞来源、细胞培养条件、纯化方法等影响,而合理调整细胞培养条件能有效提高间充质干细胞分泌的外泌体治疗活性。
2.4 细胞培养条件对间充质干细胞分泌的外泌体治疗骨关节炎潜能的影响 外泌体是细胞分泌的具有膜结构的小囊泡,其特征由母细胞的特征和状态所决定,而细胞培养条件能够显著影响细胞的状态。因此,在制备间充质干细胞或间充质干细胞分泌的外泌体用于骨关节炎治疗时,可以在细胞培养过程中,引入一些有利于软骨细胞的因素,来提高其治疗活性。图4归纳了通过调整细胞培养方案提升间充质干细胞分泌的外泌体治疗骨关节炎潜能的相关研究[43-58]。在培养基中加入一些具有关节软骨保护功能的分子,可以提高间充质干细胞分泌的外泌体的骨关节炎治疗活性;同时,成软骨分化后的间充质干细胞也能分泌有利于软骨修复的外泌体;此外,在体内软骨细胞处于无血管的低氧环境中,同时软骨细胞生长在致密的细胞外基质中,因此支架辅助/三维培养和低氧等的培养环境有利于软骨细胞的基质合成,也有利于间充质干细胞的成软骨分化;间充质干细胞的免疫调控功能还会受到炎性因子的激活。

2.4.1 软骨保护因子刺激 软骨保护因子可以通过调节细胞信号通路,来促进软骨细胞的增殖和基质合成,或者抑制炎性软骨细胞的凋亡和基质代谢。相应的信号通路也可能在间充质干细胞被激活或抑制,最终改变间充质干细胞分泌的外泌体的活性成分。
转化生长因子β家族蛋白在软骨细胞中发挥重要的调控功能,能够有效促进软骨细胞的生长和基质合成,临床试验表明高表达转化生长因子β1的软骨细胞可用于骨关节炎治疗[59]。使用转化生长因子β刺激间充质干细胞,可以显著提高其分泌外泌体的调控功能[43-44,60-62]。WANG等[44,60-61]使用转化生长因子β1刺激间充质干细胞,所得的间充质干细胞分泌的外泌体高表达miR-135b,能够下调软骨细胞中特异性蛋白1,促进软骨细胞增殖;能够靶向滑膜巨噬细胞中的MAPK6,抑制炎症;抑制破骨细胞中的MAPK通路,抑制破骨细胞形成,从而阻止软骨退化、阻止异常的血管生成、缓解疼痛,最终促进软骨修复。
Kartogenin (KGN)是2012年科学家筛选出的具有软骨保护功能的小分子,它能通过调控CBFβ-RUNX1通路诱导间充质干细胞的成软骨分化[63],因此KGN处理也能提升间充质干细胞分泌的外泌体的骨关节炎治疗活性[45,64-65]。LIU等[45]使用KGN处理细胞48 h,发现处理组间充质干细胞分泌的外泌体能更好地维持软骨细胞的表型,相较于对照间充质干细胞分泌的外泌体,处理组间充质干细胞分泌的外泌体不抑制RUNX1,这可能是KGN处理提升间充质干细胞分泌的外泌体治疗活性的内在分子机制;同时处理组间充质干细胞分泌的外泌体还能有效降低骨关节炎软骨组织中Ⅰ型胶原的表达。
姜黄素是一种多酚类化合物,具有抗氧化作用,可抑制骨关节炎的发生和发展。有研究指出,骨关节炎患者每日摄取姜黄素,可有效缓解疼痛、改善行动能力[66]。由姜黄素处理间充质干细胞后分泌的外泌体可以促进软骨细胞的增殖、抑制凋亡,并有效缓解骨关节炎病变[46],机制研究发现姜黄素处理间充质干细胞后分泌的外泌体高表达miR-124和miR-143,分别靶向抑制核因子激活的B细胞的κ-轻链增强和ROCK1/TLR9信号通路,从而抑制软骨降解。
人甲状旁腺素1-34[parathyroid hormone (1-34),PTH (1-34)]是一种治疗骨质疏松症的多肽类激素,研究表明PTH (1-34)通过调节PI3K/AKT信号通路,促进软骨细胞增殖和基质合成,抑制软骨降解,具有骨关节炎治疗潜力[67]。使用PTH (1-34)预处理的间充质干细胞所分泌的外泌体通过抑制炎症因子的产生,显现出了更强的抑炎活性,并促进软骨细胞的增殖、迁移和基质合成[47]。
软骨保护因子刺激的方法通过使用活性分子刺激间充质干细胞,其细胞培养液的成分相对简单,处理时间较短。使用的活性分子中,PTH(1-34)是临床药物,姜黄素是食物提取物,安全性好,可用于制备临床用途的间充质干细胞分泌的外泌体。此外,褪黑素、淫羊藿苷和二甲双胍等也被报道具有骨关节炎治疗活性[68-70]。因此,在间充质干细胞细胞培养过程中,加入软骨保护因子进行刺激,可有效提升间充质干细胞分泌的外泌体的骨关节炎疗效[71-75],具体相关研究进展见表1。
2.4.2 成软骨诱导间充质干细胞 在特定培养条件下,间充质干细胞可以定向诱导分化为脂肪、骨及软骨等细胞,在细胞定向分化过程中,其分泌的外泌体性质也会发生变化。WANG等[71]发现,不同分化阶段间充质干细胞产生的外泌体具有不同的生物学特征,分化阶段末期分泌的外泌体具有分化诱导功能。因此,对间充质干细胞进行成软骨诱导,也是一种提高间充质干细胞分泌的外泌体骨关节炎治疗活性的有效方法[72-78],见表2。

与软骨细胞共培养,是一种有效的间充质干细胞成软骨诱导方案,在共培养体系中,软骨细胞与间充质干细胞互相调控,诱导效率高,并能有效抑制肥大化[72]。因此,与软骨细胞共培养也是一种提高间充质干细胞分泌的外泌体骨关节炎治疗活性的策略。HOSSEINZADEH等[73]将不同比例的间充质干细胞和软骨细胞共培养,所得的间充质干细胞分泌的外泌体能促进间充质干细胞的成软骨分化,提高共培养体系中软骨细胞的比例(4∶1)可进一步提高促软骨分化能力和骨关节炎治疗活性。ESMAEILI等[49]使用三维生物反应器共培养软骨细胞和间充质干细胞,提高了间充质干细胞分泌的外泌体的产量,还进一步加强了其骨关节炎治疗活性。
除与软骨细胞共培养外, 转化生长因子β3也是一种常用的成软骨诱导因子,转化生长因子β3可以通过Smad通路上调SOX9的表达,从而促进细胞外基质Ⅱ型胶原等的表达[74]。MAO等[48]使用含转化生长因子β3的成软骨诱导培养基诱导间充质干细胞,所得的间充质干细胞分泌的外泌体高表达miR-92a-3p,而miR-92a-3p可以通过靶向软骨细胞中的Wnt5a促进间充质干细胞增殖、促进软骨细胞的基质合成、抑制动物模型中的骨关节炎进展。此外,成软骨诱导也能使间充质干细胞分泌的外泌体中高表达miR-320c、circRNA_0001236和miR-455等具有软骨保护功能的RNA分子[75-77]。CASANOVA等[78]还发现,成软骨诱导会改变间充质干细胞分泌的外泌体的蛋白表达谱,从而提高其成软骨诱导能力。
原弹性蛋白是一种可变剪切的细胞外基质蛋白,它可以促进间充质干细胞的黏附、增殖、表型维持、成软骨分化,还可以通过促进间充质干细胞的关节腔黏附和迁移来抑制骨关节炎的进展[79]。MENG等[50]发现使用原弹性蛋白处理间充质干细胞,可以提高间充质干细胞分泌的外泌体中miR-451-5p的表达水平,并提高软骨细胞的基质合成水平和骨关节炎治疗活性。
综上所述,对间充质干细胞进行成软骨诱导,可以显著提高间充质干细胞分泌的外泌体的骨关节炎治疗活性。但成软骨诱导过程需使用复杂的诱导条件,且诱导时间较长,不利于临床转化。 2.4.3 应用生物支架材料 在生理状态下,软骨细胞生长在细胞外基质中,与细胞外基质之间的相互作用有利于维持其表型[80]。由软骨细胞外基质构成的支架材料还能诱导间充质干细胞成软骨分化,ZHENG等[81]发现,基于胶原蛋白的水凝胶可以诱导间充质干细胞的体内成软骨分化。因此,将间充质干细胞培养在生物材料支架上,也可提高其间充质干细胞分泌的外泌体的调控功能[51-52],见表3。
生物支架材料培养间充质干细胞可以通过提高间充质干细胞分泌的外泌体表面特定受体的表达,来提高受体细胞对间充质干细胞分泌的外泌体的吸收[51]。RAGNI等[51]使用透明质酸涂层的培养皿培养间充质干细胞,得到的间充质干细胞分泌的外泌体高表达CD44(透明质酸受体),间充质干细胞分泌的外泌体上的CD44可以通过与成纤维样滑膜细胞表面自然分泌的透明质酸识别,从而提高细胞对间充质干细胞分泌的外泌体的摄取,最终实现骨关节炎治疗。
脱细胞细胞外基质是由胶原和非胶原蛋白构成的纤维网状支架,它通过模拟细胞外基质环境可以促进间充质干细胞增殖和成软骨分化,因此受到软骨组织工程领域的瞩目[82]。脱细胞细胞外基质也可以提高间充质干细胞的旁分泌功能,ZHANG等[52]发现使用脱细胞细胞外基质支架培养的间充质干细胞分泌的外泌体,可以更有效地促进骨关节炎样软骨细胞的增殖、合成、迁移和凋亡抑制,并促进骨关节炎小鼠模型中的软骨再生,机制研究发现脱细胞细胞外基质培养可以提高间充质干细胞分泌的外泌体中的miR-3473b,实现软骨细胞中PTEN/AKT信号通路激活。
生物材料支架通过模拟细胞微环境,可以提高间充质干细胞的增殖和定向分化能力,是软骨组织工程的三要素之一。而上述研究结果表明,支架材料还可用于制备高骨关节炎治疗潜能的间充质干细胞分泌的外泌体,这进一步拓宽了生物支架材料的应用范围。不过,目前用于临床骨关节炎或软骨缺损治疗的支架材料造价高,不利于大规模制备和成本控制,因此临床转化潜能有限。
2.4.4 不同的物理培养条件对间充质干细胞分泌的外泌体活性的影响 软骨细胞生长在致密无血管的软骨组织中,因此,在体外培养时使用细胞微团及低氧等方式培养的软骨细胞更能维持正常活性和功能[83-84]。此外,正常的软骨组织在关节活动中,会遭受到各种应力的作用,如剪切力、压力等,因此适当的力学刺激也能促进软骨细胞的机制合成[85]。此外,脉冲磁场等物理刺激被发现具有促进骨、软骨再生的潜能[86]。而相应地,这些物理刺激作用于间充质干细胞,也能促进其成软骨分化[85,87],提高间充质干细胞分泌的外泌体的骨关节炎治疗活性。
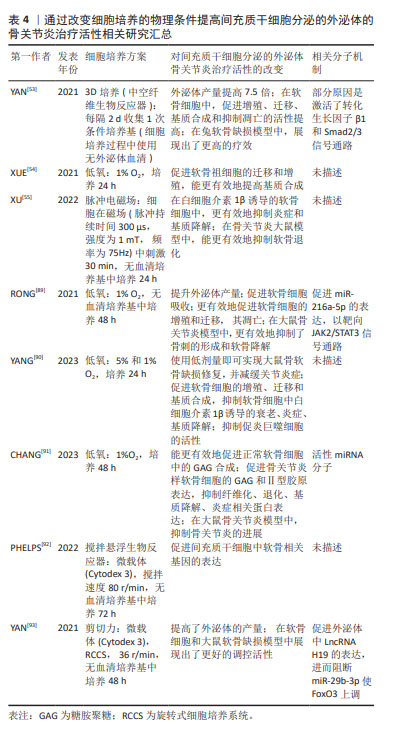
研究表明,通过低氧(1%-5%O2)刺激间充质干细胞,能提高间充质干细胞的增殖潜能和旁分泌功能,而对于软骨细胞来说,低氧刺激可以诱导表达缺氧诱导因子1,调控自噬等通路抑制软骨细胞的退化[84]。使用低氧刺激间充质干细胞,能够有效提高间充质干细胞分泌的外泌体的治疗活性,包括心肌损伤[2]、脊髓损伤[88]、骨关节炎等[54,89-91]。多项研究表明,低氧培养条件下制备所得的间充质干细胞分泌的外泌体能更有效地促进软骨细胞的增殖和合成、抑制凋亡和分解[54,89-91]。一些活性miRNA分子被认为是低氧间充质干细胞分泌的外泌体活性提高的原因,如RONG等[89]发现低氧刺激下,间充质干细胞高表达缺氧诱导因子1α,促使其分泌的外泌体高表达miR-216a-5p,miR-216a-5p靶向抑制JAK2,从而调控JAK2/STAT3信号通路实现其对软骨细胞的调控作用。YANG等[90]证实低氧条件下,间充质干细胞的旁分泌调控功能显著提高,而间充质干细胞分泌的外泌体在其中发生关键作用。
生理状态下,软骨细胞生长在有细胞外基质构成的三维支架中,而关节处于持续活动中,因此3D培养、支架材料、适当的应力刺激等对软骨细胞维持正常功能和间充质干细胞成软骨分化都是有利因素。3D细胞培养生物反应器通过使用支架载体实现3D培养,并通过搅拌和旋转等对细胞产生应力刺激[92-93],相比二维培养可以更准确地模拟细胞在体内的生理环境,不仅能满足规模化生产的需求,还能提高细胞或细胞产物的治疗活性。3D生物反应器也可用于提高间充质干细胞分泌的外泌体的骨关节炎治疗活性,如YAN等[53]使用中空纤维支持生物反应器培养间充质干细胞,其分泌外泌体的产量提高了7.5倍,其软骨调控活性也显著增加,在兔软骨缺损模型中也显现出了更有效的修复效果,进一步的机制研究表明,3D生物反应器培养所得的间充质干细胞分泌的外泌体可以激活转化生长因子β1和Smad2/3 信号通路,实现其调控功能的提升。PHELPS等[92]使用搅拌悬浮生物反应器培养间充质干细胞,所得的间充质干细胞分泌的外泌体可促进间充质干细胞中软骨相关基因的表达;通过模拟微重力而开发的旋转细胞培养系统(Rotatory cell culture system,RCCS)通过细胞产生应力刺激来改变细胞的状态,使用RCCS培养间充质干细胞,不仅能提高间充质干细胞分泌的外泌体的产量,还能提高其对骨关节炎软骨细胞的调控活性,进一步的机制研究发现,RCCS能够上调间充质干细胞分泌的外泌体中的LncH19的表达水平,通过阻断骨关节炎软骨细胞中的miR-29b-3p使FoxO3上调,从而实现功能提升。
除模拟软骨细胞生理状态的物理刺激外,脉冲式磁场刺激可用于临床骨关节炎治疗,刺激软骨组织再生[86]。因此,当使用脉冲式磁场刺激间充质干细胞时,所得的间充质干细胞分泌的外泌体能更有效地抑制软骨细胞和软骨组织的退化。
使用低氧、生物反应器、应力或磁场刺激能提高间充质干细胞分泌的外泌体的骨关节炎治疗活性;与使用化学分子或细胞因子刺激相比,改变这些物理条件不会在最终的间充质干细胞分泌的外泌体产物中引入额外的因子,相对来说是一种更为清洁的、可提升间充质干细胞分泌的外泌体活性的方法;同时生物反应器是规模化生产间充质干细胞分泌的外泌体的一种培养方式,因此,上述方法均具有巨大的临床应用前景。相关的细胞培养方案也被用于制备临床用途的间充质干细胞[94-95],FREITAG等[95]使用低氧的方法培养间充质干细胞,用于骨关节炎治疗,通过30例临床患者验证了间充质干细胞治疗骨关节炎的有效性。
因此,通过改变细胞培养的物理条件提高间充质干细胞分泌的外泌体的骨关节炎治疗活性,见表4。
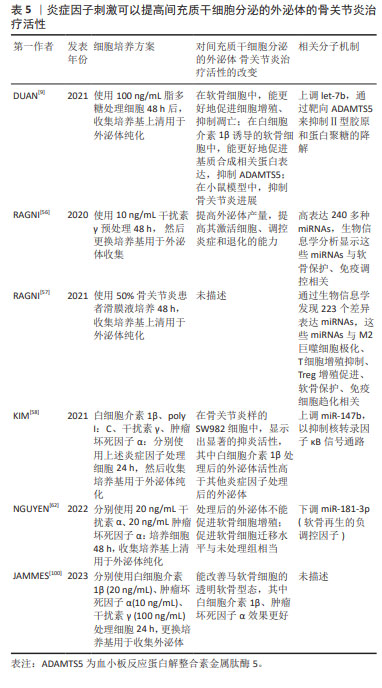
2.4.5 炎症因子刺激 间充质干细胞的调控功能受到组织来源和炎性环境的影响,如肿瘤坏死因子α、白细胞介素1β和γ干扰素等促炎因子可以激活间充质干细胞的免疫抑制表型,参与组织再生[96-97]。同样,间充质干细胞的旁分泌潜能也会受到炎性环境的影响,炎症因子刺激间充质干细胞分泌的外泌体中,蛋白和RNA组成发生了显著的改变;同时,炎症刺激还会促进外泌体的分泌[98]。
在骨关节炎生理状态下,关节腔内的间充质干细胞在炎性环境的刺激下,可以释放一些与合成代谢、抗纤维化、抗凋亡、免疫抑制等相关的因子,从而减缓关节退化[99],因此,使用骨关节炎滑膜液培养间充质干细胞,可以刺激产生促修复效果更好的外泌体。RAGNI等[57]使用含50% 骨关节炎滑膜液的培养液培养间充质干细胞,所获的外泌体较未处理组差异表达223个miRNAs,这些miRNAs与M2巨噬细胞极化、T细胞增殖抑制、Treg增殖促进、软骨保护及免疫细胞趋化相关,显现出治疗骨关节炎的潜力。
使用炎症因子刺激间充质干细胞,可以提高间充质干细胞的产量[56],也可以通过改变间充质干细胞分泌的外泌体的蛋白、RNA组成提高其抑制免疫和促进再生的功能[56,58,100]。干扰素γ是一种多效细胞因子,具有免疫调节、抗病毒及抗肿瘤活性,使用干扰素γ处理间充质干细胞,可以提高间充质干细胞分泌的外泌体的产量,并提高外泌体中与免疫调控、软骨保护相关miRNAs的表达水平[56]。除干扰素γ外,白细胞介素1β及肿瘤坏死因子α也被用于刺激间充质干细胞,提高间充质干细胞分泌的外泌体调控活性[58,100]。如KIM等[58]分别使用白细胞介素1β、干扰素γ及肿瘤坏死因子α刺激间充质干细胞,产生的间充质干细胞分泌的外泌体具有显著提高的抑炎活性,机制研究炎症因子的刺激可以上调间充质干细胞分泌的外泌体中的miR-147b,从而靶向软骨细胞中的核转录因子κB信号通路,实现炎症抑制;动物实验证明,由白细胞介素1β及干扰素γ、肿瘤坏死因子α刺激产生的间充质干细胞分泌的外泌体可再生透明软骨[100]。但炎性因子刺激并不一定会提高间充质干细胞分泌的外泌体的调控功能,如NGUYEN 等[62]发现干扰素α、肿瘤坏死因子α刺激产生的间充质干细胞分泌的外泌体虽然能下调间充质干细胞分泌的外泌体中的软骨负调控因子miR-181-3p,但细胞实验表明,该间充质干细胞分泌的外泌体并不能有效促进软骨细胞的增殖、迁移和基质合成。
除炎症因子外,脂多糖、poly I:C等免疫激活因子,也能刺激间充质干细胞,提高间充质干细胞分泌的外泌体的治疗活性。KIM等[58]发现,poly I:C与白细胞介素1β、干扰素γ及肿瘤坏死因子α等因子的刺激效果类似,可以上调间充质干细胞分泌的外泌体中的miR-147b,从而通靶向软骨细胞中的核转录因子κB信号通路抑制炎症。使用脂多糖刺激间充质干细胞后分泌的外泌体能更有效地促进软骨细胞增殖和基质合成、抑制其凋亡和分解代谢,抑制骨关节炎进展[9]。
炎症因子等的刺激,可以提高间充质干细胞分泌的外泌体的免疫调节和促修复作用,但这些方法进行临床转化存在一定困难:炎症因子价格较为昂贵,增加生产成本;受现有外泌体纯化方案的限制,引入培养液的炎症因子等,可能会残留于最终的间充质干细胞分泌的外泌体中,引起副反应;从相关文献总结可以看出,炎症因子刺激不一定能提高间充质干细胞分泌外泌体的调控活性,说明这种刺激产生的活性提升效果并不稳定,于临床转化不利。
因此,由炎症因子刺激产生的间充质干细胞分泌的外泌体也展现出了更高的骨关节炎治疗活性,见表5。
| [1] LOHMANDER LS, ROOS EM. Clinical update: treating osteoarthritis. Lancet. 2007;370(9605):2082-2084. [2] SONG Y, WANG B, ZHU X, et al. Human umbilical cord blood-derived MSCs exosome attenuate myocardial injury by inhibiting ferroptosis in acute myocardial infarction mice. Cell Biol Toxicol. 2021;37(1):51-64. [3] MIANEHSAZ E, MIRZAEI HR, MAHJOUBIN-TEHRAN M, et al. Mesenchymal stem cell-derived exosomes: a new therapeutic approach to osteoarthritis? Stem Cell Res Ther. 2019;10(1):340. [4] MATHIEU M, MARTIN-JAULAR L, LAVIEU G, et al. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat Cell Biol. 2019;21(1):9-17. [5] XIANG XN, ZHU SY, HE HC, et al. Mesenchymal stromal cell-based therapy for cartilage regeneration in knee osteoarthritis. Stem Cell Res Ther. 2022;13(1):14. [6] ZHANG S, CHU WC, LAI RC, et al. Exosomes derived from human embryonic mesenchymal stem cells promote osteochondral regeneration. Osteoarthr Cartilage. 2016;24(12):2135-2140. [7] TAO SC, YUAN T, ZHANG YL, et al. Exosomes derived from miR-140-5p-overexpressing human synovial mesenchymal stem cells enhance cartilage tissue regeneration and prevent osteoarthritis of the knee in a rat model. Theranostics. 2017;7(1):180-95. [8] WANG Z, YAN K, GE G, et al. Exosomes derived from miR-155-5p-overexpressing synovial mesenchymal stem cells prevent osteoarthritis via enhancing proliferation and migration, attenuating apoptosis, and modulating extracellular matrix secretion in chondrocytes. Cell Biol Toxicol. 2021;37(1):85-96. [9] DUAN A, SHEN K, LI B, et al. Extracellular vesicles derived from LPS-preconditioned human synovial mesenchymal stem cells inhibit extracellular matrix degradation and prevent osteoarthritis of the knee in a mouse model. Stem Cell Res Ther. 2021;12(1):427. [10] QUICKE JG, CONAGHAN PG, CORP N, et al. Osteoarthritis year in review 2021: epidemiology & therapy. Osteoarthr Cartilage. 2022;30(2):196-206. [11] ABRAMOFF B, CALDERA FE. Osteoarthritis: pathology, diagnosis, and treatment options. Med Clin North Am. 2020;104(2):293-311. [12] FOLDAGER CB. Advances in autologous chondrocyte implantation and related techniques for cartilage repair. Dan Med J. 2013;60(4):B4600. [13] MATAS J, ORREGO M, AMENABAR D, et al. Umbilical cord-derived mesenchymal stromal cells (MSCs) for knee osteoarthritis: repeated MSC dosing is superior to a single MSC dose and to hyaluronic acid in a controlled randomized phase I/II trial. Stem Cell Transl Med. 2019; 8(3):215-224. [14] PARK YB, HA CW, LEE CH, et al. Cartilage regeneration in osteoarthritic patients by a composite of allogeneic umbilical cord blood-derived mesenchymal stem cells and hyaluronate hydrogel: results from a clinical trial for safety and proof-of-concept with 7 years of extended follow-up. Stem Cell Transl Med. 2017;6(2):613-621. [15] LIM HC, PARK YB, HA CW, et al. Allogeneic umbilical cord blood-derived mesenchymal stem cell implantation versus microfracture for large, full-thickness cartilage defects in older patients: a multicenter randomized clinical trial and extended 5-year clinical follow-up. Orthop J Sports Med. 2021;9(1):2325967120973052. [16] XU X, LIANG Y, LI X, et al. Exosome-mediated delivery of kartogenin for chondrogenesis of synovial fluid-derived mesenchymal stem cells and cartilage regeneration. Biomaterials. 2021;269:120539. [17] JIA Z, LIU Q, LIANG Y, et al. Repair of articular cartilage defects with intra-articular injection of autologous rabbit synovial fluid-derived mesenchymal stem cells. J Transl Med. 2018;16(1):123. [18] KIM YG, CHOI J, KIM K. Mesenchymal stem cell-derived exosomes for effective cartilage tissue repair and treatment of osteoarthritis. Biotechnol J. 2020;15(12):e2000082. [19] ZHANG S, CHUAH SJ, LAI RC, et al. MSC exosomes mediate cartilage repair by enhancing proliferation, attenuating apoptosis and modulating immune reactivity. Biomaterials. 2018;156:16-27. [20] TOFIÑO-VIAN M, GUILLÉN MI, PÉREZ DEL CAZ MD, et al. Microvesicles from human adipose tissue-derived mesenchymal stem cells as a new protective strategy in osteoarthritic chondrocytes. Cell Physiol Biochem. 2018;47(1):11-25. [21] GUILLÉN MI, TOFIÑO-VIAN M, SILVESTRE A, et al. Role of peroxiredoxin 6 in the chondroprotective effects of microvesicles from human adipose tissue-derived mesenchymal stem cells. J Orthop Transl. 2021; 30:61-69. [22] CHEN X, SHI Y, XUE P, et al. Mesenchymal stem cell-derived exosomal microRNA-136-5p inhibits chondrocyte degeneration in traumatic osteoarthritis by targeting ELF3. Arthritis Res Ther. 2020;22(1):256. [23] XIA Q, WANG Q, LIN F, et al. miR-125a-5p-abundant exosomes derived from mesenchymal stem cells suppress chondrocyte degeneration via targeting E2F2 in traumatic osteoarthritis. Bioengineered. 2021; 12(2):11225-11238. [24] XU H, XU B. BMSC-derived exosomes ameliorate osteoarthritis by inhibiting pyroptosis of cartilage via delivering miR-326 targeting HDAC3 and STAT1//NF-κB p65 to chondrocytes. Mediat Inflamm. 2021; 2021:9972805. [25] DONG J, LI L, FANG X, et al. Exosome-encapsulated microRNA-127-3p released from bone marrow-derived mesenchymal stem cells alleviates osteoarthritis through regulating CDH11-mediated Wnt/β-catenin pathway. J Pain Res. 2021;14:297-310. [26] YE P, MI Z, WEI D, et al. miR-3960 from mesenchymal stem cell-derived extracellular vesicles inactivates SDC1/Wnt/β-catenin axis to relieve chondrocyte injury in osteoarthritis by targeting PHLDA2. Stem Cells Int. 2022;2022:9455152. [27] WU J, KUANG L, CHEN C, et al. miR-100-5p-abundant exosomes derived from infrapatellar fat pad MSCs protect articular cartilage and ameliorate gait abnormalities via inhibition of mTOR in osteoarthritis. Biomaterials. 2019;206:87-100. [28] LI F, XU Z, XIE Z, et al. Adipose mesenchymal stem cells-derived exosomes alleviate osteoarthritis by transporting microRNA -376c-3p and targeting the WNT-beta-catenin signaling axis. Apoptosis. 2023; 28(3-4):362-378. [29] WANG K, LI F, YUAN Y, et al. Synovial mesenchymal stem cell-derived EV-packaged miR-31 downregulates histone demethylase KDM2A to prevent knee osteoarthritis. Mol Ther Nucleic Acids. 2020;22:1078-1091. [30] QIU M, LIU D, FU Q. MiR-129-5p shuttled by human synovial mesenchymal stem cell-derived exosomes relieves IL-1β induced osteoarthritis via targeting HMGB1. Life Sci. 2021;269:118987. [31] LU L, WANG J, FAN A, et al. Synovial mesenchymal stem cell-derived extracellular vesicles containing microRN555A-26a-5p ameliorate cartilage damage of osteoarthritis. J Gene Med. 2021;23(11):e3379. [32] HU H, DONG L, BU Z, et al. miR-23a-3p-abundant small extracellular vesicles released from Gelma/nanoclay hydrogel for cartilage regeneration. J Extracell Vesicles. 2020;9(1):1778883. [33] ZHOU H, SHEN X, YAN C, et al. Extracellular vesicles derived from human umbilical cord mesenchymal stem cells alleviate osteoarthritis of the knee in mice model by interacting with METTL3 to reduce m6A of NLRP3 in macrophage. Stem Cell Res Ther. 2022;13(1):322. [34] ZHANG Q, CAO L, ZOU S, et al. Human umbilical cord mesenchymal stem cell-derived extracellular vesicles carrying microRNA-181c-5p promote BMP2-induced repair of cartilage injury through inhibition of SMAD7 expression. Stem Cells Int. 2022;2022:1157498. [35] CHEN P, TANG S, GAO H, et al. Wharton’s jelly mesenchymal stem cell-derived small extracellular vesicles as natural nanoparticles to attenuate cartilage injury via microRNA regulation. Int J pharmaceut. 2022;623:121952. [36] LIU Y, ZOU R, WANG Z, et al. Exosomal KLF3-AS1 from hMSCs promoted cartilage repair and chondrocyte proliferation in osteoarthritis. Biochemi J. 2018;475(22):3629-3638. [37] LIU Y, LIN L, ZOU R, et al. MSC-derived exosomes promote proliferation and inhibit apoptosis of chondrocytes via lncRNA-KLF3-AS1/miR-206/GIT1 axis in osteoarthritis. Cell cycle (Georgetown, Tex). 2018;17(21-22): 2411-2422. [38] WEN C, LIN L, ZOU R, et al. Mesenchymal stem cell-derived exosome mediated long non-coding RNA KLF3-AS1 represses autophagy and apoptosis of chondrocytes in osteoarthritis. Cell cycle (Georgetown, Tex). 2022;21(3):289-303. [39] JIN Y, XU M, ZHU H, et al. Therapeutic effects of bone marrow mesenchymal stem cells-derived exosomes on osteoarthritis. J Cell Mol Med. 2021;25(19):9281-9294. [40] PAN C, HUANG W, CHEN Q, et al. LncRNA malat-1 from MSCs-derived extracellular vesicles suppresses inflammation and cartilage degradation in osteoarthritis. Front Bioeng Biotech. 2021;9:772002. [41] AI M, HOTHAM WE, PATTISON LA, et al. Role of human mesenchymal stem cells and derived extracellular vesicles in reducing sensory neuron hyperexcitability and pain behaviors in murine osteoarthritis. Arthritis Rheumatol. 2023;75(3):352-363. [42] ARÉVALO-TURRUBIARTE M, BARATTA M, PONTI G, et al. Extracellular vesicles from equine mesenchymal stem cells decrease inflammation markers in chondrocytes in vitro. Equine Vet J. 2022;54(6):1133-1143. [43] COSENZA S, RUIZ M, TOUPET K, et al. Mesenchymal stem cells derived exosomes and microparticles protect cartilage and bone from degradation in osteoarthritis. Sci Rep. 2017;7(1):16214. [44] WANG R, XU B, XU H. TGF-β1 promoted chondrocyte proliferation by regulating Sp1 through MSC-exosomes derived miR-135b. Cell Cycle. 2018;17(24):2756-2765. [45] LIU C, LI Y, YANG Z, et al. Kartogenin enhances the therapeutic effect of bone marrow mesenchymal stem cells derived exosomes in cartilage repair. Nanomedicine (London, England). 2020;15(3):273-288. [46] QIU B, XU X, YI P, et al. Curcumin reinforces MSC-derived exosomes in attenuating osteoarthritis via modulating the miR-124/NF-kB and miR-143/ROCK1/TLR9 signalling pathways. J Cell Mol Med. 2020; 24(18):10855-10865. [47] SHAO LT, LUO L, QIU JH, et al. PTH (1-34) enhances the therapeutic effect of bone marrow mesenchymal stem cell-derived exosomes by inhibiting proinflammatory cytokines expression on OA chondrocyte repair in vitro. Arthritis Res Ther. 2022;24(1):96. [48] MAO G, ZHANG Z, HU S, et al. Exosomes derived from miR-92a-3p-overexpressing human mesenchymal stem cells enhance chondrogenesis and suppress cartilage degradation via targeting WNT5A. Stem Cell Res Ther. 2018;9(1):247. [49] ESMAEILI A, HOSSEINI S, KAMALI A, et al. Co-aggregation of MSC/chondrocyte in a dynamic 3D culture elevates the therapeutic effect of secreted extracellular vesicles on osteoarthritis in a rat model. Sci Rep. 2022;12(1):19827. [50] MENG S, TANG C, DENG M, et al. Tropoelastin-pretreated exosomes from adipose-derived stem cells improve the synthesis of cartilage matrix and alleviate osteoarthritis. J Funct Biomater. 2023;14(4):203. [51] RAGNI E, PERUCCA ORFEI C, DE LUCA P, et al. Interaction with hyaluronan matrix and miRNA cargo as contributors for in vitro potential of mesenchymal stem cell-derived extracellular vesicles in a model of human osteoarthritic synoviocytes. Stem Cell Res Ther. 2019;10(1):109. [52] ZHANG Y, QI G, YAN Y, et al. Exosomes derived from bone marrow mesenchymal stem cells pretreated with decellularized extracellular matrix enhance the alleviation of osteoarthritis through miR-3473b/phosphatase and tensin homolog axis. J Gene Med. 2023;25(8):e3510. [53] YAN L, WU X. Exosomes produced from 3D cultures of umbilical cord mesenchymal stem cells in a hollow-fiber bioreactor show improved osteochondral regeneration activity. Cell Biol Toxicol. 2020;36(2):165-178. [54] XUE K, JIANG Y, ZHANG X, et al. Hypoxic ADSCs-derived EVs promote the proliferation and chondrogenic differentiation of cartilage stem/progenitor cells. Adipocyte. 2021;10(1):322-337. [55] XU Y, WANG Q, WANG XX, et al. The effect of different frequencies of pulsed electromagnetic fields on cartilage repair of adipose mesenchymal stem cell-derived exosomes in osteoarthritis. Cartilage. 2022:13(4):200-212. [56] RAGNI E, PERUCCA ORFEI C, DE LUCA P, et al. Inflammatory priming enhances mesenchymal stromal cell secretome potential as a clinical product for regenerative medicine approaches through secreted factors and EV-miRNAs: the example of joint disease. Stem Cell Res Ther. 2020;11(1):165. [57] RAGNI E, COLOMBINI A, VIGANÒ M, et al. Cartilage protective and immunomodulatory features of osteoarthritis synovial fluid-treated adipose-derived mesenchymal stem cells secreted factors and extracellular vesicles-embedded miRNAs. Cells. 2021;10(5):1072. [58] KIM M, SHIN DI, CHOI BH, et al. Exosomes from IL-1β-primed mesenchymal stem cells inhibited IL-1β- and TNF-α-mediated inflammatory responses in osteoarthritic SW982 cells. Tissue Eng Regen Med. 2021;18(4):525-536. [59] HA CW, NOH MJ, CHOI KB, et al. Initial phase I safety of retrovirally transduced human chondrocytes expressing transforming growth factor-beta-1 in degenerative arthritis patients. Cytotherapy. 2012; 14(2):247-256. [60] WANG R, XU B. TGF-β1-modified MSC-derived exosomal miR-135b attenuates cartilage injury via promoting M2 synovial macrophage polarization by targeting MAPK6. Cell Tissue Res. 2021;384(1):113-127. [61] WANG R, XU B. TGFβ1-modified MSC-derived exosome attenuates osteoarthritis by inhibiting PDGF-BB secretion and H-type vessel activity in the subchondral bone. Acta Histochem. 2022;124(7):151933. [62] NGUYEN TH, DAO HH, DUONG CM, et al. Cytokine-primed umbilical cord mesenchymal stem cells enhanced therapeutic effects of extracellular vesicles on osteoarthritic chondrocytes. Front Immunol. 2022;13:1041592. [63] JOHNSON K, ZHU S, TREMBLAY MS, et al. A stem cell-based approach to cartilage repair. Science. 2012;336(6082):717-721. [64] SHAO J, ZHU J, CHEN Y, et al. Exosomes from kartogenin-pretreated infrapatellar fat pad mesenchymal stem cells enhance chondrocyte anabolism and articular cartilage regeneration. Stem Cells Int. 2021; 2021:6624874. [65] XIE A, XUE J, WANG Y, et al. Kartogenin induced adipose-derived stem cell exosomes enhance the chondrogenic differentiation ability of adipose-derived stem cells. Dis Markers. 2022;2022:6943630. [66] ZENG L, YU G, HAO W, et al. The efficacy and safety of Curcuma longa extract and curcumin supplements on osteoarthritis: a systematic review and meta-analysis. Biosci Rep. 2021;41(6):BSR20210817. [67] LI G, LIU S, CHEN Y, et al. An injectable liposome-anchored teriparatide incorporated gallic acid-grafted gelatin hydrogel for osteoarthritis treatment. Nat Commun. 2023;14(1):3159. [68] ZHANG Y, LIU T, YANG H, et al. Melatonin: a novel candidate for the treatment of osteoarthritis. Ageing Res Rev. 2022;78:101635. [69] ZHANG J, FAN F, LIU A, et al. Icariin: a potential molecule for treatment of knee osteoarthritis. Front Pharmacol. 2022;13:811808. [70] LI J, ZHANG B, LIU WX, et al. Metformin limits osteoarthritis development and progression through activation of AMPK signalling. Ann Rheum Dis. 2020;79(5):635-645. [71] WANG X, OMAR O, VAZIRISANI F, et al. Mesenchymal stem cell-derived exosomes have altered microRNA profiles and induce osteogenic differentiation depending on the stage of differentiation. PLoS One. 2018;13(2):e0193059. [72] BIAN L, ZHAI DY, MAUCK RL, et al. Coculture of human mesenchymal stem cells and articular chondrocytes reduces hypertrophy and enhances functional properties of engineered cartilage. Tissue Eng Part A. 2011;17(7-8):1137-1145. [73] HOSSEINZADEH M, KAMALI A, BAGHABAN ESLAMINEJAD M, et al. Higher ratios of chondrocyte to mesenchymal stem cells elevate the therapeutic effects of extracellular vesicles harvested from chondrocyte/mesenchymal stem cell co-culture on osteoarthritis in a rat model. Cell Tissue Res. 2023;394(1):145-162. [74] DU X, CAI L, XIE J, et al. The role of TGF-beta3 in cartilage development and osteoarthritis. Bone Res. 2023;11(1):2. [75] SUN H, HU S, ZHANG Z, et al. Expression of exosomal microRNAs during chondrogenic differentiation of human bone mesenchymal stem cells. J Cell Biochem. 2019;120(1):171-181. [76] MAO G, XU Y, LONG D, et al. Exosome-transported circRNA_0001236 enhances chondrogenesis and suppress cartilage degradation via the miR-3677-3p/Sox9 axis. Stem Cell Res Ther. 2021;12(1):389. [77] SUN Y, ZHAO J, WU Q, et al. Chondrogenic primed extracellular vesicles activate miR-455/SOX11/FOXO axis for cartilage regeneration and osteoarthritis treatment. NPJ Regen Med. 2022;7(1):53. [78] CASANOVA MR, OSÓRIO H, REIS RL, et al. Chondrogenic differentiation induced by extracellular vesicles bound to a nanofibrous substrate. NPJ Regen Med. 2021;6(1):79. [79] YANG J, WANG X, FAN Y, et al. Tropoelastin improves adhesion and migration of intra-articular injected infrapatellar fat pad MSCs and reduces osteoarthritis progression. Bioact Mater. 2022;10:443-459. [80] SVOBODA KK. Chondrocyte-matrix attachment complexes mediate survival and differentiation. Microsc Res Tech. 1998;43(2):111-122. [81] ZHENG L, FAN HS, SUN J, et al. Chondrogenic differentiation of mesenchymal stem cells induced by collagen-based hydrogel: an in vivo study. J Biomed Mater Res A. 2010;93(2):783-792. [82] PEI M, HE F, KISH VL. Expansion on extracellular matrix deposited by human bone marrow stromal cells facilitates stem cell proliferation and tissue-specific lineage potential. Tissue Eng Part A. 2011;17(23-24): 3067-3076. [83] GRECO KV, IQBAL AJ, RATTAZZI L, et al. High density micromass cultures of a human chondrocyte cell line: a reliable assay system to reveal the modulatory functions of pharmacological agents. Biochem Pharmacol. 2011;82(12):1919-1929. [84] MURPHY CL, THOMS BL, VAGHJIANI RJ, et al. Hypoxia. HIF-mediated articular chondrocyte function: prospects for cartilage repair. Arthritis Res Ther. 2009;11(1):213. [85] WEISS WM, MULET-SIERRA A, KUNZE M, et al. Coculture of meniscus cells and mesenchymal stem cells in simulated microgravity. NPJ Microgravity. 2017;3:28. [86] YANG X, HE H, YE W, et al. Effects of pulsed electromagnetic field therapy on pain, stiffness, physical function, and quality of life in patients with osteoarthritis: a systematic review and meta-analysis of randomized placebo-controlled trials. Phys Ther. 2020;100(7):1118-1131. [87] GÓMEZ-LEDUC T, DESANCÉ M, HERVIEU M, et al. Hypoxia is a critical parameter for chondrogenic differentiation of human umbilical cord blood mesenchymal stem cells in type I/III collagen sponges. Int J Mol Sci. 2017;18(9):1933. [88] LIU W, RONG Y, WANG J, et al. Exosome-shuttled miR-216a-5p from hypoxic preconditioned mesenchymal stem cells repair traumatic spinal cord injury by shifting microglial M1/M2 polarization. J Neuroinflammation. 2020;17(1):47. [89] RONG Y, ZHANG J, JIANG D, et al. Hypoxic pretreatment of small extracellular vesicles mediates cartilage repair in osteoarthritis by delivering miR-216a-5p. Acta Biomater. 2021;122:325-342. [90] YANG Y, WU Y, YANG D, et al. Secretive derived from hypoxia preconditioned mesenchymal stem cells promote cartilage regeneration and mitigate joint inflammation via extracellular vesicles. Bioact Mater. 2023;27:98-112. [91] CHANG LH, WU SC, CHEN CH, et al. Exosomes derived from hypoxia-cultured human adipose stem cells alleviate articular chondrocyte inflammaging and post-traumatic osteoarthritis progression. Int J Mol Sci. 2023;24(17):13414. [92] PHELPS J, LEONARD C, SHAH S, et al. Production of mesenchymal progenitor cell-derived extracellular vesicles in suspension bioreactors for use in articular cartilage repair. Stem Cells Transl Med. 2022;11(1):73-87. [93] YAN L, LIU G, WU X. Exosomes derived from umbilical cord mesenchymal stem cells in mechanical environment show improved osteochondral activity via upregulation of LncRNA H19. J Orthop Translat. 2021;26:111-120. [94] DOS SANTOS F, CAMPBELL A, FERNANDES-PLATZGUMMER A, et al. A xenogeneic-free bioreactor system for the clinical-scale expansion of human mesenchymal stem/stromal cells. Biotechnol Bioeng. 2014; 111(6):1116-1127. [95] FREITAG J, BATES D, WICKHAM J, et al. Adipose-derived mesenchymal stem cell therapy in the treatment of knee osteoarthritis: a randomized controlled trial. Regen Med. 2019;14(3):213-230. [96] WATERMAN RS, TOMCHUCK SL, HENKLE SL, et al. A new mesenchymal stem cell (MSC) paradigm: polarization into a pro-inflammatory MSC1 or an Immunosuppressive MSC2 phenotype. PLoS One. 2010;5(4): e10088. [97] BARRACHINA L, REMACHA AR, ROMERO A, et al. Assessment of effectiveness and safety of repeat administration of proinflammatory primed allogeneic mesenchymal stem cells in an equine model of chemically induced osteoarthritis. BMC Vet Res. 2018;14(1):241. [98] WOZNIAK AL, ADAMS A, KING KE, et al. The RNA binding protein FMR1 controls selective exosomal miRNA cargo loading during inflammation. J Cell Biol. 2020;219(10):e201912074. [99] MCGONAGLE D, BABOOLAL TG, JONES E. Native joint-resident mesenchymal stem cells for cartilage repair in osteoarthritis. Nat Rev Rheumatol. 2017;13(12):719-730. [100] JAMMES M, CASSÉ F, VELOT E, et al. Pro-inflammatory cytokine priming and purification method modulate the impact of exosomes derived from equine bone marrow mesenchymal stromal cells on equine articular chondrocytes. Int J Mol Sci. 2023;24(18):14169. |
| [1] | 张艺博, 卢健棋, 毛美玲, 庞 延, 董 礼, 杨尚冰, 肖 湘. 类风湿关节炎与冠状动脉粥样硬化的因果关系:GWAS数据库血清代谢物和炎症因子数据[J]. 中国组织工程研究, 2025, 29(在线): 1-9. |
| [2] | 赖鹏宇, 梁 冉, 沈 山. 组织工程技术修复颞下颌关节:问题与挑战[J]. 中国组织工程研究, 2025, 29(在线): 1-9. |
| [3] | 马 驰, 王 宁, 陈 拥, 魏志晗, 刘逢纪, 朴成哲. 3D打印个体化截骨导板结合定制钢板在开放楔形胫骨高位截骨中的应用[J]. 中国组织工程研究, 2025, 29(9): 1863-1869. |
| [4] | 余 帅, 刘家伟, 朱 彬, 潘 檀, 李兴龙, 孙广峰, 于海洋, 丁 亚, 王宏亮. 小分子药物治疗骨关节炎的热点问题及应用前景[J]. 中国组织工程研究, 2025, 29(9): 1913-1922. |
| [5] | 赵济宇, 王少伟. 叉头框转录因子O1信号通路与骨代谢[J]. 中国组织工程研究, 2025, 29(9): 1923-1930. |
| [6] | 孙韫頔, 程露露, 万海丽, 常 赢, 熊雯娟, 夏 渊. 神经肌肉训练对膝骨关节炎患者疼痛和功能影响的Meta分析[J]. 中国组织工程研究, 2025, 29(9): 1945-1952. |
| [7] | 邓柯淇, 李光第, GOSWAMI ASHUTOSH, 刘星余, 何孝勇. 基于生物信息学对骨关节炎铁超载关键基因的筛选与验证[J]. 中国组织工程研究, 2025, 29(9): 1972-1980. |
| [8] | 王秋月, 靳 攀, 蒲 锐. 运动干预与细胞焦亡在骨关节炎中的作用[J]. 中国组织工程研究, 2025, 29(8): 1667-1675. |
| [9] | 陈跃平, 陈 锋, 彭清林, 陈荟伊, 董盼锋. 三七治疗骨关节炎机制:基于UHPLC-QE-MS、网络药理学及分子动力学模拟[J]. 中国组织工程研究, 2025, 29(8): 1751-1760. |
| [10] | 尹 路, 蒋川锋, 陈俊杰, 易 明, 王子赫, 石厚银, 汪国友, 沈骅睿. 沙苑子苷A对关节软骨细胞凋亡的影响[J]. 中国组织工程研究, 2025, 29(8): 1541-1547. |
| [11] | 王佩光, 张小文, 麦美斯, 黎璐茜, 黄 浩. 广义估计方程评估浮针法联合穴位埋线治疗不同分期膝骨关节炎的疗效[J]. 中国组织工程研究, 2025, 29(8): 1565-1571. |
| [12] | 胡涛涛, 刘 兵, 陈 诚, 殷宗银, 阚道洪, 倪 杰, 叶凌霄, 郑祥兵, 严 敏, 邹 勇. 过表达神经调节蛋白1的人羊膜间充质干细胞促进小鼠皮肤创面愈合[J]. 中国组织工程研究, 2025, 29(7): 1343-1349. |
| [13] | 金 凯, 唐 婷, 李美乐, 谢裕安. 人脐带间充质干细胞条件培养基及外泌体对肝癌细胞增殖、迁移、侵袭和凋亡的影响[J]. 中国组织工程研究, 2025, 29(7): 1350-1355. |
| [14] | 李帝均, 酒精卫, 刘海峰, 闫 磊, 李松岩, 王 斌. 明胶三维微球装载人脐带间充质干细胞修复慢性肌腱病[J]. 中国组织工程研究, 2025, 29(7): 1356-1362. |
| [15] | 刘 琪, 李林臻, 李玉生, 焦泓焯, 杨 程, 张君涛. 淫羊藿苷含药血清促进3种细胞共培养体系中软骨细胞增殖和干细胞成软骨分化[J]. 中国组织工程研究, 2025, 29(7): 1371-1379. |
过去几十年来,用于治疗各种疾病和促进组织再生的细胞疗法发展迅速,为治疗骨关节炎提供了新的途径。间充质干细胞是一种可自我更新的多能干细胞,可定向分化成软骨细胞实现软骨再生,而且间充质干细胞可以对关节腔微环境进行调控实现透明软骨再生,是治疗骨关节炎的理想种子细胞[2]。而随着该研究领域的发展,更多的证据表明,间充质干细胞的调控功能在其促修复中起关键作用,间充质干细胞通过分泌旁分泌因子调节关节微环境,抑制炎症反应,促进组织再生[3]。
在间充质干细胞的旁分泌因子中,含有蛋白质、核酸和脂质的外泌体在不同疾病中显示出应用前景。外泌体是由细胞分泌而来的微小囊泡,粒径为30-150 nm,外泌体通过将核酸、蛋白质等从母细胞递送至受体细胞,在细胞间通讯中发挥重要作用[4]。由间充质干细胞分泌的外泌体具有与其母细胞相似的调控功能,可通过调控免疫微环境、启动修复和再生来恢复关键细胞的功能,实现疾病的治疗,有望发展为可替代间充质干细胞疗法的无细胞治疗方案[5]。
在2016年,ZHANG等[6]首次报道了胚胎干细胞来源的间充质干细胞分泌的外泌体具有促进软骨缺损修复的功能。之后大量研究证实多种不同组织来源的间充质干细胞分泌的外泌体均具有治疗骨关节炎的潜能[5]。但间充质干细胞分泌的外泌体并不总是有效,如TAO等[7]和WANG等[8]发现,来源于滑膜的间充质干细胞分泌的外泌体虽然能提高软骨细胞的增殖和迁移,但抑制或不促进其软骨细胞外基质的合成。而同样是滑膜液来源的间充质干细胞分泌的外泌体,DUAN等[9]的结果表明其不仅能促进软骨细胞的增殖和迁移,也能促进细胞外基质Ⅱ型胶原和蛋白聚糖的表达,并抑制分解代谢相关蛋白血小板反应蛋白分解整合素金属肽酶的表达。由此说明,间充质干细胞分泌的外泌体的活性在一定范围内,受细胞培养条件、外泌体纯化方法等影响。
因此,为了最大程度地提高间充质干细胞分泌的外泌体的活性,优化其制备过程中的细胞培养和外泌体纯化储存等方案至关重要。而目前尚未有文章综述细胞培养方案对间充质干细胞分泌的外泌体活性的影响,该文将首次总结和讨论细胞培养条件对间充质干细胞分泌的外泌体活性的影响。通过总结分析发现,在间充质干细胞培养过程中,提供有利于软骨细胞生长的物理环境,或添加软骨保护小分子、成软骨诱导因子、炎症因子等刺激细胞,可以实现间充质干细胞分泌的外泌体调控功能的提升。通过理解细胞培养过程中的各种因素对间充质干细胞分泌的外泌体活性的影响,可为开发新型的细胞培养方案打下基础,同时也为临床用途间充质干细胞分泌外泌体的制备提供参考。
中国组织工程研究杂志出版内容重点:干细胞;骨髓干细胞;造血干细胞;脂肪干细胞;肿瘤干细胞;胚胎干细胞;脐带脐血干细胞;干细胞诱导;干细胞分化;组织工程
1.1.1 检索人及检索时间 第一作者于2023年8月进行检索。
1.1.2 检索文献时限 2010年1月至2023年8月。
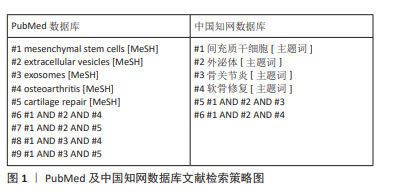
1.1.3 检索数据库 PubMed和中国知网数据库。
1.1.4 检索词 英文检索词为“exosomes,extracellular vesicles,mesenchymal stem cells,osteoarthritis,cartilage repair”;中文检索词为“外泌体,间充质干细胞,骨性关节炎,软骨修复”。
1.1.5 检索文献类型 研究原著和综述。
1.1.6 检索策略 以PubMed及中国知网数据库检索策略为例,见图1。
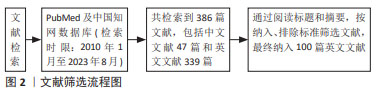
1.1.7 检索文献量 初步共检索到文献386篇,包括中文文献47篇(中国知网数据库),英文文献339篇(PubMed数据库),最后纳入100篇,均为英文文献(PubMed数据库)。
1.2 入组标准1.2.1 纳入标准 通过文章标题与摘要进行初步筛选,通过精读与泛读筛选出与研究相关的文献。
1.2.2 排除标准 研究内容与此综述无关的文章和重复性研究。
1.3 文献质量评估和数据提取 对386篇文献阅读标题和摘要内容进行初步筛选,最终通过全文阅读,共选择100篇相关文献进行总结和讨论,具体流程见图2。
中国组织工程研究杂志出版内容重点:干细胞;骨髓干细胞;造血干细胞;脂肪干细胞;肿瘤干细胞;胚胎干细胞;脐带脐血干细胞;干细胞诱导;干细胞分化;组织工程
文章选择“调整细胞培养条件对间充质干细胞分泌的外泌体活性影响”这一主题进行总结,通过讨论发现,模拟软骨细胞体内微环境、软骨有利因子、间充质干细胞免疫抑制刺激因子等,均能诱导间充质干细胞分泌有利于软骨细胞维持表型、促进软骨组织再生的活性因子于间充质干细胞分泌的外泌体中,进而提高其骨关节炎治疗活性。
3.2 作者综述区别于他人他篇的特点 文章全面概述了调整细胞培养条件对提高间充质干细胞分泌的外泌体治疗骨关节炎潜能的影响,而相关领域的其他综述文章主要是分析间充质干细胞或间充质干细胞分泌的外泌体在骨关节炎治疗中的应用。文章中,详细讨论了多种可调整的细胞培养条件,包括低氧、生物反应器、应力或磁场刺激、成软骨诱导、软骨保护分子和炎症因子刺激、以及生物支架材料细胞培养等,可以为读者提供全面的视角和深入的理解。此外,文章还针对不同的细胞培养方案进行了深入的分析和讨论,探讨其可能的机制和临床转化的潜力,可以为相关领域的研究者提供参考。
3.3 综述的局限性 该综述存在一定的局限性,仅分析了改变细胞培养条件对间充质干细胞分泌的外泌体活性的影响。此外,文章的讨论主要集中在实验室基础研究上,临床实践数据较少。
3.4 综述的重要意义 文章对于理解细胞培养条件对间充质干细胞分泌的外泌体活性的影响,以及如何通过调整细胞培养条件来提高其治疗活性,具有重要的意义。常规的细胞培养条件与体内微环境存在差异,低氧、生物反应器及生物支架材料等培养条件可以模拟体内微环境,使得间充质干细胞分泌出更有利于软骨细胞维持表型、软骨组织再生的因子,从而提高其骨关节炎治疗活性;除物理微环境外,成软骨诱导因子或软骨保护分子的使用,能够激发间充质干细胞分泌有利于软骨细胞增殖、合成的因子,从而协同间充质干细胞的调控功能,实现骨关节炎软骨的再生;使用炎症因子预处理间充质干细胞,可以激活间充质干细胞的免疫抑制表型,从而调控骨关节炎关节腔内的免疫微环境,促进组织再生。
此外,文章还分析了上述不同方法的临床转化潜能。低氧、生物反应器、脉冲电磁场等物理刺激,以及姜黄素等软骨保护小分子的使用,具有清洁及造价低的优点,可用于制备临床使用的间充质干细胞分泌的外泌体,有广阔的应用前景。
3.5 课题组专家的意见和建议 通过总结和分析细胞培养方案对间充质干细胞分泌的外泌体活性的影响,不仅可以开发新型的细胞培养方案用于提升间充质干细胞分泌外泌体的骨关节炎治疗活性,也能为临床转化提供参考。除骨关节炎外,间充质干细胞分泌的外泌体在其他疾病中也展现出了巨大的应用前景,如皮肤损伤修复等。通过文献调研可以发现,低氧诱导下产生的间充质干细胞分泌外泌体的皮肤修复功能也得到了提升,这说明细胞培养方案对间充质干细胞分泌外泌体活性的影响具有通用性,而非局限于骨关节炎治疗,因此,文章也可为开发新型的细胞培养方案以提高间充质干细胞分泌的外泌体疾病治疗活性提供参考。
中国组织工程研究杂志出版内容重点:干细胞;骨髓干细胞;造血干细胞;脂肪干细胞;肿瘤干细胞;胚胎干细胞;脐带脐血干细胞;干细胞诱导;干细胞分化;组织工程
文题释义:
骨关节炎:是一种慢性关节疾病,主要病理特征是关节软骨破坏、骨质增生以及关节边缘的骨质增生(骨刺)。在全球范围内,骨关节炎是最常见的关节疾病之一,是关节疼痛和残疾的主要原因,然而现有临床策略仅能缓解骨关节炎的症状,不能实现疾病的修正治疗。
间充质干细胞外泌体:具有治疗骨关节炎的潜能,其能减轻炎症反应,促进软骨细胞的增殖和软骨基质的合成,抑制软骨细胞的凋亡和软骨基质的降解,实现软骨组织的修复和再生,具有临床转化潜能。
中国组织工程研究杂志出版内容重点:干细胞;骨髓干细胞;造血干细胞;脂肪干细胞;肿瘤干细胞;胚胎干细胞;脐带脐血干细胞;干细胞诱导;干细胞分化;组织工程
调整细胞培养条件:尽管大量研究表明间充质干细胞外泌体具有治疗骨关节炎的潜能,但间充质干细胞外泌体并不总是有效,间充质干细胞外泌体的活性受细胞培养条件等影响。通过文献综述发现,改变细胞培养条件,如改变细胞培养的物理环境、使用成软骨诱导培养基培养细胞、使用软骨保护分子/炎症因子刺激细胞、或将细胞培养于生物材料支架上等,均能实现间充质干细胞外泌体治疗骨关节炎活性的提升。
中国组织工程研究杂志出版内容重点:干细胞;骨髓干细胞;造血干细胞;脂肪干细胞;肿瘤干细胞;胚胎干细胞;脐带脐血干细胞;干细胞诱导;干细胞分化;组织工程
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||