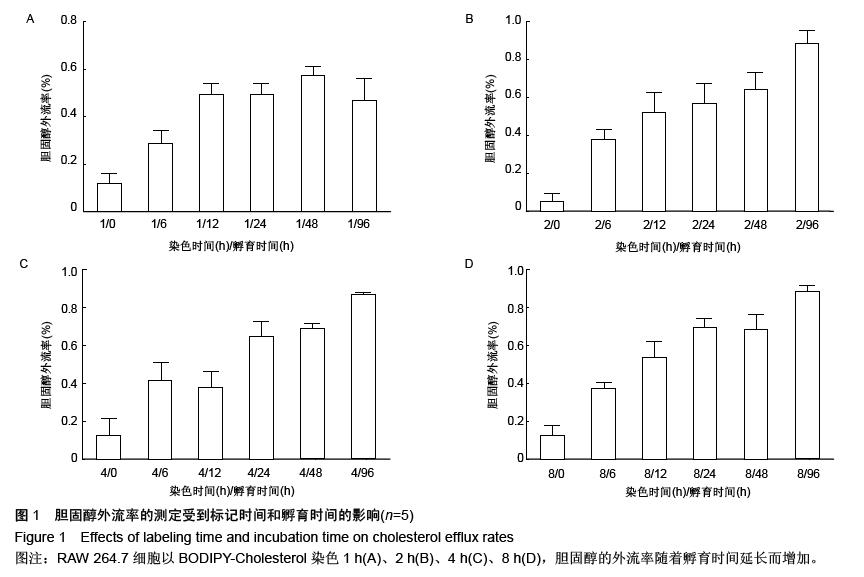
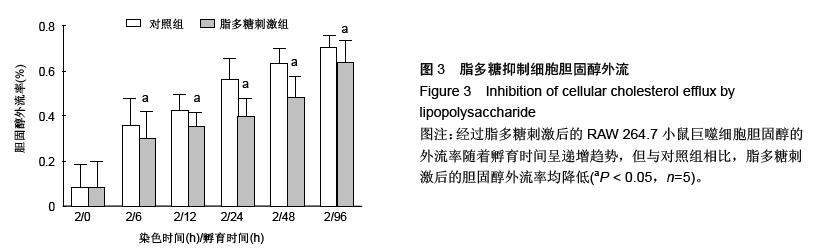
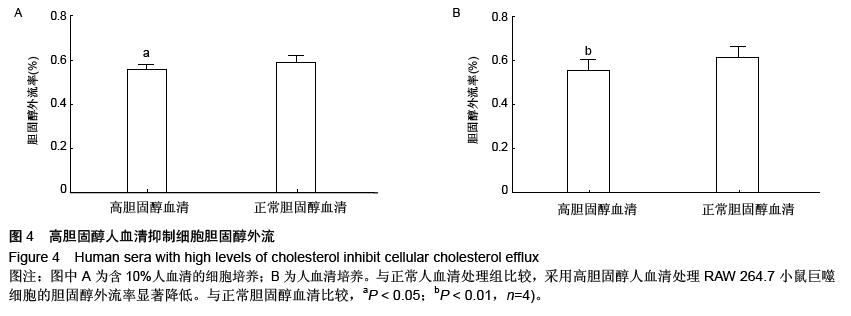
| [1] Mao M, Lei H, Liu Q,et al.Effects of miR-33a-5P on ABCA1/G1-mediated cholesterol efflux under inflammatory stress in THP-1 macrophages. PLoS One.2014;9(10): e109722.
[2] Pisetsky DS, Spencer DM. Effects of progesterone and estradiol sex hormones on the release of microparticles by RAW 264.7 macrophages stimulated by Poly(I:C). Clin Vaccine Immunol.2011;18(9): 1420-1426.
[3] 王智.他汀与动脉粥样硬化斑块逆转[J].临床荟萃.2013, 28(7): 831-834.
[4] Iizuka M, Ayaori M, Uto-Kondo H,et al.Astaxanthin enhances ATP-binding cassette transporter A1/G1 expressions and cholesterol efflux from macrophages. J Nutr Sci Vitaminol (Tokyo).2012;58(2): 96-104.
[5] Yvan-Charvet L, Wang N, Tall A R. Role of HDL, ABCA1, and ABCG1 transporters in cholesterol efflux and immune responses. Arterioscler Thromb Vasc Biol.2010; 30(2): 139-143.
[6] Li Z,Mintzer E,Bittman R.First synthesis of free cholesterol- BODIPY conjugates. J Org Chem. 2006; 71(4):1718-1721.
[7] 黄柳倩. BODIPY基荧光传感器对半胱氨酸的检测[J]. 杭州师范大学学报:自然科学版, 2014, 13(5): 499-502.
[8] Gao S, Wang L, Liu W, et al.The synergistic effect of homocysteine and lipopolysaccharide on the differentiation and conversion of raw264.7 macrophages. J Inflamm (Lond).2014;11:13.
[9] Cho W, Kang JL, Park YM. Corticotropin-Releasing Hormone (CRH) Promotes Macrophage Foam Cell Formation via Reduced Expression of ATP Binding Cassette Transporter-1 (ABCA1). PLoS One.2015; 10(6): e0130587.
[10] 洪雪华,生瑜. BODIPY类荧光染料的研究进展[J].广州化工, 2012, 40(7): 65-68.
[11] Solanko LM, Honigmann A, Midtiby HS, et al. Membrane orientation and lateral diffusion of BODIPY-cholesterol as a function of probe structure. Biophys J. 2013;105(9): 2082-2092.
[12] Ariola FS, Li ZG, Cornejo C, et al. Membrane Fluidity and Lipid Order in Ternary Giant Unilamellar Vesicles Using a New Bodipy-Cholesterol Derivative. Biophys J. 2009;96(7): 2696-2708.
[13] Holtta-Vuori M, Uronen RL, Repakova J, et al. BODIPY-Cholesterol: A New Tool to Visualize Sterol Trafficking in Living Cells and Organisms. Traffic. 2008;9(11): 1839-1849.
[14] Wustner D, Solanko L, Sokol E, et al. Quantitative assessment of sterol traffic in living cells by dual labeling with dehydroergosterol and BODIPY- cholesterol. Chemistry and Physics of Lipids.2011; 164(3):221-235.
[15] 宋玮.荧光标记胆固醇用于测定人单核巨噬细胞脂质外流[J].中国动脉硬化杂志, 2012, 20(8):749-754.
[16] Zhang J,Cai S,Peterson BR,et al.Development of a cell-based, high-throughput screening assay for cholesterol efflux using a fluorescent mimic of cholesterol. Assay Drug Dev Technol. 2011;9(2): 136-146.
[17] Kanerva K, Uronen RL, Blom T, et al. LDL cholesterol recycles to the plasma membrane via a Rab8a- Myosin5b-actin-dependent membrane transport route. Dev Cell. 2013;27(3): 249-262.
[18] 张晔.细胞骨架改构在LPS作用后VE-Cad胞吞途径转化中的作用[J].中国普外基础与临床杂志, 2015, 22(3): 269-273.
[19] 贝克曼库尔特商贸(中国)有限公司对尿酸检测试剂盒、肌酐测定试剂盒、乳酸测定试剂盒、脂肪酶测定试剂盒、胆固醇测定试剂盒主动召回[J].中国医疗设备, 2015, 30(3):120.
[20] Ladeiras-Lopes R, Agewall S, Tawakol A, et al. Atherosclerosis: Recent trials, new targets and future directions. Int J Cardiol.2015;192:72-81.
[21] 吴先杰.动脉粥样硬化发生机制研究现状及思路[J].中华实用诊断与治疗杂志, 2012, 26(7): 629-631.
[22] Meiler S, Baumer Y, Toulmin E, et al. MicroRNA 302a Is a Novel Modulator of Cholesterol Homeostasis and Atherosclerosis. Arterioscl Throm Vas.2015; 35(2): 323-331.
[23] Li X H, Li Y, Cheng ZY, et al.The Effects of Phellinus linteus Polysaccharide Extracts on Cholesterol Efflux in Oxidized Low-Density Lipoprotein-Loaded THP-1 Macrophages. J Invest Med.2015;63(5): 752-757. |
.jpg)