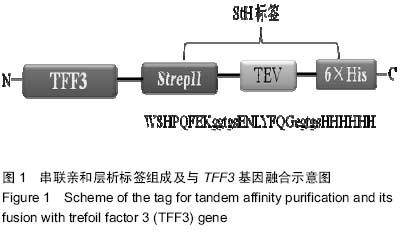
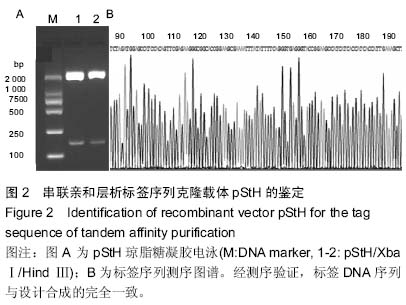
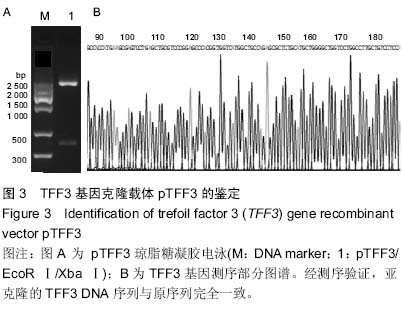
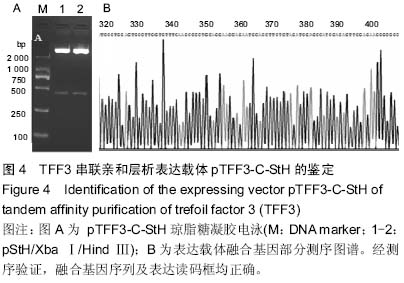
| [1] Taupin D,Podolsky DK. Trefoil factors: initiators of mucosal healing.Nat Rev Mol Cell Bio.2003;4(9):721-732.
[2] Shirazi T, Longman RJ,Corfield AP, et al.Mucins and inflammatory bowel disease.Postgrad Med J.2000;76(698): 473-478.
[3] Mashimo H,Wu DC, Podolsky DK, et al. Impaired defense of intestinal mucosa in mice lacking intestinal trefoil factor. Science.1996;274(5285):262-265.
[4] Emami S, Le Floch N, Bruyneel E, et al. Induction of scattering and cellular invasion by trefoil pep tides in sac and RhoA2transformed kidney and colonic ep ithelialcells. FASEB J.2001;15 (2):351-361.
[5] Beck PL,Wong JF, Li Y, et al. Chemotherapy - and radiotherapy induced intestinal damage is regulated by intestinal trefoil factor. Gas troenterology.2004;126(3): 796-808.
[6] Kaise, M,Miwa J,Tashiro J,et al.The combination of serum trefoil factor3 and pepsinogen testing is a valid non-endoscopic biomarker for predicting the presence of gastric cancer: a new marker for gastric cancer risk.J Gastroenterol.2011 ;46(6):736-745.
[7] Kaise M,Miwa J,Fujimoto A,et al.Influence of Helicobacter pylori status and eradication on the serum levels of trefoil factors and pepsinogen test: serum trefoil factor 3 is a stable biomarker.Gastric Cancer.2013;16(3):329-337.
[8] Aikou S,Ohmoto Y,Gunji T,et al.Tests for Serum Levels of Trefoil Factor Family Proteins Can Improve Gastric Cancer Screening.Gastroenterology.2011;141 (3):837-845.
[9] Huang Z,Zhang X,Lu H,et al.Serum refoil factor 3 is a promising non-invasive biomarker for gastric cancer screening: A monocentric cohort study in China.BMC Gastroenterol.2014 ;14(74): doi 10.1186/1471-230X-14-74.
[10] May FE.The potential of trefoil proteins as biomarkers in human cancer.Biomark Med.2012;6(3):301-304.
[11] Thim L, Mortz E. Isolation and characterization of putative trefoil peptide receptors. Regul Pept.2000;90(1–3):61–68.
[12] Otto WR, Thim L.Trefoil factor family-interacting proteins.Cell Mol Life Sci.2005;62(24):2939-2946.
[13] Rigaut, G., Shevchenko A, Rutz, B, et al.A generic protein purification method for protein complex characterization and proteome exploration.Nat. Biotechnol.1999;17(10):1030-1032.
[14] Puig O, Caspary F, Rigaut G, et al.The tandem affinity purification (TAP) method: a general procedure of protein complex purification. Methods. 2001;24 (3):218-229.
[15] Forler D, Kocher T, Rode M,et al. An efficient protein complex purification method for functional proteomics in higher eukaryotes. Nat.Biotechnol.2003; 21(1): 89-92.
[16] Knuesel M, WanY, Xiao Z, et al.Identification of novel protein–protein interactions using a versatile mammalian tandem affinity purification expression system. Mol Cell. Proteomics.2003;2(11):1225-1233.
[17] Guleng B, Han J, Yang JQ, et al.TFF3 mediated induction of VEGF via hypoxia in human gastric cancer SGC-7901 cells. Mol Biol Rep. 2012;(4):4127-4134.
[18] Rodrigues S, Van Aken E, Van Bocxlaer S,et al. Trefoil peptides as proangiogenic factors in vivo and in vitro: implication of cyclooxygenase-2 and EGF receptor signaling. FASEB J. 2003;17(1):7-16.
[19] Lin N, Xu LF, Sun M. The protective effect of trefoil factor 3 on the intestinal tight junction barrier is mediated by toll-like receptor 2 via a PI3K/Akt dependent mechanism. Biochem Biophys Res Commun.2013;440(1):143-149.
[20] Zheng Q, Gao J, Li H,et al.Trefoil factor 3 peptide regulates migration via a Twist-dependent pathway in gastric cell.Biochem Biophys Res Commun.2013;438(1):6-12.
[21] Gloeckner CJ,Boldt K,Schumacher A,et al.A novel tandem affinity purification strategy for the efficient isolation and characterization of native protein complexs.proteinomics. 2007;7(5)4228-4234.
[22] Giannone RJ,McDonald WH, Hurst GB,et al. Dual-tagging system for the affinity purification of mammalian protein complexs.Biotechnique.2007;43(3): 296-302.
[23] Tsai A,Carstens RP.An optimized protocol for protein purification in cultured mammalian cells using a tandem affinity purification approach.Nature protocols.2006;1(6): 2820-2827. |