[1] URITANI D, KODA H, YASUURA Y, et al. Factors associated with subjective knee joint stiffness in people with knee osteoarthritis: A systematic review. Int J Rheum Dis. 2023;26(3):425-436.
[2] LONG H, LIU Q, YIN H, et al. Prevalence trends of site-specific osteoarthritis from 1990 to 2019: Findings from the global burden of disease study 2019. Arthritis Rheumatol. 2022;74(7):1172-1183.
[3] DI JK, BAI J, ZHANG JR, et al. Regional disparities, age-related changes and sex-related differences in knee osteoarthritis. BMC Musculoskelet Disord. 2024;25(1):66.
[4] SHAO W, HOU H, HAN Q, et al. Prevalence and risk factors of knee osteoarthritis: a cross-sectional survey in Nanjing, China. Front Public Health. 2024;12:1441408.
[5] BIJLSMA JW, BERENBAUM F, LAFEBER FP. Osteoarthritis: an update with relevance for clinical practice. Lancet. 2011;377(9783):2115-2126.
[6] WANG ST, NI GX. Depression in Osteoarthritis: Current Understanding. Neuropsychiatr Dis Treat. 2022;18:375-389.
[7] GBD 2021 OSTEOARTHRITIS COLLABORATORS. Global, regional, and national burden of osteoarthritis, 1990-2020 and projections to 2050: a systematic analysis for the Global Burden of Disease Study 2021. Lancet Rheumatol. 2023;5(9):e508-e522.
[8] ZENG C, DOHERTY M, PERSSON MSM, et al. Comparative efficacy and safety of acetaminophen, topical and oral non-steroidal anti-inflammatory drugs for knee osteoarthritis: evidence from a network meta-analysis of randomized controlled trials and real-world data. Osteoarthritis Cartilage. 2021;29(9):1242-1251.
[9] ZENG F, WANG K, DUAN H, et al. Diacerein versus non-steroidal anti-inflammatory drugs in the treatment of knee osteoarthritis: a meta-analysis. J Orthop Surg Res. 2023;18(1):308.
[10] STACEY SK, MCELENEY M. Topical corticosteroids: choice and application. Am Fam Physician. 2021;103(6):337-343.
[11] RABADE A, VISWANATHA GL, NANDAKUMAR K, et al. Evaluation of efficacy and safety of glucosamine sulfate, chondroitin sulfate, and their combination regimen in the management of knee osteoarthritis: a systematic review and meta-analysis. Inflammopharmacology. 2024;32(3):1759-1775.
[12] DAVIES NM, HOLMES MV, DAVEY SMITH GD. Reading mendelian randomisation studies: a guide, glossary, and checklist for clinicians. BMJ. 2018;362(12):k601.
[13] BURGESS S, SCOTT RA, TIMPSON NJ, et al. Using published data in Mendelian randomization: a blueprint for efficient identification of causal risk factors. Eur J Epidemiol. 2015;30(7):543-552.
[14] SANDERSON E, GLYMOUR MM, HOLMES MV, et al. Mendelian randomization. Nat Rev Method Prim. 2022;2(1):6.
[15] ZOU MR, SHAO ZX. Proteome-wide Mendelian randomization and colocalization analysis identify therapeutic targets for knee and hip osteoarthritis. Biomolecules. 2024;14(3):355.
[16] WANG YZ, SHEN HB. Challenges and factors that influencing causal inference and interpretation, based on Mendelian randomization studies. Zhonghua Liu Xing Bing Xue Za Zhi. 2020;41(8):1231-1236.
[17] LEVIN MG, BURGESS S. Mendelian randomization as a tool for cardiovascular research: A review. JAMA Cardiol. 2024;9(1):79-89.
[18] LIU CY, YANG YS, PEI MQ, et al. Mendelian randomization analysis reveals causal association of anthropometric measures on sepsis risk and mortality. PLoS One. 2024;19(9):e0310898.
[19] LIN LJ, WEI YY, ZHANG RY, et al. Application of Mendelian randomization methods in causal inference of observational study. Zhonghua Yu Fang Yi Xue Za Zhi. 2019;53(6):619-624.
[20] LIU HH, JIANG X, DENG GH, et al. Mendelian randomization analysis of the causal relationship between asthma, allergic rhinitis, and chronic sinusitis. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2024; 59(8):820-827.
[21] MINELLI C, DEL GRECO MF, VAN DER PLAAT DA, et al. The use of two-sample methods for Mendelian randomization analyses on single large datasets. Int J Epidemiol. 2021;50(5):1651-1659.
[22] JIANG X, ALFREDSSON L. Modifiable environmental exposure and risk of rheumatoid arthritis-current evidence from genetic studies. Arthritis Res Ther. 2020;22(1):154.
[23] KURKI MI, KARJALAINEN J, PALTA P, et al. Author correction: FinnGen provides genetic insights from a well-phenotyped isolated population. Nature. 2023;615(7952):e19.
[24] KURKI MI, KARJALAINEN J, PALTA P, et al. FinnGen provides genetic insights from a well-phenotyped isolated population. Nature. 2023; 613(7944):508-518.
[25] TACHMAZIDOU I, HATZIKOTOULAS K, SOUTHAM L, et al. Identification of new therapeutic targets for osteoarthritis through genome-wide analyses of UK Biobank data. Nat Genet. 2019;51(2):230-236.
[26] CEREZO M, SOLLIS E, JI Y, et al. The NHGRI-EBI GWAS Catalog: standards for reusability, sustainability and diversity. Nucleic Acids Res. 2024;2024.10.23.619767
[27] VÕSA U, CLARINGBOULD A, WESTRA HJ, et al. Large-scale cis- and trans-eQTL analyses identify thousands of genetic loci and polygenic scores that regulate blood gene expression. Nat Genet. 2021;53(9):1300-1310.
[28] KINTU C, SOREMEKUN O, KAMIZA AB, et al. The causal effects of lipid traits on kidney function in Africans: bidirectional and multivariable Mendelian-randomization study. EBioMedicine. 2023;90:104537.
[29] BULL CJ, HAZELWOOD E, LEGGE DN, et al. Impact of weight loss on cancer-related proteins in serum: results from a cluster randomised controlled trial of individuals with type 2 diabetes. EBioMedicine. 2024;100:104977.
[30] BURGESS S, DUDBRIDGE F, THOMPSON SG. Combining information on multiple instrumental variables in Mendelian randomization: comparison of allele score and summarized data methods. Stat Med. 2016;35(11):1880-1906.
[31] HEMANI G, BOWDEN J, SMITH GD. Evaluating the potential role of pleiotropy in Mendelian randomization studies. Hum Mol Genet. 2018;27(R2): R195-R208.
[32] GIAMBARTOLOMEI C, VUKCEVIC D, SCHADT EE, et al. Bayesian test for colocalisation between pairs of genetic association studies using summary statistics. PLoS Genet. 2014;10(5):e1004383.
[33] CHEN SH, LI ZY, HUANG WY, et al. Prognostic and Therapeutic Significance of BTN3A Proteins in Tumors. J Cancer. 2021;12(15):4505-4512.
[34] RHODES DA, CHEN HC, PRICE AJ, et al. Activation of human γδ T cells by cytosolic interactions of BTN3A1 with soluble phosphoantigens and the cytoskeletal adaptor periplakin. J Immunol. 2015;194(5):2390-2398.
[35] YU HL, MI CH, WANG Q, et al. Long noncoding RNA profiling reveals that LncRNA BTN3A2 inhibits the host inflammatory response to Eimeria tenella infection in chickens. Front Immunol. 2022;13:891001.
[36] GALLO J, RASKA M, KRIEGOVA E, et al. Inflammation and its resolution and the musculoskeletal system. J Orthop Transl. 2017;10:52-67.
[37] REN H, LI SL, LIU X, et al. Multi-omics analysis of the expression and prognostic value of the butyrophilins in breast cancer. J Leukoc Biol. 2021;110(6):1181-1195.
[38] LIU BH, WANG XY, YANG ZR, et al. A genetic study to identify pathogenic mechanisms and drug targets for benign prostatic hyperplasia: a multi-omics Mendelian randomization study. Sci Rep. 2024;14(1):23120.
[39] DIKIY S, RUDENSKY AY. Principles of regulatory T cell function. Immunity. 2023;56(2):240-255.
[40] HAN S. Osteoarthritis year in review 2022: biology. Osteoarthritis Cartilage. 2022;30(12):1575-1582.
[41] CAI PA, LU ZH, WU JJ, et al. BTN3A2 serves as a prognostic marker and favors immune infiltration in triple-negative breast cancer. J Cell Biochem. 2020;121(3):2643-2654.
[42] YANG K, XU JJ, FAN M, et al. Lactate suppresses macrophage pro-inflammatory response to LPS stimulation by inhibition of YAP and NF-κB activation via GPR81-mediated signaling. Front Immunol. 2020;11: 587913.
[43] YE QY, XU HK, LIU SY, et al. Apoptotic extracellular vesicles alleviate Pg-LPS induced inflammatory responses of macrophages via AMPK/SIRT1/NF-κB pathway and inhibit osteoclast formation. J Periodont. 2022;93(11):1738-1751.
[44] LIN YS, ZHOU H, LI SJ. BTN3A2 expression Is connected with favorable prognosis and high infiltrating immune in lung adenocarcinoma. Front Genet. 2022;13:848476.
[45] SHAPOURI-MOGHADDAM A, MOHAMMADIAN S, VAZINI H, et al. Macrophage plasticity, polarization, and function in health and disease. J Cell Physiol. 2018;233(9):6425-6440.
[46] ZHAO K, RUAN JQ, NIE LY, et al. Effects of synovial macrophages in osteoarthritis. Front Immunol. 2023;14:1164137.
[47] MEHANA ESE, KHAFAGA AF, EL-BLEHI SS. The role of matrix metalloproteinases in osteoarthritis pathogenesis: An updated review. Life Sci. 2019;234:116786.
[48] RAMSEWAK RS, DEWITT DL, NAIR MG. Cytotoxicity, antioxidant and anti-inflammatory activities of curcumins I-III from curcuma longa. Phytomedicine. 2000;7(4):303-308.
[49] SI SC, LIU HY, XU L, et al. Identification of novel therapeutic targets for chronic kidney disease and kidney function by integrating multi-omics proteome with transcriptome. Genome Med. 2024;16(1):84.
[50] ZENG C, WEI J, PERSSON MSM, et al. Relative efficacy and safety of topical non-steroidal anti-inflammatory drugs for osteoarthritis: a systematic review and network meta-analysis of randomised controlled trials and observational studies. Br J Sports Med. 2018;52(10):642-650.
[51] AFRACHE H, PONTAROTTI P, ABI-RACHED L, et al. Evolutionary and polymorphism analyses reveal the central role of BTN3A2 in the concerted evolution of the BTN3 gene family. Immunogenetics. 2017; 69(6):379-390.
[52] CAO JX, WANG YL, WANG ZX. Advances in precise regulation of CRISPR/Cas9 gene editing technology. Yi Chuan. 2020;42(12):1168-1177.
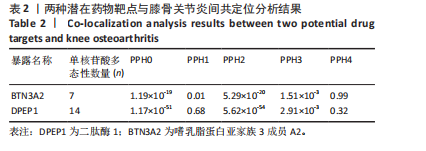
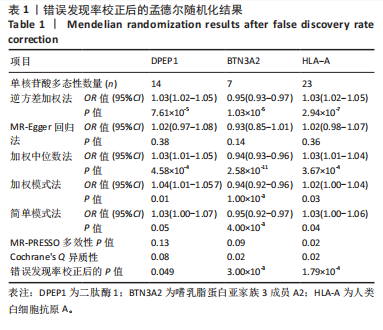
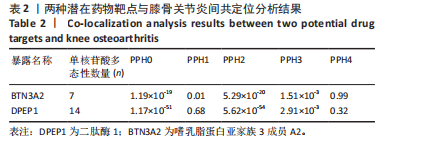
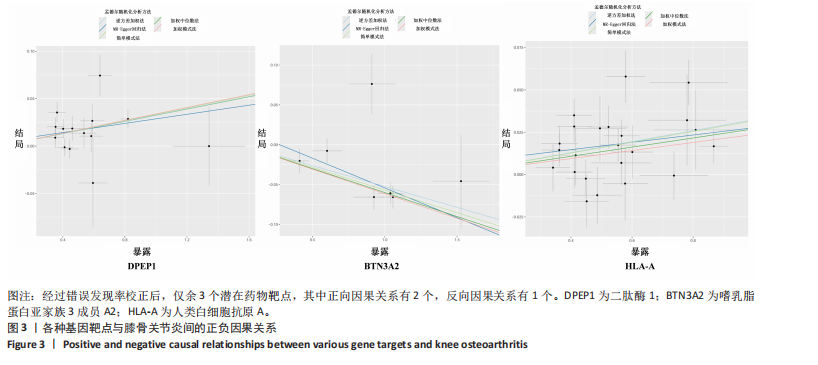
|