中国组织工程研究 ›› 2025, Vol. 29 ›› Issue (13): 2764-2773.doi: 10.12307/2025.017
• 干细胞综述 stem cell review • 上一篇 下一篇
不同来源间充质干细胞治疗卵巢早衰的机制
刘艳艳,马园园,黄向华,张敬坤
- 河北医科大学第二医院,河北省石家庄市 050000
-
收稿日期:2023-11-06接受日期:2024-02-05出版日期:2025-05-08发布日期:2024-09-11 -
通讯作者:张敬坤,博士,副教授,河北医科大学第二医院,河北省石家庄市 050000 -
作者简介:刘艳艳,女,1997年生,内蒙古自治区通辽市人,汉族,河北医科大学妇产科在读硕士,医师,主要从事卵巢早衰、再生医学研究。 -
基金资助:河北省自然科学基金(H2021206463),项目负责人:张敬坤;河北省医学科学研究课题计划项目(20210080,20240487),项目负责人:张敬坤
Mechanism of different sources of mesenchymal stem cells in treatment of premature ovarian failure
Liu Yanyan, Ma Yuanyuan, Huang Xianghua, Zhang Jingkun
- The Second Hospital of Hebei Medical University, Shijiazhuang 050000, Hebei Province, China
-
Received:2023-11-06Accepted:2024-02-05Online:2025-05-08Published:2024-09-11 -
Contact:Zhang Jingkun, MD, Associate professor, The Second Hospital of Hebei Medical University, Shijiazhuang 050000, Hebei Province, China -
About author:Liu Yanyan, Master candidate, Physician, The Second Hospital of Hebei Medical University, Shijiazhuang 050000, Hebei Province, China -
Supported by:Hebei Provincial Natural Science Foundation, No. H2021206463 (to ZJK); Hebei Province Medical Science Research Project, Nos. 20210080, 20240487 (to ZJK)
摘要:
文题释义:
间充质干细胞:来源于发育早期的中胚层和外胚层,属于多能干细胞,具有多向分化、造血支持、促进干细胞植入、免疫调控和自我复制等潜能。间充质干细胞主要存在于结缔组织和器官间质中,包括骨髓、胎盘、脐带、脂肪、黏膜、骨骼、肌肉、肺、肝、胰腺、羊水、羊膜、脐血等。卵巢早衰:指女性40岁以前出现闭经、促性腺激素水平升高和雌激素水平降低,并伴有不同程度的低雌激素症状,是早发性卵巢功能不全的终末阶段,严重影响女性生理及心理健康。
摘要
背景:已经有大量细胞及动物实验证实了间充质干细胞具有改善卵巢功能的作用,部分临床试验也已完成并初步证实了其有效性,为卵巢早衰女性带来了希望。
目的:总结并分析近年来关于不同来源间充质干细胞治疗卵巢早衰的机制、研究进展及相关临床试验,为间充质干细胞治疗卵巢早衰的进一步研究及临床应用提供理论依据。
方法:以“间充质干细胞,卵巢早衰”及“mesenchymal stem cells,premature ovarian failure”分别作为中、英文关键词,在中国知网、万方数据库、中华医学库及PubMed数据库对相关文献进行检索,最终纳入符合要求的72篇文献进行综述。
结果与结论:目前常用于治疗卵巢早衰的间充质干细胞种类有7类,分别为脐带间充质干细胞、骨髓间充质干细胞、胎盘间充质干细胞、经血间充质干细胞、羊膜间充质干细胞、羊水间充质干细胞及脂肪间充质干细胞,涉及作用机制主要包括抑制凋亡促进增殖、抗炎及抑制氧化应激、归巢、促血管生成、抗纤维化、旁分泌、免疫调节、自噬及改善微环境。细胞和动物实验均表明不同来源间充质干细胞可以通过各种机制对卵巢早衰有较好的干预效果,一定程度上延缓了卵巢早衰的进展。如果将来能成功运用于临床,能够很大程度上缓解患者来自心理及生理上的痛苦。但目前临床研究因干细胞来源伦理、具体给药方式及剂量、是否引发严重不良反应等方面缺乏全面且准确的证据,故在未来仍需进行深入探讨,同时远期安全性也有待进一步观察。
https://orcid.org/0009-0009-0610-0276 (刘艳艳)
中国组织工程研究杂志出版内容重点:干细胞;骨髓干细胞;造血干细胞;脂肪干细胞;肿瘤干细胞;胚胎干细胞;脐带脐血干细胞;干细胞诱导;干细胞分化;组织工程
中图分类号:
引用本文
刘艳艳, 马园园, 黄向华, 张敬坤. 不同来源间充质干细胞治疗卵巢早衰的机制[J]. 中国组织工程研究, 2025, 29(13): 2764-2773.
Liu Yanyan, Ma Yuanyuan, Huang Xianghua, Zhang Jingkun. Mechanism of different sources of mesenchymal stem cells in treatment of premature ovarian failure[J]. Chinese Journal of Tissue Engineering Research, 2025, 29(13): 2764-2773.
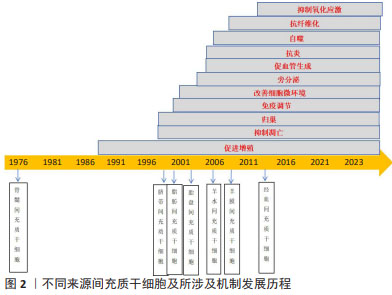
2.2 脐带间充质干细胞治疗卵巢早衰的机制研究 脐带间充质干细胞是一种存在于新生儿脐带组织中的多功能干细胞,可以在特定条件下持续增殖并分化成一种或多种细胞类型,具有易于获得、分离、培养和纯化等优点[6]。 脐带间充质干细胞作为一种更新能力强和分化能力高的细胞,已经在临床许多疾病的治疗中展现了广阔的前景,在卵巢早衰中的作用机制如表1所示。

2.2.1 抑制凋亡、促进增殖 WANG等[7]研究发现当脐带间充质干细胞移植入卵巢早衰小鼠后,Caspase-3的表达下降、增殖细胞核抗原表达升高,进而抑制颗粒细胞凋亡、促进增殖,并诱导卵泡开始正常发育,恢复卵巢功能。QU等[8]通过实验表明:在体外,脐带间充质干细胞来源外泌体中miR-126-3p通过PIK3R2/PI3K/AKT/mTOR轴抑制颗粒细胞凋亡;在体内,通过上调Bcl-2蛋白的表达,下调Bax和Caspase-3蛋白的表达发挥作用,此结果为外泌体在卵巢早衰中的作用提供了新思路。赵莹莹等[9]研究发现卵巢早衰大鼠Bax和Caspase-3表达水平升高,Ras和Erk表达水平降低,经脐带间充质干细胞来源外泌体干预后可逆转这些基因表达,说明脐带间充质干细胞来源外泌体能够有效调节卵巢功能,促进细胞增殖,其机制可能与调节Ras/ Erk信号通路有关。GAO等[10]研究中同样发现脐带间充质干细胞来源外泌体miR-22-3p可以通过下调KLF6表达抑制ATF4-ATF3-CHOP轴,进而减轻顺铂诱发的颗粒细胞凋亡,改善卵巢功能。随着研究深入发展,NIU等[11]在2022年首次发现脐带间充质干细胞通过甲基化miR-100-5p来抑制NOX4/NLRP3信号轴,从而减少细胞焦亡以改善卵巢早衰,这为后续探讨机制提供了新思路。
2.2.2 抗炎、抑制氧化应激 谭丽等[12]发现脐带间充质干细胞移植后能改善氧化酶系统紊乱状态,抗氧化酶超氧化物歧化酶1使体内的活性氧水平降低,从而改善氧化应激状态,最终保护卵巢线粒体形态结构及功能。SHI等[13]通过组织病理学发现卵巢局部炎症细胞浸润是注射脐带间充质干细胞后的主要反应。DENG 等[14]发现脐带间充质干细胞处理显著下调了白细胞介素6和白细胞介素1β的mRNA表达,上调了白细胞介素10、肿瘤坏死因子α诱导蛋白6和血管内皮生长因子的mRNA表达,亦证明脐带间充质干细胞可通过分泌抗炎因子减少卵巢损伤,恢复功能。
2.2.3 归巢 付霞菲等[15]在卵巢局部移植脐带间充质干细胞,可见到较多绿色荧光,说明脐带间充质干细胞能够在卵巢组织中定居、分布,具有向创伤组织定向趋化迁移的能力。近些年随着研究深入,大部分人认为脐带间充质干细胞归于卵巢髓质,而非卵泡细胞,可能与器官缺血、缺氧、损伤等有关。JALALIE 等[16]发现卵巢髓质中CM-Dil标记的脐带间充质干细胞数量高于卵巢皮质和生发上皮细胞层,此外皮质区标记的脐带间充质干细胞平均数量明显高于生发上皮细胞层,脐带间充质干细胞向髓质归巢可能是由于髓质主要由基质组织组成,同时富含血管。
2.2.4 抗纤维化 ZHOU等[17]发现移植脐带间充质干细胞组卵巢早衰大鼠的卵巢纤维化率明显降低,提示脐带间充质干细胞可能通过减轻卵巢组织纤维化,恢复卵巢功能。既往研究表明组织内基质金属蛋白酶组织抑制物 1、纤连蛋白和纤维形成相关因子基质金属蛋白酶9等与卵巢组织发生纤维化和功能异常密切相关。在张娟等[18]研究中发现脐带间充质干细胞能够达到改善卵巢功能的作用,是因为一方面通过自身释放血管内皮生长因子,另一方面通过上调卵巢组织内基质金属蛋白酶组织抑制物 1和纤连蛋白的表达、下调基质金属蛋白酶9的表达来实现的。
2.2.5 促血管生成 张娟等[19]发现卵巢早衰家兔移植脐带间充质干细胞后可以上调富半胱氨酸蛋白61和结缔组织生长因子表达,进而影响血管内皮生长因子表达,促进新生血管的形成,减轻卵巢损伤。QU等[8]同时发现miR-126-3p亦可在体内促进血管内皮生长因子、胰岛素样生长因子1和成纤维细胞生长因子的表达进而促进血管生成,恢复卵巢功能。
2.2.6 旁分泌 静脉注射脐带间充质干细胞可迁移到卵巢,但不能分化为卵泡成分细胞,而脐带间充质干细胞归巢能力是基于细胞黏附分子的表达量来决定,这有助于细胞迁移到目标组织。LI等[20]发现脐带间充质干细胞可分泌肝细胞生长因子、血管生长因子和胰岛素样生长因子。将脐带间充质干细胞经尾静脉移植到围绝经期大鼠后,卵巢中血管生长因子、肝细胞生长因子和胰岛素样生长因子1的表达明显增加,说明脐带间充质干细胞通过旁分泌机制改善围绝经期大鼠的卵巢储备功能。
2.2.7 免疫调节 LU等[21]研究发现,脐带间充质干细胞移植后,卵巢早衰小鼠健康卵泡总数增加,闭锁卵泡减少,子宫内膜中HOXA10基因表达明显增加,而CD56+CD16-uNK细胞表达减少,Th1/Th2比值亦显著降低,说明Th1/Th2细胞因子比例和uNK细胞的分泌调控可能参与了脐带间充质干细胞移植后卵巢早衰小鼠卵巢功能恢复和内皮接受性,但具体机制还需进一步研究。
2.2.8 自噬 自噬是一种通过溶酶体在细胞内部降解功能失调的细胞组分的复杂过程。YIN等[22]发现脐带间充质干细胞通过血红素加氧酶1激活JNK/Bcl-2信号通路调节自噬,上调CD8+CD28- T细胞的表达,帮助恢复卵巢早衰小鼠的卵巢功能。
2.3 骨髓间充质干细胞治疗卵巢早衰的机制探究 骨髓间充质干细胞是具有多向分化潜能的干细胞,能够增强内源性细胞功能,重建组织微环境,进而修复组织损伤;作为最先发现的间充质干细胞种类以及器官移植治疗的关键干细胞,具有向损伤组织趋化特性和多向分化的潜能。在卵巢早衰中的作用机制如表2所示。
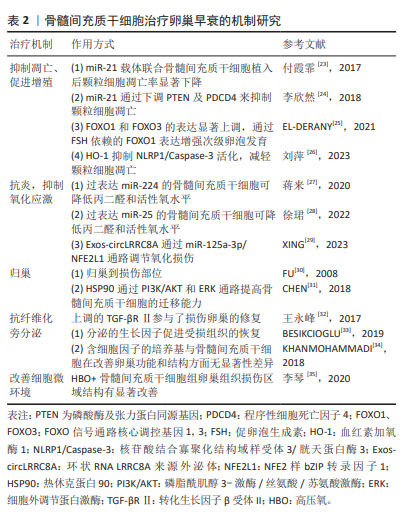
2.3.1 抑制凋亡、促进增殖 付霞菲等[23]研究发现转染miRNA-21慢病毒载体的骨髓间充质干细胞植入后颗粒细胞凋亡率显著下降,表明miR-21表达上调能抑制颗粒细胞的凋亡,增强其存活能力,但具体机制有待进一步研究。在后续的研究中,李欣然等[24]发现miR-21通过下调磷酸酶及张力蛋白同源基因和程序性细胞死亡因子4抑制颗粒细胞凋亡,增加骨髓间充质干细胞在化疗性卵巢早衰中的治疗潜能。EL-DERANY等[25]在研究中发现骨髓间充质干细胞处理后FOXO信号通路核心调控基因1和3的表达显著上调,通过促卵泡生成素依赖的FOXO信号通路核心调控基因1表达增强次级卵泡发育进而促进卵巢功能的恢复。血红素加氧酶1是机体内血红素氧化分解代谢过程中的限速酶,作为应急时相反应蛋白,具有抗凋亡作用。刘萍等[26]研究发现血红素加氧酶1基因转染骨髓间充质干细胞后NLRP1、Caspase-3蛋白相对表达水平均显著降低,说明血红素加氧酶1发挥抗凋亡作用可能是通过抑制NLRP1/Caspase-3通路活化实现。
2.3.2 抗炎、抑制氧化应激 氧化应激指体内氧化与抗氧化作用失衡的一种状态,倾向于氧化,导致中性粒细胞炎性浸润、蛋白酶分泌增加,产生大量氧化中间产物,被认为是导致衰老和疾病的一个重要因素。蒋来等[27]发现移植骨髓间充质干细胞可降低丙二醛和活性氧水平,且过表达miR-224的骨髓间充质干细胞亦可进一步降低丙二醛和活性氧水平,说明miR-224可通过降低氧化应激水平抑制顺铂诱导的卵巢颗粒细胞凋亡。后续研究中,徐珺等[28]发现转染过表达miR-25的骨髓间充质干细胞亦可进一步降低丙二醛和活性氧水平进而改善环磷酰胺诱导的卵巢早衰。在最新的一项研究中首次报道了环状RNA——circLRRC8A在卵巢中作用,它在衰老颗粒细胞中表达下调。XING等[29]研究显示环状RNA LRRC8A来源外泌体通过miR-125a-3p/NFE2L1信号通路调节颗粒细胞的氧化损伤进而减缓衰老,恢复卵巢功能。
2.3.3 归巢 归巢是指间充质干细胞在目标组织的脉管系统里被捕获,随后跨越血管内皮细胞迁移至目标组织的过程。早在2008年FU 等[30]就提出骨髓间充质干细胞的治疗作用主要是通过归巢到损伤部位和分泌旁分泌因子如血管生长因子、胰岛素样生长因子1和碱性成纤维细胞生长因子的观点。随着研究深入,在一项热休克预处理骨髓间充质干细胞对颗粒细胞凋亡的研究中发现,热休克预处理可诱导热休克转录因子的生成,从而激活特异性信号通路(如HSF1/miR-34a/HSP70)产生多种热休克蛋白,热休克蛋白90可通过PI3K/AKT和ERK通路提高骨髓间充质干细胞的迁移能力[31]。
2.3.4 抗纤维化 转化生长因子β受体Ⅱ具有传递信息和修复的作用。骨髓间充质干细胞移植后上调转化生长因子β受体Ⅱ抑制卵巢纤维化,促进损伤卵巢的修复,使化疗后卵巢能够有较多的卵泡存活[32]。
2.3.5 旁分泌 BESIKCIOGLU 等[33]认为骨髓间充质干细胞不仅直接影响受损组织,而且具有旁分泌和自分泌活性,受损组织的恢复是由干细胞分泌的生长因子、免疫调节剂、蛋白质和趋化因子促进的。KHANMOHAMMADI等[34]对比条件培养基(含分泌因子)与骨髓间充质干细胞在改善卵巢功能和结构方面无显著差异,说明骨髓间充质干细胞可以通过旁分泌途径分泌卵泡发育所需要的各种生长因子、趋化因子等促进功能与结构的恢复。
2.3.6 改善细胞微环境 李琴等[35]在高压氧联合骨髓间充质干细胞对卵巢早衰大鼠卵巢功能重建的研究中发现,当高压氧+骨髓间充质干细胞治疗后,通过苏木精-伊红染色可以观察到实验组卵巢组织损伤区域结构较卵巢早衰大鼠有显著改善,提示在高压氧作用下,骨髓间充质干细胞存活增加,迁移到损伤组织的数量增加,分泌更多营养因子,从而改善移植骨髓间充质干细胞生存微环境,更好地发挥受损卵巢的重建作用,为进一步探讨骨髓间充质干细胞对卵巢早衰的作用提供了新的方向。
2.4 人胎盘间充质干细胞治疗卵巢早衰的机制研究 人胎盘间充质干细胞因易于获得、分化和增殖潜能高、免疫原性低,被认为是临床治疗疾病的理想来源。在卵巢早衰中的作用机制如表3所示。
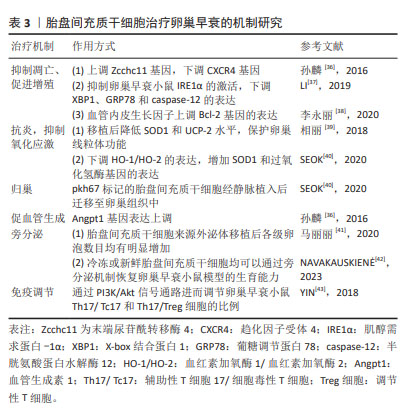
2.4.1 抑制凋亡、促进增殖 孙麟[36]发现移植人胎盘间充质干细胞后可以上调末端尿苷酰转移酶 4基因表达,下调趋化因子受体 4基因的表达。趋化因子受体 4基因表达下降,表明胎盘间充质干细胞可以促进原始卵泡发育为成熟卵泡;末端尿苷酰转移酶 4基因表达上调,表明胎盘间充质干细胞可以减少卵巢早衰介导的细胞凋亡,从而促进原始卵泡发育为成熟卵泡,这为进一步探究抗凋亡蛋白Bcl-2、促凋亡蛋白Bax/caspsase家族与末端尿苷酰转移酶 4基因是否有关联提供了新思路。在一项研究自身免疫卵巢早衰小鼠凋亡机制的研究中发现人胎盘间充质干细胞移植后抑制卵巢早衰小鼠肌醇需求蛋白1α的激活,下调X-box 结合蛋白1、葡糖调节蛋白78和caspase-12的表达,从而减少颗粒细胞凋亡,说明人胎盘间充质干细胞可以通过抑制肌醇需求蛋白-1α内质网应激通路的过度激活来减少颗粒细胞的凋亡[37]。李永丽等[38]研究发现随着培养时间延长,人胎盘间充质干细胞培养液中血管内皮生长因子分泌水平逐渐增加,通过上调Bcl-2基因的表达,从而调节卵巢细胞凋亡相关蛋白的表达以减少颗粒细胞的凋亡,但具体机制仍需进一步研究。
2.4.2 抗炎、抑制氧化应激 超氧化物歧化酶1和解耦联蛋白2在氧化应激中发挥重要作用。相丽等[39]发现人胎盘间充质干细胞移植后可改善大鼠氧化应激,超氧化物歧化酶1和解偶联蛋白2水平降低,保护卵巢线粒体功能。SEOK等[40]研究发现人胎盘间充质干细胞可以通过抑制卵巢组织中血红素加氧酶1/血红素加氧酶2的表达,增加超氧化物歧化酶1和过氧化氢酶基因的表达,进而提高抗氧化效果。
2.4.3 归巢 SEOK等[40]除了发现抗氧化机制,还发现有pkh67标记的人胎盘间充质干细胞会表达在卵巢组织中,卵巢组织中hAlu重复序列(这种DNA序列中有限制性内切核酸酶Alu的识别序列AGCT)的mRNA表达证明移植的人胎盘间充质干细胞在卵巢中显示出归巢活性。
2.4.4 促血管生成 血管生成素 1是一种血管生成调节蛋白,被认为是维持血管稳定性和完整性的关键因素。它有助于抑制血管渗漏和炎症反应,促进血管内皮细胞的成熟和稳定,从而对维持正常血管系统功能至关重要。孙麟[36]在研究中除发现末端尿苷酰转移酶 4、趋化因子受体 4凋亡相关基因变化,还发现植入人胎盘间充质干细胞后血管生成素 1的表达上调。上述研究充分表明人胎盘间充质干细胞有分化为血管内皮细胞的潜能。
2.4.5 旁分泌 间充质干细胞能够表达、合成、分泌各类生长因子、细胞因子、调节因子、信号肽等多种生物活性分子,为干细胞免疫调节、抗凋亡等提供了适宜的环境。马丽丽等[41]使用人胎盘间充质干细胞来源外泌体移植治疗化疗致卵巢损伤大鼠,结果显示卵巢内各级卵泡数目均有明显增加,证实了人胎盘间充质干细胞来源外泌体能够通过旁分泌的方式对化疗所致卵巢损伤发挥保护作用。在最新的一项对比低温保存胎盘组织和新鲜胎盘组织中分离出来的人胎盘间充质干细胞研究中发现,从低温保存胎盘组织中分离出的人胎盘间充质干细胞中促炎细胞因子基因表达升高;在淋巴细胞、单核细胞和中性粒细胞的趋化过程中起着关键作用的趋化因子CXCL12、CXCL6、CCL2和CXCL1的表达水平较高;此外,血管生成相关基因ANGPTL1、ANGPTL2、PDGFB和PDGFD在低温细胞中相对低表达,这可能会对卵巢早衰后血管发育和卵巢恢复产生负面影响。但无论是新鲜胎盘组织还是低温保存胎盘组织,以上各种细胞因子的分泌与表达均通过旁分泌途径[42]。
2.4.6 免疫调节 胎盘组织因其低免疫原性得到广泛关注。YIN等[43]通过研究发现人胎盘间充质干细胞移植后Treg细胞比例增加,Th17表达降低、血清白细胞介素17水平也降低,人胎盘间充质干细胞通过PI3K/Akt信号通路进而调节卵巢早衰小鼠的Th17/Tc17和Th17/Treg细胞比例,进而参与了卵巢功能的恢复。
2.5 经血来源间充质干细胞治疗卵巢早衰的机制研究 经血来源间充质干细胞作为一种成体干细胞,具有来源丰富、无创获取、易分离培养、增殖率高、传代次数较多、低免疫原性、无伦理争议等独特优势,在适当的刺激下可分化为骨细胞、脂肪细胞、软骨细胞、肝细胞和心肌细胞等,在再生医学中治疗多种疾病具有广阔的临床应用前景[44]。在卵巢早衰中的作用机制如表4所示。
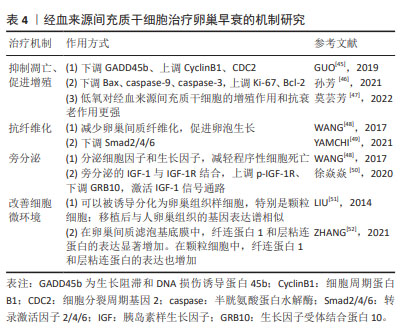
2.5.1 抑制凋亡、促进增殖 GUO等[45]在研究中发现植入经血来源间充质干细胞后会下调GADD45b表达、上调CyclinB1和CDC2表达,提示经血来源间充质干细胞可以通过抑制颗粒细胞凋亡减轻卵巢功能损伤。在卵巢生长发育及月经周期中,促性腺激素释放激素发挥重要作用。孙芳等[46]在一项将促性腺激素释放激素与经血来源间充质干细胞联合治疗对小鼠卵巢功能影响的研究中发现,联合治疗后可明显抑制小鼠卵巢颗粒细胞中 Bax、caspase-9、caspase-3的蛋白表达,促进Ki-67、Bcl-2的蛋白表达,为以后探索经血来源间充质干细胞的研究提供了新的方向。此外,莫芸芳等[47]通过对比在低氧条件下脐带间充质干细胞与经血来源间充质干细胞的各自生物特性得出结论:生理性低氧均可以促进两种细胞贴壁、增殖和代谢,但相较于脐带间充质干细胞,低氧对经血来源间充质干细胞的增殖作用和抗衰老作用更强。这对于改善经血来源间充质干细胞这种培养困难的细胞具有重大的意义。
2.5.2 抗纤维化 WANG等[48]在经血源间充质干细胞对小鼠卵巢早衰的修复作用研究中观察到注射到小鼠体内的浓缩条件培养基中含有丰富的成纤维细胞生长因子2,可以减少卵巢间质纤维化,促进卵泡生长,有效修复卵巢功能;但当使用siRNA敲除成纤维细胞生长因子2的表达时,经血来源间充质干细胞的修复活性显著消失,说明成纤维细胞生长因子2对血管生成和子宫内膜细胞的增殖和重塑至关重要。YAMCHI等[49]发现,当小鼠发生卵巢早衰后,促纤维化Smad2/4的表达增加,植入CD146+ 经血来源间充质干细胞后Smad2/4的表达减少;在经血来源间充质干细胞移植8周后,抑制性Smad6的mRNA水平显著下调,并恢复至基础水平,这充分说明经血来源间充质干细胞移植可通过TGFβ/Smad通路介导卵巢纤维化的逆转。但这种修复作用是否通过经典纤维化通路TGFβ/Smad文章中未做表述,这为后续研究提供了新思路。
2.5.3 旁分泌 WANG 等[48]发现经血来源间充质干细胞通过旁分泌途径分泌的细胞因子和生长因子,可以减轻程序性细胞死亡,进而促进卵泡发育和生长。徐焱焱等[50]研究发现经血来源间充质干细胞移植后,通过干细胞旁分泌的胰岛素样生长因子1与受体结合,促进磷酸化胰岛素样生长因子1受体的表达以及下调卵巢靶细胞GRB10的表达,激活胰岛素样生长因子1信号通路,促进PI3K-AKT-FOXO3a途径下游因子p-FOXO3a的表达,从而激活原始卵泡发育,改善卵巢早衰,充分说明了旁分泌机制在经血来源间充质干细胞改善卵巢早衰中的重要作用。
2.5.4 改善细胞微环境 细胞微环境不仅对细胞起支持、保护、连结和营养作用,而且与细胞的增殖、分化、代谢等基本生命活动密切相关。早在2014年LIU 等[51]就对经血来源间充质干细胞展开了研究,他们发现在卵巢早衰小鼠模型的卵巢组织中,经血来源间充质干细胞可以被诱导分化为卵巢组织样细胞,特别是卵巢颗粒样细胞。cDNA芯片检测结果显示,诱导前的经血来源间充质干细胞很少高水平表达在人类卵巢组织中发现的基因;然而,移植后的经血来源间充质干细胞在卵巢早衰卵巢中存活了几天,并观察到与人类卵巢组织中相似的基因表达谱,有力地说明了经血来源间充质干细胞被诱导分化为卵巢组织样细胞。ZHANG等[52]研究发现:在经血来源间充质干细胞和经血来源间充质干细胞外泌体中纤连蛋白1和层粘连蛋白的表达均显著增加,在颗粒细胞中纤连蛋白1和层粘连蛋白的表达也增加。既往有实验证明层粘连蛋白被认为是人类卵泡发育的重要细胞外基质成分。层粘连蛋白由卵母细胞分泌,已被证明在体外促进卵巢干细胞分化,充分说明经血来源间充质干细胞可以通过改善细胞微环境,进而促进卵巢功能恢复。
2.6 羊膜间充质干细胞治疗卵巢早衰的机制研究 羊膜间充质干细胞是由胎盘靠近胎儿一侧的羊膜组织分离而来的干细胞,具有多能干性、无限增殖性和抗炎特性、低免疫原性,是再生医学和细胞治疗临床应用的理想种子细胞。但目前有关羊膜间充质干细胞的研究较少,机制还需要更深一步研究。羊膜间充质干细胞在卵巢早衰中的作用机制研究如表5所示。
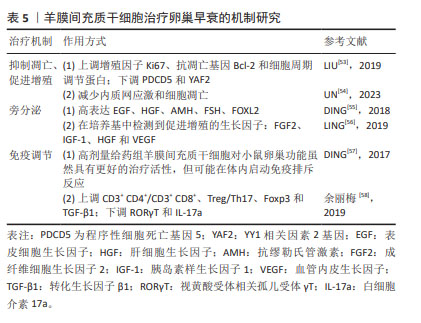
2.6.1 抑制凋亡、促进增殖 LIU等[53]研究发现羊膜间充质干细胞来源外泌体可上调增殖因子Ki67、抗凋亡基因Bcl-2、细胞周期调节蛋白CDK1、CDK2、CDK4、CDK5、CCND3和CCNH的表达,下调PDCD5和YAF2的表达,提示羊膜间充质干细胞来源外泌体对颗粒细胞的治疗作用是通过抑制YAF2/PDCD5/p53的表达来实现。UN 等[54]研究表明羊膜间充质干细胞条件培养基可以通过减少内质网应激和细胞凋亡,在电离辐射诱导的卵巢早衰中发挥治疗作用。
2.6.2 旁分泌 在DING等[55]研究中将含有羊膜间充质干细胞分泌的各种细胞因子和微囊泡的条件培养基浓缩并注射到自然衰老的大鼠双侧卵巢中,注射后高表达表皮生长因子和肝细胞生长因子,同时抗苗勒氏管激素、促卵泡生成素、FOXL2表达水平也有明显升高,充分说明羊膜间充质干细胞可通过旁分泌作用改善卵巢功能。LING 等[56]的研究中,除发现抑制凋亡、促进增殖机制外,同时还体现了旁分泌机制。在羊膜间充质干细胞条件培养基中可以检测到成纤维细胞生长因子2、胰岛素样生长因子1、肝细胞生长因子和血管内皮生长因子这些生长因子。
2.6.3 免疫调节 DING等[57]通过检测人卵巢颗粒细胞的增殖情况和人外周血单核细胞(CD4、CD11b、CD19、CD56)的免疫排斥反应来比较人羊膜上皮干细胞和人羊膜间充质干细胞之间的细胞生物学特性,如胶原分泌水平、端粒酶活性、免疫分子表达水平(HLA-ABC和HLA-DR)和细胞因子(生长因子、趋化因子、炎症因子、凋亡因子等),结果表明高剂量给药组人羊膜间充质干细胞对小鼠卵巢功能具有更好的治疗活性,可以促进卵巢早衰患者卵巢颗粒细胞增殖,可能与胶原蛋白的大量分泌有关;虽然人羊膜间充质干细胞比人羊膜上皮干细胞分泌更多的生长因子,但人羊膜间充质干细胞的转录因子OCT4和NANOG表达量高于人羊膜上皮干细胞,这可能导致在体内启动免疫排斥反应。余丽梅等[58]发现人羊膜间充质干细胞移植后上调CD3+ CD4+/CD3+ CD8+和Treg/Th17细胞比例,同时Treg细胞的标志基因Foxp3和转化生长因子β1 mRNA 表达亦上调,而Th17的标志基因RORγT 和白细胞介素17a表达下调,为进一步探索免疫调节机制提供了初步方向。
2.7 羊水间充质干细胞在卵巢早衰中的作用机制 羊水间充质干细胞具有易获取、低免疫原性、增殖稳定的特性,同时具有强大的自体细胞修复和再生能力。
2.7.1 抑制凋亡促进增殖 LIU等[59]发现CD44+/CD105+羊水间充质干细胞可以在卵巢早衰小鼠卵巢内存活至少3周,且CD44+/CD105+羊水间充质干细胞中增殖标志物Ki67和生存素水平显著高于CD44-/CD105- 羊水间充质干细胞。既往有研究表明受体激活C激酶1、肿瘤坏死因子受体相关因子6和BCL2L11基因与细胞凋亡相关,XIAO等[60]发现移植羊水间充质干细胞来源外泌体miR-10a后上述相关凋亡基因及Caspase-9表达显著下降,同时miR-146a和miR-10a可能协同发挥抗凋亡作用。GENG 等[61]研究发现羊水间充质干细胞来源外泌体可下调凋亡基因(Caspase-3和Caspase-9)、上调凋亡抗性基因(Bcl-2和Survivin),可能是通过miR-369-3p/YAF2/PDCD5/p53通路发挥作用。
2.7.2 旁分泌 HUANG等[62]在研究中发现羊水间充质干细胞可将正常衰老卵巢中不同发育阶段的标记物(血清中雌二醇、抗苗勒管激素、促卵泡生成素、FOXL2、CYP19A1)表达恢复到正常水平,说明羊水间充质干细胞可以通过分泌细胞因子来抵抗DNA损伤。
2.8 脂肪间充质干细胞在卵巢早衰中的作用机制 脂肪间充质干细胞具有取材容易、可获取量大、对机体损伤小等优点,逐渐成为研究热点[63]。宋开静等[64]研究发现不同浓度的脂肪间充质干细胞均能促进CD4+CD25+Foxp3+Treg细胞增殖,并上调Foxp3 mRNA的表达,且该作用随脂肪间充质干细胞数量的增高而增强,证明脂肪间充质干细胞能促进卵巢早衰患者外周血Treg细胞的增殖,从而发挥免疫调节作用。
2.9 其他来源间充质干细胞对卵巢早衰的治疗机制 除上述几种目前常见来源间充质干细胞,随着研究深入还发现了几种其他来源间充质干细胞对卵巢早衰的作用。如人绒毛膜来源间充质干细胞[65]、子宫内膜来源间充质干细胞[66-67]、脐血来源间充质干细胞[68]、皮肤来源间充质干细胞[69],这些干细胞来源大多亦通过抑制凋亡、促进增殖及归巢途径发挥作用。
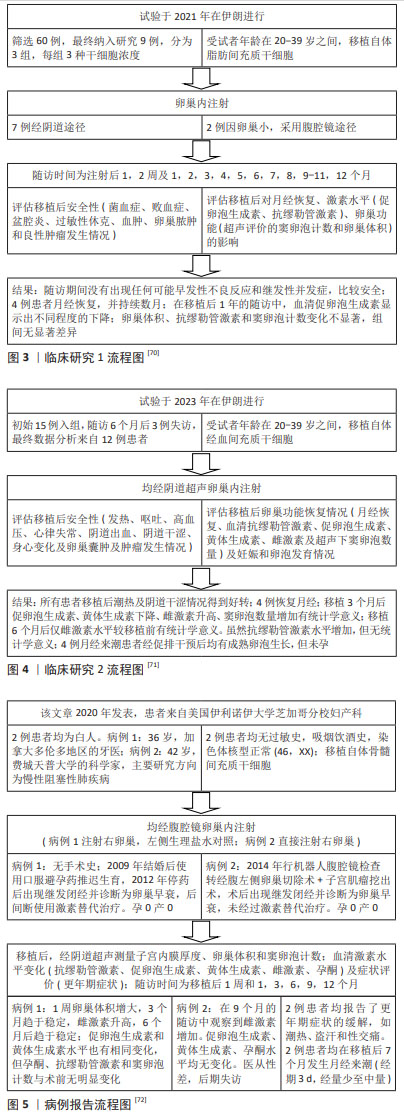
2.10 间充质干细胞的临床研究 因干细胞来源特殊,涉及伦理问题,故相关临床研究较少。目前中国尚无卵巢早衰临床研究,国外成功开展试验且报道的有2篇[70-71],另有一篇以病例报告形式报道[72]。3篇临床试验情况说明,见图3-5。
| [1] 郁琦,唐瑞怡.全方位提升早发性卵巢功能不全的诊治水平[J].中国实用妇科与产科杂志,2023,39(9):865-868. [2] LUBORSKY JL, MEYER P, SOWERS MF, et al. Premature menopause in a multi-ethnic population study of the menopause transition. Hum Reprod. 2003;18(1):199-206. [3] LIU W, NGUYEN TN, TRAN THI TV, et al. Kuntai Capsule plus Hormone Therapy vs. Hormone Therapy Alone in Patients with Premature Ovarian Failure: A Systematic Review and Meta-Analysis. Evid Based Complement Alternat Med. 2019;2019:2085804. [4] 吴洁,陈蓉.早发性卵巢功能不全的激素补充治疗专家共识[J].中华妇产科杂志,2016,51(12):881-886. [5] DING L, YAN G, WANG B, et al. Transplantation of UC-MSCs on collagen scaffold activates follicles in dormant ovaries of POF patients with long history of infertility. Sci China Life Sci. 2018; 61(12):1554-1565. [6] 尤斯涵,杨宋蕊,郭春燕,等.脐带间充质干细胞临床应用进展[J].河北北方学院学报(自然科学版),2022,38(2):57-60. [7] WANG J, ZHAO Y, ZHENG F, et al. Activated Human Umbilical Cord Blood Platelet-Rich Plasma Enhances the Beneficial Effects of Human Umbilical Cord Mesenchymal Stem Cells in Chemotherapy-Induced POF Rats. Stem Cells Int. 2021;2021:8293699. [8] QU Q, LIU L, CUI Y, et al. miR-126-3p containing exosomes derived from human umbilical cord mesenchymal stem cells promote angiogenesis and attenuate ovarian granulosa cell apoptosis in a preclinical rat model of premature ovarian failure. Stem Cell Res Ther. 2022;13(1):352. [9] 赵莹莹,陈莉莎,闫丽,等.脐带间充质干细胞外泌体对大鼠卵巢功能作用及机制研究[J].临床军医杂志,2022,50(11):1155-1158. [10] GAO T, CHEN Y, HU M, et al. MicroRNA-22-3p in human umbilical cord mesenchymal stem cell-secreted exosomes inhibits granulosa cell apoptosis by targeting KLF6 and ATF4-ATF3-CHOP pathway in POF mice. Apoptosis. 2023;28(7-8):997-1011. [11] NIU J, YU F, LUO X, et al. Human Umbilical Cord Mesenchymal Stem Cells Improve Premature Ovarian Failure through Cell Apoptosis of miR-100-5p/NOX4/NLRP3. Biomed Res Int. 2022;2022:3862122. [12] 谭丽,毛熙光,钟影, 等.人脐带间充质干细胞移植修复大鼠的卵巢早衰[J].中国比较医学杂志,2019,29(10):85-91. [13] SHI L, ZHANG Y, DONG X, et al. Toxicity from a single injection of human umbilical cord mesenchymal stem cells into rat ovaries. Reprod Toxicol. 2022;110:9-18. [14] DENG T, HE J, YAO Q, et al. Human Umbilical Cord Mesenchymal Stem Cells Improve Ovarian Function in Chemotherapy-Induced Premature Ovarian Failure Mice Through Inhibiting Apoptosis and Inflammation via a Paracrine Mechanism. Reprod Sci. 2021;28(6): 1718-1732. [15] 付霞霏,何援利.脐带间充质干细胞卵巢局部移植在卵巢早衰大鼠体内的分布[J].临床医学工程,2013,20(9):1081-1083. [16] JALALIE L, REZAIE MJ, JALILI A, et al. Distribution of the CM-Dil-Labeled Human Umbilical Cord Vein Mesenchymal Stem Cells Migrated to the Cyclophosphamide-Injured Ovaries in C57BL/6 Mice. Iran Biomed J. 2019;23(3):200-208. [17] ZHOU Y, ZHOU J, XU X, et al. Matrigel/Umbilical Cord-Derived Mesenchymal Stem Cells Promote Granulosa Cell Proliferation and Ovarian Vascularization in a Mouse Model of Premature Ovarian Failure. Stem Cells Dev. 2021;30(15):782-796. [18] 张娟,周丽,段瑶,等.脐带间充质干细胞对卵巢早衰模型家兔TIMP1、FN和MMP9表达的影响[J].中华生殖与避孕杂志,2021, 41(12):1071-1078. [19] 张娟,王晶,周丽,等.脐带间充质干细胞对卵巢早衰家兔性激素、CYR61和CTGF表达的影响[J].中国比较医学杂志,2020,30(3): 56-62. [20] LI J, MAO Q, HE J, et al. Human umbilical cord mesenchymal stem cells improve the reserve function of perimenopausal ovary via a paracrine mechanism. Stem Cell Res Ther. 2017;8(1):55. [21] LU X, CUI J, CUI L, et al. The effects of human umbilical cord-derived mesenchymal stem cell transplantation on endometrial receptivity are associated with Th1/Th2 balance change and uNK cell expression of uterine in autoimmune premature ovarian failure mice. Stem Cell Res Ther. 2019;10(1):214. [22] YIN N, WU C, QIU J, et al. Protective properties of heme oxygenase-1 expressed in umbilical cord mesenchymal stem cells help restore the ovarian function of premature ovarian failure mice through activating the JNK/Bcl-2 signal pathway-regulated autophagy and upregulating the circulating of CD8+CD28- T cells. Stem Cell Res Ther. 2020;11(1):49. [23] 付霞霏,何援利,王雪峰,等.miR-21慢病毒载体构建及对骨髓间充质干细胞凋亡的影响[J].中国组织工程研究,2017,21(1):1-5. [24] 李欣然,何援利,王雪峰,等.过表达miR-21的骨髓间充质干细胞在化疗性卵巢早衰大鼠模型中的治疗潜能及机制[J].现代妇产科进展,2018,27(12):924-928. [25] EL-DERANY MO, SAID RS, EL-DEMERDASH E. Bone Marrow-Derived Mesenchymal Stem Cells Reverse Radiotherapy-Induced Premature Ovarian Failure: Emphasis on Signal Integration of TGF-β, Wnt/β-Catenin and Hippo Pathways. Stem Cell Rev Rep. 2021;17(4):1429-1445. [26] 刘萍,黄健,谢宝国.血红素加氧酶-1基因转染骨髓间充质干细胞移植对化疗所致卵巢早衰的保护作用[J].中国临床药理学杂志, 2023,39(5):679-683. [27] 蒋来,陈玲,彭影,等.过表达miRNA-224骨髓间充质干细胞对卵巢颗粒细胞增殖和氧化应激的影响[J].陕西医学杂志,2020,49(2): 131-134. [28] 徐珺,陈珂,赵静.过表达miR-25的BMMSCs对POF动物模型卵巢超微结构和储备功能的影响[J].解剖学研究,2022,44(4):337-343. [29] XING J, ZHANG M, ZHAO S, et al. EIF4A3-Induced Exosomal circLRRC8A Alleviates Granulosa Cells Senescence Via the miR-125a-3p/NFE2L1 axis. Stem Cell Rev Rep. 2023;19(6):1994-2012. [30] FU X, HE Y, XIE C, et al. Bone marrow mesenchymal stem cell transplantation improves ovarian function and structure in rats with chemotherapy-induced ovarian damage. Cytotherapy. 2008;10(4): 353-363. [31] CHEN X, WANG Q, LI X, et al. Heat shock pretreatment of mesenchymal stem cells for inhibiting the apoptosis of ovarian granulosa cells enhanced the repair effect on chemotherapy-induced premature ovarian failure. Stem Cell Res Ther. 2018;9(1):240. [32] 王永峰,马会明,相丽,等.BMSCs移植对大鼠卵巢早衰的修复及TGF-βRⅡ表达的影响[J].宁夏医科大学学报,2017,39(6):627-630, 644. [33] BESIKCIOGLU HE, SARıBAS GS, OZOGUL C, et al. Determination of the effects of bone marrow derived mesenchymal stem cells and ovarian stromal stem cells on follicular maturation in cyclophosphamide induced ovarian failure in rats. Taiwan J Obstet Gynecol. 2019;58(1): 53-59. [34] KHANMOHAMMADI N, SAMENI HR, MOHAMMADI M, et al. Effect of Transplantation of Bone Marrow Stromal Cell- Conditioned Medium on Ovarian Function, Morphology and Cell Death in Cyclophosphamide-Treated Rats. Cell J. 2018;20(1):10-18. [35] 李琴,敖洪峰,李蕾,等.高压氧暴露协同骨髓间充质干细胞移植对卵巢早衰大鼠卵巢功能重建的研究[J].重庆医科大学学报, 2020,45(11):1551-1556. [36] 孙麟.人胎盘间充质干细胞治疗小鼠卵巢早衰的分子机制研究[J].实用妇科内分泌电子杂志,2016,3(21):64-65. [37] LI H, ZHAO W, WANG L, et al. Human placenta-derived mesenchymal stem cells inhibit apoptosis of granulosa cells induced by IRE1α pathway in autoimmune POF mice. Cell Biol Int. 2019;43(8):899-909. [38] 李永丽,陈冬梅,徐仙,等.胎盘间充质干细胞培养液对卵巢早衰模型大鼠卵巢BCL-2表达的影响[J].宁夏医学杂志,2020,42(8): 673-676. [39] 相丽,马会明,何艳桃,等.人胎盘间充质干细胞移植通过降低超氧化物歧化酶1和解耦联蛋白-2的表达提高卵巢功能[J].中华生殖与避孕杂志,2018,38(2):101-108. [40] SEOK J, PARK H, CHOI JH, et al. Placenta-Derived Mesenchymal Stem Cells Restore the Ovary Function in an Ovariectomized Rat Model via an Antioxidant Effect. Antioxidants (Basel). 2020;9(7):591. [41] 马丽丽,杨玲玲,杨海燕,等.胎盘间充质干细胞外泌体对大鼠卵巢早衰修复作用的研究[J].世界最新医学信息文摘(连续型电子期刊),2020,20(45):1-2,5. [42] NAVAKAUSKIENĖ R, ŽUKAUSKAITĖ D, BORUTINSKAITĖ VV, et al. Effects of human placenta cryopreservation on molecular characteristics of placental mesenchymal stromal cells. Front Bioeng Biotechnol. 2023;11:1140781. [43] YIN N, WANG Y, LU X, et al. hPMSC transplantation restoring ovarian function in premature ovarian failure mice is associated with change of Th17/Tc17 and Th17/Treg cell ratios through the PI3K/Akt signal pathway. Stem Cell Res Ther. 2018;9(1):37. [44] 许思娟.月经血源性间充质干细胞在女性生殖系统疾病中的应用进展[J].实用妇产科杂志,2021,37(5):354-357. [45] GUO F, XIA T, ZHANG Y, et al. Menstrual blood derived mesenchymal stem cells combined with Bushen Tiaochong recipe improved chemotherapy-induced premature ovarian failure in mice by inhibiting GADD45b expression in the cell cycle pathway. Reprod Biol Endocrinol. 2019;17(1):56. [46] 孙芳,韦伟.GnRH激动剂和经血源性干细胞联合治疗对小鼠卵巢功能的影响[J].南方医科大学学报,2021,41(12):1850-1856. [47] 莫芸芳,王泽剑,齐念民,等.生理性低氧条件下脐带和经血来源间充质干细胞的生物学特性[J].中国组织工程研究,2022,26(30): 4819-4825. [48] WANG Z, WANG Y, YANG T, et al. Study of the reparative effects of menstrual-derived stem cells on premature ovarian failure in mice. Stem Cell Res Ther. 2017;8(1):11. [49] YAMCHI NN, RAHBARGHAZI R, BEDATE AM, et al. Menstrual blood CD146+ mesenchymal stem cells reduced fibrosis rate in the rat model of premature ovarian failure. Cell Biochem Funct. 2021;39(8):998-1008. [50] 徐焱焱,颜贝,王锐,等.经血间充质干细胞通过IGF-1信号通路改善小鼠卵巢早衰[J].山东大学学报(医学版),2020,58(2):13-20. [51] LIU T, HUANG Y, ZHANG J, et al. Transplantation of human menstrual blood stem cells to treat premature ovarian failure in mouse model. Stem Cells Dev. 2014;23(13):1548-1557. [52] ZHANG S, HUANG B, SU P, et al. Concentrated exosomes from menstrual blood-derived stromal cells improves ovarian activity in a rat model of premature ovarian insufficiency. Stem Cell Res Ther. 2021;12(1):178. [53] LIU R, ZHANG X, FAN Z, et al. Human amniotic mesenchymal stem cells improve the follicular microenvironment to recover ovarian function in premature ovarian failure mice. Stem Cell Res Ther. 2019;10(1):299 [54] UN B, CETINKAYA-UN B, AKPOLAT M, et al. The effects of human amnion membrane-derived mesenchymal stem cells conditioned medium on ionizing radiation-induced premature ovarian failure and endoplasmic reticulum stress-related apoptosis mechanism. Eur J Obstet Gynecol Reprod Biol. 2023;288:191-197. [55] DING C, ZOU Q, WANG F, et al. Human amniotic mesenchymal stem cells improve ovarian function in natural aging through secreting hepatocyte growth factor and epidermal growth factor. Stem Cell Res Ther. 2018;9(1):55. [56] LING L, FENG X, WEI T, et al. Human amnion-derived mesenchymal stem cell (hAD-MSC) transplantation improves ovarian function in rats with premature ovarian insufficiency (POI) at least partly through a paracrine mechanism. Stem Cell Res Ther. 2019;10(1):46. [57] DING C, LI H, WANG Y, et al. Different therapeutic effects of cells derived from human amniotic membrane on premature ovarian aging depend on distinct cellular biological characteristics. Stem Cell Res Ther. 2017;8(1):173. [58] 余丽梅,刘荣霞,张小雨,等.人羊膜间充质干细胞治疗卵巢早衰的作用和机制[J].中国药理学与毒理学杂志,2019,33(10):919-920. [59] LIU T, HUANG Y, GUO L, et al. CD44+/CD105+ human amniotic fluid mesenchymal stem cells survive and proliferate in the ovary long-term in a mouse model of chemotherapy-induced premature ovarian failure. Int J Med Sci. 2012;9(7):592-602. [60] XIAO GY, CHENG CC, CHIANG YS, et al. Exosomal miR-10a derived from amniotic fluid stem cells preserves ovarian follicles after chemotherapy. Sci Rep. 2016;6:23120. [61] GENG Z, CHEN H, ZOU G, et al. Human Amniotic Fluid Mesenchymal Stem Cell-Derived Exosomes Inhibit Apoptosis in Ovarian Granulosa Cell via miR-369-3p/YAF2/PDCD5/p53 Pathway. Oxid Med Cell Longev. 2022;2022:3695848. [62] HUANG B, DING C, ZOU Q, et al. Human Amniotic Fluid Mesenchymal Stem Cells Improve Ovarian Function During Physiological Aging by Resisting DNA Damage. Front Pharmacol. 2020;11:272. [63] 王丽娟,梁晓磊,杨永秀.干细胞治疗卵巢早衰研究进展[J].中华生殖与避孕杂志,2021,41(1):85-88. [64] 宋开静,何援利,蔡慧华,等.异体脂肪间充质干细胞对卵巢早衰患者外周血调节性T细胞的影响[J].解放军医学杂志,2018,43(4): 294-298. [65] LI J, YU Q, HUANG H, et al. Human chorionic plate-derived mesenchymal stem cells transplantation restores ovarian function in a chemotherapy-induced mouse model of premature ovarian failure. Stem Cell Res Ther. 2018;9(1):81. [66] LAI D, WANG F, YAO X, et al. Human endometrial mesenchymal stem cells restore ovarian function through improving the renewal of germline stem cells in a mouse model of premature ovarian failure. J Transl Med. 2015;13:155. [67] YAN Z, GUO F, YUAN Q, et al. Endometrial mesenchymal stem cells isolated from menstrual blood repaired epirubicin-induced damage to human ovarian granulosa cells by inhibiting the expression of Gadd45b in cell cycle pathway. Stem Cell Res Ther. 2019;10(1):4. [68] ELFAYOMY AK, ALMASRY SM, EL-TARHOUNY SA, et al. Human umbilical cord blood-mesenchymal stem cells transplantation renovates the ovarian surface epithelium in a rat model of premature ovarian failure: Possible direct and indirect effects. Tissue Cell. 2016;48(4):370-382. [69] LAI D, WANG F, DONG Z, et al. Skin-derived mesenchymal stem cells help restore function to ovaries in a premature ovarian failure mouse model. PLoS One. 2014;9(5):e98749. [70] MASHAYEKHI M, MIRZADEH E, CHEKINI Z, et al. Evaluation of safety, feasibility and efficacy of intra-ovarian transplantation of autologous adipose derived mesenchymal stromal cells in idiopathic premature ovarian failure patients: non-randomized clinical trial, phase I, first in human. J Ovarian Res. 2021;14(1):5. [71] ZAFARDOUST S, KAZEMNEJAD S, DARZI M, et al. Intraovarian Administration of Autologous Menstrual Blood Derived-Mesenchymal Stromal Cells in Women with Premature Ovarian Failure. Arch Med Res. 2023;54(2):135-144. [72] IGBOELI P, EL ANDALOUSSI A, SHEIKH U, et al. Intraovarian injection of autologous human mesenchymal stem cells increases estrogen production and reduces menopausal symptoms in women with premature ovarian failure: two case reports and a review of the literature. J Med Case Rep. 2020;14(1):108. |
| [1] | 周盼盼, 崔应麟, 张文涛, 王姝瑞, 陈佳慧, 杨 潼. 细胞自噬在脑缺血损伤中的作用及中药调控机制[J]. 中国组织工程研究, 2025, 29(8): 1650-1658. |
| [2] | 杨治航, 孙祖延, 黄文良, 万 喻, 陈仕达, 邓 江. 神经生长因子促进兔骨髓间充质干细胞软骨分化并抑制肥大分化[J]. 中国组织工程研究, 2025, 29(7): 1336-1342. |
| [3] | 胡涛涛, 刘 兵, 陈 诚, 殷宗银, 阚道洪, 倪 杰, 叶凌霄, 郑祥兵, 严 敏, 邹 勇. 过表达神经调节蛋白1的人羊膜间充质干细胞促进小鼠皮肤创面愈合[J]. 中国组织工程研究, 2025, 29(7): 1343-1349. |
| [4] | 金 凯, 唐 婷, 李美乐, 谢裕安. 人脐带间充质干细胞条件培养基及外泌体对肝癌细胞增殖、迁移、侵袭和凋亡的影响[J]. 中国组织工程研究, 2025, 29(7): 1350-1355. |
| [5] | 李帝均, 酒精卫, 刘海峰, 闫 磊, 李松岩, 王 斌. 明胶三维微球装载人脐带间充质干细胞修复慢性肌腱病[J]. 中国组织工程研究, 2025, 29(7): 1356-1362. |
| [6] | 刘 琪, 李林臻, 李玉生, 焦泓焯, 杨 程, 张君涛. 淫羊藿苷含药血清促进3种细胞共培养体系中软骨细胞增殖和干细胞成软骨分化[J]. 中国组织工程研究, 2025, 29(7): 1371-1379. |
| [7] | 章镇宇, 梁秋健, 杨 军, 韦相宇, 蒋 捷, 黄林科, 谭 桢. 新橙皮苷治疗骨质疏松症的靶点及对骨髓间充质干细胞成骨分化的作用[J]. 中国组织工程研究, 2025, 29(7): 1437-1447. |
| [8] | 李佳林, 张耀东, 娄艳茹, 于 洋, 杨 蕊. 间充质干细胞分泌组发挥作用的分子机制[J]. 中国组织工程研究, 2025, 29(7): 1512-1522. |
| [9] | 何 波, 陈 文, 马岁录, 何志军, 宋 渊, 李金鹏, 刘 涛, 魏晓涛, 王威威, 谢 婧. 皮瓣缺血再灌注损伤的发病机制及治疗进展[J]. 中国组织工程研究, 2025, 29(6): 1230-1238. |
| [10] | 赵晓璇, 刘帅祎, 李 奇, 邢 政, 李庆雯, 褚晓蕾. 不同运动方式促进周围神经损伤后的功能恢复[J]. 中国组织工程研究, 2025, 29(6): 1248-1256. |
| [11] | 张文华, 李 荀, 张伟超, 李欣颖, 马帼澳, 王孝强. SphK1/S1P/S1PR2信号通路促进肌生成:运动改善骨骼肌健康的新视角[J]. 中国组织工程研究, 2025, 29(6): 1265-1275. |
| [12] | 吴广涛, 秦 刚, 何凯毅, 范以东, 李威材, 朱宝刚, 曹 英. 免疫细胞与膝骨关节炎之间因果作用:一项两样本双向孟德尔随机化分析[J]. 中国组织工程研究, 2025, 29(5): 1081-1090. |
| [13] | 孙现娟, 王秋花, 张锦艺, 杨杨杨, 王文双, 张晓晴. 不同静电纺丝膜上骨髓间充质干细胞的黏附、增殖与成血管平滑肌分化[J]. 中国组织工程研究, 2025, 29(4): 661-669. |
| [14] | 张 敏, 张霓霓, 黄桂林, 李壮壮, 王 雪, 王辉科. 人羊膜间充质干细胞外泌体修复大鼠放射性颌下腺损伤[J]. 中国组织工程研究, 2025, 29(36): 7804-7815. |
| [15] | 王清芳, 张 芬, 昌广萍, 李子晗, 邢 岚, 彭 浩, 曾秀萍, 钟桂强, 陈 辉, 刘 波, 刘振宇, 梁 晓. 一种新型冷冻保护剂冻存组织和细胞的效果[J]. 中国组织工程研究, 2025, 29(36): 7816-7826. |
目前卵巢早衰的主要治疗措施包括调整生活方式、激素替代治疗、供卵体外受精-胚胎移植等[4],这些临床治疗方法虽然能够改善卵巢早衰症状并促进生育,但无法从根本上逆转卵巢功能,且供卵存在伦理争议等问题。因此,寻找一种能够安全有效地逆转卵巢功能的治疗手段是今后主要的研究方向。
间充质干细胞可以从各种组织中分离出来,主要来源于脐带、骨髓、胎盘、经血、羊膜、羊水、脂肪等。2018年中国通过一项单中心随机对照试验表明胶原蛋白联合脐带间充质干细胞能明显改善卵巢早衰患者的卵巢功能,并通过试管技术可使患者成功受孕[5]。
该文章就不同来源间充质干细胞改善卵巢早衰的机制及相关临床研究进行综述,从而为间充质干细胞的深入研究提供理论依据。
中国组织工程研究杂志出版内容重点:干细胞;骨髓干细胞;造血干细胞;脂肪干细胞;肿瘤干细胞;胚胎干细胞;脐带脐血干细胞;干细胞诱导;干细胞分化;组织工程
1.1.1 检索人及检索时间 第一作者在2023年10月进行检索。
1.1.2 检索文献时限 2010年1月至2023年10月。
1.1.3 检索数据库 中国知网、万方数据库、中华医学库及PubMed数据库。
1.1.4 检索词 中文检索词为“间充质干细胞,卵巢早衰”;英文检索词为“(mesenchymal stem cells) AND (premature ovarian failure)”。
1.1.5 检索文献类型 基础研究、临床研究及综述类文献。
1.1.6 检索文献量 共检索到408篇文献,包括中文文献292篇,英文文献116篇。
1.2 纳入和排除标准
1.2.1 纳入标准 ①各类间充质干细胞治疗卵巢早衰的研究;②各类间充质干细胞治疗卵巢早衰的机制研究;③各类间充质干细胞来源外泌体治疗卵巢早衰及机制研究;④间充质干细胞与miRNA共同治疗卵巢早衰的机制研究;⑤各类间充质干细胞的临床研究。
1.2.2 排除标准 ①重复性及相似性研究:机制内容相同或者十分相似的文章,则只选取发布较早的文献加以综述;②缺乏可靠数据及数据不严谨的文献;③单纯功能性研究,未涉及治疗机制的文献。
1.3 文献筛选过程及质量评估 共检索到408篇文献,其中中国知网147篇,万方数据库140篇,中华医学库5篇,PubMed数据库116篇。排除与研究目的不符、内容重复、数据不可靠的文章,资料收集者仔细阅读筛选,互相评估纳入文献的适用性,最终纳入72篇文献。文献筛选流程图,见图1。
中国组织工程研究杂志出版内容重点:干细胞;骨髓干细胞;造血干细胞;脂肪干细胞;肿瘤干细胞;胚胎干细胞;脐带脐血干细胞;干细胞诱导;干细胞分化;组织工程
除外共有途径,每种细胞又有各自的侧重机制。如脐带间充质干细胞在抗炎及氧化应激和促血管生成方面研究较多,在自噬方面研究较少;骨髓间充质干细胞在抗炎及氧化应激方面研究较多,但在改善细胞微环境及抗纤维化方面研究较少;胎盘间充质干细胞同样在抗炎及氧化应激方面研究较多,但其他机制如促血管生成、归巢、免疫调节方面还较少;经血来源间充质干细胞在抗纤维化及改善细胞微环境方面研究相当,但是否会有其他机制如促血管生成、免疫调节未见报道;羊膜间充质干细胞除共有机制外,仅有免疫调节机制已研究,其他机制仍需进一步研究;羊水间充质干细胞及脂肪间充质干细胞目前研究较少:羊水间充质干细胞除共有机制外,未见其他机制报道;脂肪间充质干细胞虽然没有共有机制发挥作用,但免疫调节机制已有研究。
无论哪种机制,在发挥作用时都不是独立发挥作用,而是相辅相成、共同参与的。
3.2 作者综述区别于他人他篇的特点 文章全面且系统地从目前细胞及动物实验阶段所涉及的常用间充质干细胞种类、作用机制进行综合阐述,区别于此前分别阐述间充质干细胞作用或机制研究的综述;另外将现有临床研究也纳入,为间充质干细胞能有效改善卵巢早衰患者卵巢功能提供了有力证据,为后续深入研究提供了新的方向及理论基础。此外,卵巢早衰作为早发性卵巢功能不全的终末阶段,在研究时易混淆,在选取参考文献时严格参考卵巢早衰诊断标准。
3.3 综述的局限性 间充质干细胞的研究追溯至今已有47年历史,但该文仅选取了近10年内的部分代表性文献进行综述,可能会对早期的重要文献有所疏漏。虽然该文纳入的参考文献中关于脂肪间充质干细胞研究较少,但脂肪间充质干细胞的发展历程与脐带间充质干细胞相当,课题组后续将持续关注脂肪间充质干细胞的研究方向。另外该文章仅综述了卵巢早衰的相关文献,对早发性卵巢功能不全未进行综述。
3.4 综述的重要意义 近年卵巢早衰的发病率呈逐年上升趋势,但随着研究深入,需要对现有研究种类及机制进行归纳总结。首先,目前机制研究较透彻的为脐带间充质干细胞,其次为骨髓间充质干细胞,其余各种来源间充质细胞的作用机制尚且存在空白区域,尤其是经血来源间充质干细胞及脂肪间充质干细胞,这将给后续科研提供了新的方向。其次,虽然目前国内外临床研究已逐渐开展,但所包含样本量较少,不足以证明间充质干细胞能够在无不良反应或者不良反应较小的情况下改善患者卵巢功能甚至促进生育,为后续开展临床研究提供了改进方案。最后,间充质干细胞作为一种具有多向分化功能且免疫原性低、易获得等特点的细胞,相信在不久的将来能够应用于临床,能够改善更多卵巢早衰患者的生活质量。
中国组织工程研究杂志出版内容重点:干细胞;骨髓干细胞;造血干细胞;脂肪干细胞;肿瘤干细胞;胚胎干细胞;脐带脐血干细胞;干细胞诱导;干细胞分化;组织工程
文题释义:
间充质干细胞:来源于发育早期的中胚层和外胚层,属于多能干细胞,具有多向分化、造血支持、促进干细胞植入、免疫调控和自我复制等潜能。间充质干细胞主要存在于结缔组织和器官间质中,包括骨髓、胎盘、脐带、脂肪、黏膜、骨骼、肌肉、肺、肝、胰腺、羊水、羊膜、脐血等。#br#卵巢早衰:指女性40岁以前出现闭经、促性腺激素水平升高和雌激素水平降低,并伴有不同程度的低雌激素症状,是早发性卵巢功能不全的终末阶段,严重影响女性生理及心理健康。
#br#
中国组织工程研究杂志出版内容重点:干细胞;骨髓干细胞;造血干细胞;脂肪干细胞;肿瘤干细胞;胚胎干细胞;脐带脐血干细胞;干细胞诱导;干细胞分化;组织工程
间充质干细胞是一种多能干细胞,可以从脐带、骨髓、血管外周以及脂肪中获取,对治疗脑卒中、肝硬化、糖尿病、心血管疾病等具有重要意义。根据最新的Clinical Trials.gov临床注册数据显示,目前全球与间充质干细胞相关的临床试验有1600多项,覆盖1170多种疾病,其中涉及卵巢早衰的临床试验有15例,中国注册的临床试验有3项,但成功完成的仅有1例。这说明间充质干细胞逐渐走进研究人员视野,不过应用于卵巢早衰方面还有限。相信在不久的将来,关于卵巢早衰的临床试验也会陆续开展,给卵巢早衰的患者带来福音。
#br#
中国组织工程研究杂志出版内容重点:干细胞;骨髓干细胞;造血干细胞;脂肪干细胞;肿瘤干细胞;胚胎干细胞;脐带脐血干细胞;干细胞诱导;干细胞分化;组织工程
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||