| [1] Liao D, Miller EC, Teravskis PJ.Tau Acts as a Mediator for Alzheimer's Disease-Related Synaptic Deficits. Eur J Neurosci.2014;39(7): 1202-1213.
[2] Kovalevich J, Corley G, Yen W, et al.Cocaine decreases expression of neurogranin via alterations in thyroid receptor /retinoid X receptor signaling. J Neurochem. 2012;121(2): 302-313.
[3] Zhong L, Kaleka KS, Gerges NZ. Neurogranin phosphorylation fine-tunes long-term potentiation. Eur J Neurosci. 2011;33(2): 244-250.
[4] Burger LL, Haisenleder DJ, Aylor KW, et al..Regulation of Intracellular Signaling Cascades by GNRH Pulse Frequency in the Rat Pituitary: Roles for CaMK II,ERK,and JNK Activation. Biol Reprod. 2008;79(5):947-953.
[5] Waggener CT, Dupree JL, Elgersma Y. CaMKIIβ Regulates Oligodendrocyte Maturation and CNS Myelination. J Neurosci. 2013;33(25):10453-10458.
[6] Tsai NP, Wilkerson JR, Guo W, et al.Multiple autism-linked genes mediate synapse elimination via proteasomal degradation of a synaptic scaffold PSD-95.J Cell.2012;151(7): 1581-1594.
[7] Bonnet SA, Akad DS, Samaddar T, et al.Synaptic State-Dependent Functional Interplay between Postsynaptic Density-95 and Synapse-Associated Protein 102. J Neurosci. 2013;33(33):13398-13409.
[8] Xu W, Schlüter OM, Steiner P, et al.Molecular dissociation of the role of PSD-95 in regulating synaptic strength and LTD.J Neuron.2008;57(2):248-262.
[9] Lee YW, Stachowiak EK, Birkaya B, et al.NGF-Induced Cell Differentiation and Gene Activation is Mediated by Integrative Nuclear FGFR1 Signaling(INFS).J PLoS ONE.2013;8(7): e68931.
[10] 王建秀,王德生,段淑荣.Aβ25-35对PC12细胞损伤作用的研究[J].中风与神经疾病杂志,2007;24(2):148.
[11] Guo YB, Shao YM, Chen J, et al..Ethyl acetate extracts of Fructus Ligustri Lucide induce cell apoptosis in human neuroglioma cell.Int J Clin Exp Med.2015;8(3):3833-3840.
[12] 唐薇婷,冯莉,肖波,等.QDs-SA 和Cy3荧光标记阿尔茨海默病细胞模型β淀粉样蛋白的比较研究[J].中国临床神经科学, 2010, 18(3):225-230.
[13] 陈萌,任宁,刘文君,等.免疫荧光双标法在小鼠表皮干细胞分子标记筛选中的应用.山东大学学报:医学版,2011,49(4):1671-7554.
[14] Prà ID, Chiarini A, Armato U. Antagonizing amyloid-β/ calcium-sensing receptor signaling in human astrocytes and neurons: a key to halt Alzheimer’s disease progression?. Neural Regen Res. 2015; 10(2): 213-218.
[15] Bass B, Upson S, Roy K, et al. Glycogen and amyloid-beta: key players in the shift from neuronal hyperactivity to hypoactivity observed in Alzheimer’s disease?. Neural Regen Res. 2015; 10(7): 1023-1025.
[16] Grasselli G, Mandolesi G, Strata P,et al.Impaired Sprouting and Axonal Atrophy in Cerebellar Climbing Fibres following In Vivo Silencing of the Growth-Associated Protein GAP-43. PLoS One. 2011;6(6):e20791.
[17] Feng X,Liang N,Zhu D,et al.Resveratrol Inhibits β-Amyloid-Induced Neuronal Apoptosis through Regulation of SIRT-ROCK1 Signaling Pathway. PLoS One. 2013;8(3): e59888.
[18] Alzheimer A.About a peculiar disease of the cerebral cortex.(Translated by Jarvik L and Greenson H).J Alzheimer Dis Assoc Disord.1987;1:3-8.
[19] Hardy JA,Higgins A.Alzheimer's disease:the amyloid cascade hypothesis.Science 1992;256(5054):184-185.
[20] Hardy J,Selkoe DJ.The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science.2002;297(5580):353-356.
[21] Tannenberg RK,Scott HL,Tannenberg AE,et al.Selective loss of synaptic proteins in Alzheimer's disease:evidence for an increased severity with APOE varepsilon4.J Neurochem Int.2006;49(7):631-639.
[22] Han SD, Gruhl J, Beckett L,et al.Beta Amyloid,Tau, Neuroimaging, and Cognition:Sequence Modeling of Biomarkers for Alzheimer's Disease.J Brain Imaging Behav. 2012;6(4):610-620.
[23] Spencer B,Rockenstein E,Crews L,et al.Novel strategies for Alzheimer's disease treatment.J Expert Opin Biol Ther.2007; 7(12):1853-1867.
[24] Rowan MJ,Klyubin I,Wang Q,et al.Synaptic memory mechanisms: Alzheimer's disease amyloid beta-peptide- induced dysfunction.J Biochem Soc Trans,2007;35(5): 1219-1223.
[25] Malabou C.Aging and psychological plasticity:contradiction or new therapeutic challenge?J Encephale.2006;32(5): S628-631.
[26] Gengler S,Gault VA,Harriott P,et al.Impairments of hippocampal synaptic plasticity induced by aggregated beta-amyloid(25-35)are dependent on stimulation protocol and genetic background.J Exp Brain Res.2007;179(4): 621-630.
[27] Small DH.Mechanisms of synaptic homeostasis in Alzheimer's disease. J Curr Alzheimer Res.2004;1(1):27-32.
[28] Wang HW,Pasternak JF,Kuo H,et al.Soluble oligomers of beta amyloid(1-42) inhibit long-term potentiation but not long-term depression in rat dentate gyrus.J Brain Res.2002;924: 133- 140.
[29] Musiek Erik S, Holtzman D M. Origins of Alzheimer's Disease: Reconciling CSF biomarker and neuropathology data regarding the temporal sequence of Aβ and tau involvement.J Curr Opin Neurol.2012;25(6): 715-720.
[30] Lacor PN,Buniel MC,Chang L,et al.Synaptic targeting by Alzheimer's-related amyloid beta oligomers.J Neurosci. 2004;24:10191-10200.
[31] Terry RD,Masliha E,Salmon D,et al.Physical basis Of cognitive alterations in Alzheimer’s disease:Synapse loss is the major correlate of cognitive impairment.J Ann Neurol.1991; 30(4):572-580.
[32] Kanekiyo T, Bu G.The low-density lipoprotein receptor-related protein 1 and amyloid-β clearance in Alzheimer’s disease. Front Aging Neurosci.2014;6:93. |
.jpg)
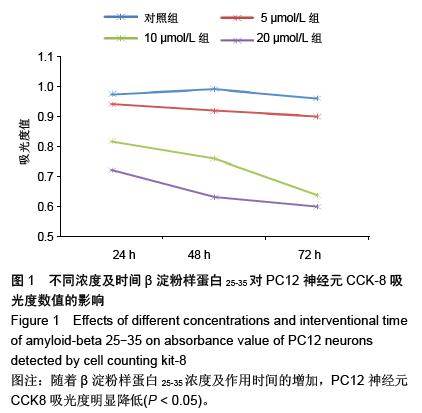
.jpg)

.jpg)

.jpg)