中国组织工程研究 ›› 2025, Vol. 29 ›› Issue (12): 2560-2568.doi: 10.12307/2025.378
• 组织构建综述 tissue construction review • 上一篇 下一篇
天然产物调控氧化应激治疗脊髓损伤
张孝炜1,闫炳翰1,仇道迪2,薛海鹏2,谭国庆2,徐展望2
- 1山东中医药大学,山东省济南市 250000;2山东中医药大学附属医院,山东省济南市 250000
-
收稿日期:2024-04-03接受日期:2024-06-11出版日期:2025-04-28发布日期:2024-09-10 -
通讯作者:徐展望,博士,主任医师,山东中医药大学附属医院,山东省济南市 250000 -
作者简介:张孝炜,男,1998年生,山东中医药大学在读硕士,主要从事脊柱脊髓损伤和脊柱关节退行性疾病相关研究。 -
基金资助:国家自然科学基金面上项目(82174410),项目负责人:徐展望;山东省自然科学基金项目(ZR2020KH011),项目负责人:徐展望;山东省自然科学基金面上项目(ZR2020MH362),项目负责人:谭国庆;全国名老中医药专家传承工作室建设项目(国中医药人教函[2022]75号),项目负责人:徐展望
Natural products regulate oxidative stress in the treatment of spinal cord injury
Zhang Xiaowei1, Yan Binghan1, Qiu Daodi2, Xue Haipeng2, Tan Guoqing2, Xu Zhanwang2
- 1Shandong University of Traditional Chinese Medicine, Jinan 250000, Shandong Province, China; 2Affiliated Hospital of Shandong University of Traditional Chinese Medicine, Jinan 250000, Shandong Province, China
-
Received:2024-04-03Accepted:2024-06-11Online:2025-04-28Published:2024-09-10 -
Contact:Xu Zhanwang, MD, Chief physician, Affiliated Hospital of Shandong University of Traditional Chinese Medicine, Jinan 250000, Shandong Province, China -
About author:Zhang Xiaowei, Master candidate, Shandong University of Traditional Chinese Medicine, Jinan 250000, Shandong Province, China -
Supported by:National Natural Science Foundation of China (General Program), No. 82174410 (to XZW); Shandong Natural Science Foundation, No. ZR2020KH011 (to XZW); Shandong Natural Science Foundation (General Project), No. ZR2020MH362 (to TGQ); National Famous Traditional Chinese Medicine Expert Inheritance Studio Construction Project, No. [2022]75 (to XZW)
摘要:
文题释义:
氧化应激:是指机体内氧化与抗氧化作用失衡,高活性分子如活性氧自由基产生过多并累积,损害细胞核酸、蛋白质、脂质和其他结构成分,导致细胞不可逆的坏死和变性。
天然产物:是指从自然界中的植物、动物、昆虫、海洋生物和微生物等体内提取或分离得到的化合物或物质,它们具有特殊的生理功能和抗氧化活性。
背景:脊髓损伤是一种严重的神经系统疾病,常导致神经功能严重受损。在脊髓损伤后的病理过程中,氧化应激是一个重要环节,导致神经细胞死亡和功能丧失。近年来,天然产物因来源广泛、结构多样且生物活性丰富,逐渐在脊髓损伤后的氧化应激治疗中展现出潜在的应用价值。
目的:讨论部分天然产物在脊髓损伤后氧化应激过程中的治疗作用以及相关作用机制,以期为脊髓损伤的抗氧化治疗提供新的思路和方向。
方法:以“spinal cord injury,oxidative stress,anti-oxidation,natural products,natural compounds,polyphenols”“脊髓损伤,氧化应激,抗氧化,天然产物,天然化合物,多酚”为关键词,在PubMed、Web of Science、Embase、Cochrane、维普、CBM、万方和中国知网数据库检索自建库以来至2024年5月的相关文献。制定纳入和排除标准,通过阅读文献标题、摘要及全文内容进行筛选,最终纳入97篇相关文献。
结果与结论:①天然产物如多酚类等可以依靠其结构中的酚羟基发挥直接清除氧化自由基的作用,减轻脊髓损伤后的氧化应激反应;②一些天然产物可以通过调控某些信号转导通路增强体内相关抗氧化酶的活性,减轻氧化应激;③部分天然产物可以通过增强自噬的方式减弱脊髓损伤后的氧化应激;④利用天然产物调控氧化应激可能成为今后临床上治疗脊髓损伤的有效工具。
https://orcid.org/0009-0003-7065-8107(张孝炜)
中国组织工程研究杂志出版内容重点:组织构建;骨细胞;软骨细胞;细胞培养;成纤维细胞;血管内皮细胞;骨质疏松;组织工程
中图分类号:
引用本文
张孝炜, 闫炳翰, 仇道迪, 薛海鹏, 谭国庆, 徐展望. 天然产物调控氧化应激治疗脊髓损伤[J]. 中国组织工程研究, 2025, 29(12): 2560-2568.
Zhang Xiaowei, Yan Binghan, Qiu Daodi, Xue Haipeng, Tan Guoqing, Xu Zhanwang . Natural products regulate oxidative stress in the treatment of spinal cord injury[J]. Chinese Journal of Tissue Engineering Research, 2025, 29(12): 2560-2568.
氢酶和谷胱甘肽过氧化物酶等),将活性氧转化为低毒性的小分子[21];当第二层防线仍不足时,各种氧化剂破坏细胞,将激活第三层防线——各种修复和清除酶,修复受损的细胞和组织[22]。当活性氧与内源性抗氧化防御机制失去平衡时,过多的活性氧会损害细胞核酸、蛋白质、脂质和其他结构成分,激活多种细胞凋亡途径,导致细胞死亡[23-25]。
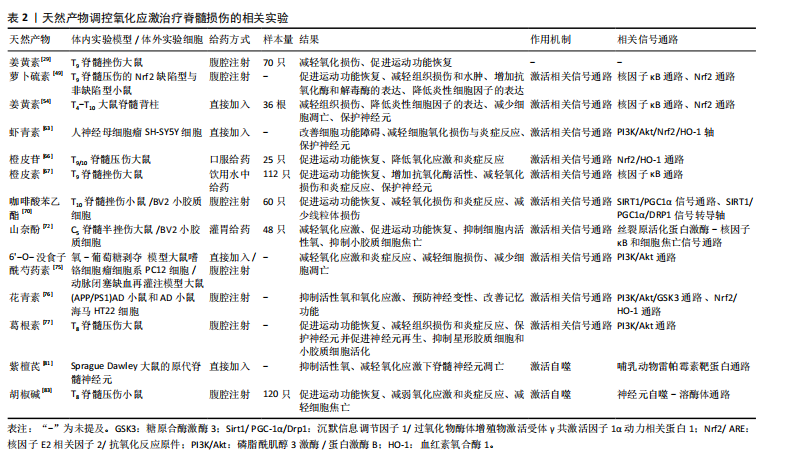
2.2 天然产物直接清除脊髓损伤自由基 常见的天然产物分类见表1。

多酚是一类广泛存在于天然植物中的化合物,因其卓越的抗氧化性能在预防医学范围内已经应用了几个世纪。近年来,许多研究表明多酚类天然产物对于活性氧自由基有直接的清除作用,姜黄素是一种天然的具有生物活性的酚类化合物,是天然中草药姜黄的提取物。AK等[26]
通过采用各种体外抗氧化测定方法,发现姜黄素具有有效的超氧阴离子、过氧化氢清除能力。过氧亚硝酸盐是由超氧阴离子和一氧化氮反应产生的细胞毒性中间体,KIM等[27]通过体外实验的方式,发现姜黄素具有过氧亚硝酸盐清除活性。SUMANONT等[28]采用硝普钠生成一氧化氮系统,通过比较多种抗氧化剂对一氧化氮自由基的清除作用,发现姜黄素锰配合物和二乙酰姜黄素锰配合物分别比其母体化合物姜黄素和乙酰姜黄素具有更强的一氧化氮自由基清除能力。
以上研究表明,姜黄素具备显著的抗氧化性能,具有较强的自由基清除作用。不止如此,多酚类天然化合物通过清除自由基保护神经损伤同样也表现出较大潜力,ALVARADO-SANCHEZ等[29]通过建立脊髓损伤大鼠模型,报道了姜黄素的抗氧化作用,他们通过对脊髓损伤大鼠模型腹腔注射姜黄素溶液,同样发现姜黄素可显著降低组织中一氧化氮、羟基自由基和脂质过氧化水平,降低氧化应激,减少脊髓组织的死亡。槲皮素是多酚中具有代表性的类黄酮化合物之一,存在于多种蔬菜、水果中,因其抗氧化、抗炎等多种药理作用而广泛应用于治疗代谢和炎症性疾病[30],同样可以通过直接清除羟基自由基、超氧阴离子等发挥神经保护作用[31-34]。MISHRA等[35]在多发性硬化(一种中枢神经系统的慢性、炎症性、脱髓鞘疾病,主要影响大脑和脊髓的白质)小鼠模型的自由基清除实验中发现一种芙蓉花萼提取物与α硫辛酸等效,并且其对缓解培养物中铁对神经元损伤的能力优于抗氧化剂α硫辛酸,而芙蓉提取物同样是多酚类天然化合物。多酚类化合物清除自由基的原理主要是因其结构中的酚羟基,酚羟基能够提供高活性的氢原子与自由基发生反应生成一种具有抗氧化性质的物质——苯酚氧自由基,从而将自由基淬灭[36]。
此外,银杏叶提取物、水飞蓟素、橄榄油酚类等多酚类物质同样是活性氧和活性氮清除剂[37-39],其作用机制与槲皮素、姜黄素相似[40]。
多酚类物质对于活性氧自由基的清除作用,已经被广泛证明,但在脊髓损伤中其直接的自由基清除作用常被其多靶点的抗氧化机制覆盖,这使得单纯针对于天然产物直接脊髓损伤清除自由基的研究较少。
在天然产物的应用方面,许多天然产物如槲皮素由于自身的特性如疏水性等因素[32],难以穿过血脑屏障,导致其不易被吸收和利用。目前,已有不少研究集中在开发和改进天然药物的递送方式上,以提高生物利用度。白藜芦醇是一种非黄酮类多酚有机化合物,天然存在于葡萄、蓝莓等多种植物的果皮中,是一种天然的抗氧化剂。白藜芦醇是一种难溶性物质,LI等[41]建立脊髓损伤小鼠模型,并设计了壳聚糖改性的空心二氧化锰纳米颗粒,用于递送白藜芦醇以帮助通过血脊髓屏障,提高其水溶性和生物利用度,他们发现白藜芦醇可在损伤部位快速释放,通过催化作用将活性氧直接分解为水和氧,缓解缺氧环境,降低氧化应激,促进了脊髓损伤小鼠运动功能恢复。
天然产物如多酚类在直接清除自由基以改善脊髓损伤后氧化应激方面展现出显著优点,它们不仅具有强大的自由基清除能力,能够有效减少氧化应激反应,保护脊髓组织,而且通常具有较高的安全性,与人体内的生物化学反应更为相容。目前,已有不少研究者着眼于开发和改进天然药物的递送方式,提高其生物利用度,以发挥更大的自由基清除效能。此外,许多天然产物对于调控氧化应激具备多靶点作用,能够发挥更全面的抗氧化能力,甚至促进受损神经细胞的修复和再生。
2.3 天然产物激活相关信号通路降低脊髓损伤氧化应激 人体主要的抗氧化酶包括超氧化物歧化酶、谷胱甘肽过氧化物酶和过氧化氢酶等[42],这些抗氧化酶通过不同的酶促反应清除不同氧化自由基,他们的存在和正常功能能够维持细胞内氧化还原平衡,减轻氧化应激对细胞的损伤。一些研究表明,通过激活某些信号转导通路如核因子E2相关因子2(Nuclear factor erythroid 2-related factor 2,Nrf2)/抗氧化反应原件(Antioxidant Response Element,ARE)通路、磷脂酰肌醇3激酶(phosphatidylinositol 3-kinase,PI3K)/蛋白激酶B(protein kinase B,PKB/Akt)通路等,可以激活抗氧化酶基因的表达,从而增强体内的抗氧化能力[43-44],幸运的是,某些天然产物也可以通过调节这些信号转导通路,增加抗氧化酶的合成和释放,减轻氧化应激。
Nrf2是一种重要的转录因子,在正常情况下,Nrf2与其抑制蛋白(Kelch-1ike ECH- associated protein 1,Keap1)在细胞质中结合,处于未激活状态。当细胞受到氧化应激等刺激时,Nrf2会被激活并进入细胞核,与抗氧化反应元件结合,从而启动下游的抗氧化酶基因转录和表达[45-48]。
MAO等[49]通过建立Nrf2保留与Nrf2缺失的脊髓压迫损伤小鼠模型,并对其腹膜内给药萝卜硫素(天然存在于多种十字花科植物中的一种异硫氰酸盐),发现缺失Nrf2的小鼠在脊髓损伤后表现出更严重的神经功能缺损和脊髓水肿,证明了萝卜硫素可以激活Nrf2导致抗氧化酶上调,减少受损脊髓组织中的炎症,并改善神经功能。
同样,有多项研究报道,姜黄素可以通过激活Keap1/Nrf2/ARE通路以发挥抗氧化作用[50-52]。PARK等[53]利用小鼠大脑皮质细胞进行体外实验,发现姜黄素处理过的细胞Nrf2表达水平增高,并表现出Nrf2向细胞核的转移和ARE基因的转录活性。他们认为这种调节机制是因为姜黄素激活了蛋白激酶Cδ,使p62(也称为螯合体 1,是一种多功能蛋白,参与各种细胞过程,包括自噬和氧化应激)的Ser-351位点磷酸化,磷酸化后,p62获得对Keap1的高亲和力,从而阻止了Keap1对Nrf2的抑制。DAVEREY等[54]通过去除大鼠脊髓背柱白质,并采用经不同溶液处理脊髓背柱的方式,在体外探讨了姜黄素的神经保护作用,发现姜黄素可有效减轻白质细胞凋亡,对于脊髓损伤相关的缺氧、炎症和细胞凋亡确有治疗作用,并且该研究表明Nrf2通路可以通过抑制核因子κB抑制蛋白降解和增加血红素氧合酶1(heme oxygenase-1,HO-1)表达来抑制核因子κB活化,从而降低活性氧产生,同样,核因子κB介导的转录也可以通过减少抗氧化反应元件基因的表达来抑制Nrf2的激活,这两种信号通路将通过串扰的方式共同发挥神经保护作用。
天然产物对于Nrf2/ARE通路的激活是多样的,鼠尾草酸是一种天然存在的邻苯二酚型多酚二萜,常见于迷迭香和鼠尾草中,其抗氧化、抗炎、抗癌和神经保护活性已有广泛报道[55-57]。SATOH等[58]通过一系列的体外实验研究了鼠尾草酸保护神经元免受氧化应激的化学和分子机制,发现鼠尾草酸通过与特定的Keap1半胱氨酸残基结合来激活Nrf2 的转录,从而保护神经元免受氧化应激和兴奋性毒性的影响,并且鼠尾草酸在体外和体内都具有神经保护作用。MILLER等[59]通过对皮质撞击性脑损伤小鼠模型应用鼠尾草酸,同样报道了鼠尾草酸通过激活Nrf2/ARE通路减少氧化损伤、减少神经元细胞骨架分解以及保护线粒体功能等作用;他们还在皮质撞击性脑损伤小鼠模型中研究了Nrf2/ARE通路在皮质和海马体表达的时间过程,发现在没有任何药理激活剂的情况下,即便Nrf2/ARE通路在发生损伤后就被激活,但由于其激活的程度和速度低,内源性抗氧化防御开启缓慢,根本无法有效对抗氧化应激损伤[60]。
PI3K/Akt信号通路是一种重要的细胞内信号转导途径,这一通路的关键基因包括PI3K和Akt,当细胞受到氧化应激刺激时,PI3K被激活并催化磷脂酰肌醇-4,5-二磷酸转化为磷脂酰肌醇-3,4,5-三磷酸,进而募集并激活下游的Akt。激活的Akt可以通过磷酸化作用影响Nrf2的上游调控蛋白,从而解除Nrf2的抑制状态,使其从细胞质转移到细胞核内,进而激活Nrf2/ARE通路,启动抗氧化基因的转录,增强细胞的抗氧化能力。研究表明,多种天然产物可以通过该途径减弱氧化应激来发挥神经保护作用,虾青素是一种天然的酮式类胡萝卜素,广泛存在于生物界中,具有极强的抗氧化能力[61-62]。BRASIL等[63]的实验表明,在抑制PI3K/Akt信号通路或者HO-1亦或是沉默Nrf2时,虾青素对于神经母细胞瘤SH-SY5Y细胞中过氧化氢诱导的氧化损伤的保护作用都会消失,他们认为虾青素是通过激活PI3K/Akt/Nrf2/HO-1轴,减轻了神经母细胞瘤SH-SY5Y细胞中的氧化应激,发挥了对氧化损伤的保护作用。
川芎嗪是从著名中药材川芎中提取的主要活性成分之一,也被称为四甲基比嗪,许多体内和体外实验研究证明川芎嗪可以通过抑制钙离子过载和谷氨酸兴奋性毒性、抗氧化/硝化应激、减轻炎症反应、抗凋亡、保护血脑屏障的完整性以及促进突触可塑性等机制在中枢神经系统疾病的治疗中发挥作用[64]。WANG等[65]通过对脊髓损伤大鼠模型注射川芎嗪、锌原卟啉(一种HO-1抑制剂)和LY294002(一种Akt抑制剂)进行分组对照实验,发现川芎嗪可以通过激活Akt/Nrf2/HO-1信号通路发挥对脊髓损伤大鼠的神经保护作用,改善脊髓损伤大鼠模型的运动功能,降低了脑脊髓屏障的通透性,减少氧化应激、炎症和细胞凋亡。
橙皮苷是来自于柑橘类水果中的一种类黄酮,HEO等[66]通过对脊髓损伤大鼠应用橙皮苷,发现橙皮苷处理显著提高了细胞质Nrf2和核易位Nrf2的蛋白水平。与溶剂处理对照组相比,橙皮苷处理组的HO-1、超氧化物歧化酶和过氧化氢酶的活性明显增加。这些结果表明,橙皮苷同样可以激活Nrf2/HO-1信号通路发挥神经保护作用。
此外,最近的研究表明橙皮苷的水解产物橙皮素可以通过增强抗氧化酶活性、抑制氧化应激、抑制细胞凋亡和缓解脊髓损伤后的炎症反应发挥对脊髓损伤大鼠的神经保护作用,最终促进脊髓神经功能和组织学恢复。橙皮素表现出一系列生物活性,是治疗脊髓损伤有前途的候选者[67]。
PI3K/Akt信号通路是细胞应对氧化应激的关键机制之一,多种天然产物如虾青素、川芎嗪和橙皮苷等,能够通过激活该通路来减弱氧化应激,发挥神经保护作用。虾青素通过激活PI3K/Akt/Nrf2/HO-1轴,减轻氧化应激对神经细胞的损伤。川芎嗪和橙皮苷同样通过激活Nrf2/HO-1信号通路,改善脊髓损伤大鼠的运动功能,降低氧化应激、炎症和细胞凋亡。这些发现为脊髓损伤的治疗提供了新策略。
沉默信息调节因子1(sirtuin1,Sirt1)/过氧化物酶体增殖物激活受体γ共激活因子1α(peroxisome proliferator-activated receptor gamma coactivator 1-alpha,PGC-1α)/动力相关蛋白1(dynamin-related protein 1,Drp1)信号通路在细胞代谢、线粒体功能和氧化应激反应等方面发挥着重要作用,同样是调控治疗脊髓损伤的研究之一,但相比与以上几种通路来说其在脊髓损伤领域的应用研究可能相对较少。在此通路中Sirt1通过去乙酰化作用激活PGC-1α,进而调节线粒体的生物合成和能量代谢。PGC-1α的激活可以进一步影响Drp1的表达和活性,从而影响线粒体的分裂过程,从而适应细胞内的能量需求和氧化应激反应[68-69]。ZHANG等[70]通过对脊髓挫伤大鼠模型腹膜注射咖啡酸苯乙酯进行分组对照实验,发现咖啡酸苯乙酯与Sirt1之间具有很强的结合亲和力,可增加Sirt1的表达,进而使PGC-1α水平升高、Drp1的表达降低,咖啡酸苯乙酯通过抑制脊髓损伤诱导的线粒体过度分裂,减少了线粒体碎片化降低了氧化应激水平。该研究表明咖啡酸苯乙酯正是通过调节Sirt1/PGC-1α/Drp1信号通路和Sirt1水平来发挥抗氧化应激和抗炎作用,它的应用减少了神经组织损伤的面积,增加了神经元存活的数量,同时减轻了脱髓鞘的程度,使得脊髓损伤小鼠获得了更好的运动功能恢复。
山奈酚是一种存在于多种水果、蔬菜和药用植物中的类黄酮化合物,具有抗氧化、抗炎和抗凋亡等多种特性,已被证明可以通过调控PI3K/Akt通路、核因子κB通路以及丝裂原活化蛋白激酶/细胞外信号调节激酶通路等多种信号通路发挥神经保护作用,包括增强神经元存活、减弱氧化应激、减少神经炎症、促进神经再生和改善认知功能[71]。LIU等[72]的体内实验表明,山奈酚的施用降低了脊髓中的小胶质细胞活化和氧化应激水平,促进了脊髓损伤大鼠后肢运动功能的恢复,改善了脊髓组织损伤;体外研究也表明,山奈酚能够显著抑制脂多糖与ATP导致的小鼠小胶质细胞(BV-2)活化,从而减轻神经炎症,用山奈酚预处理的小鼠小胶质细胞通过抑制还原型烟酰胺腺嘌呤二核苷酸磷酸氧化酶4减少了活性氧的产生,进而通过下调活性氧依赖性丝裂原活化蛋白激酶/核因子κB通路和细胞焦亡信号通路,降低了氧化应激和炎症反应。
综上,在脊髓损伤的治疗研究中,Sirt1/PGC-1α/Drp1信号通路作为潜在的治疗策略受到了关注。Sirt1通过激活PGC-1α调节线粒体功能和能量代谢,进而影响Drp1的表达和线粒体分裂过程,而咖啡酸苯乙酯通过增强Sirt1表达,展现出对脊髓损伤的抗氧化应激和抗炎作用。同时,山奈酚作为一种类黄酮化合物,通过调控多个信号通路,如丝裂原活化蛋白激酶/核因子κB通路,显示出神经保护作用,包括减轻氧化应激和炎症反应,促进脊髓损伤后的功能恢复。
此外,银杏内酯[73-74]、6’-O-没食子酰芍药素[75]、花青素[76]、葛根素等对于PI3K/Akt通路的调节与虾青素具有相似性[77],同样可以通过该途径增强抗氧化机制,发挥神经保护作用。
天然产物可以通过调控多种相关信号通路来减少脊髓损伤后的氧化应激,其作用机制错综复杂,调控方式也多种多样,具有多靶点调控、抗氧化能力强、减少炎症反应、促进神经再生和修复等多种优点,使得天然产物在脊髓损伤的治疗中表现出广阔的应用前景。实验证明,部分天然产物可以通过激活多种抗氧化酶通路来增强内源性抗氧化保护机制,这种激活方式更加温和、持久,且符合人体自身的生理需求。此外一些天然产物在促进神经细胞的再生和修复方面也有效果,它们可能通过降低氧化应激、改善炎症环境和细胞代谢、抑制细胞凋亡等过程,促进神经细胞的再生和轴突的重塑。这些作用有助于恢复脊髓损伤后的神经传导功能,改善患者的运动和感觉功能。未来需要进一步的研究来系统地确定相关天然产物在脊髓损伤治疗过程中神经保护作用的潜在机制。
2.4 天然产物激活自噬减弱氧化应激 自噬是一种细胞保护机制,可以促进受损细胞器的回收并降解错误折叠的蛋白质,在脊髓发生创伤之后,自噬的适度激活可以对脊髓神经元凋亡起保护作用[78]。有研究指出,自噬可调节Nrf2的核易位,进而激活Nrf2/ARE通路以发挥抗氧化作用[79]。
紫檀芪是一种来源于紫檀、葡萄、蓝莓等多种植物中的天然化合物,具有抗氧化、抗癌、抗炎和镇痛等多种作用[80]。血管紧张素原基因(ATG5)是自噬过程的关键参与者,HE等[81]通过抑制血管紧张素原基因的表达抑制自噬,逆转了紫檀芪对活性氧产生和细胞凋亡的抑制作用,证明紫檀芪是通过抑制哺乳动物雷帕霉素靶蛋白(参与自噬的负调控)信号通路激活脊髓神经元自噬,从而抑制脊髓神经元中活性氧的产生和脊髓神经元凋亡。
同样,胡椒碱是一种从黑胡椒中提取的天然活性生物碱,具有抗氧化、调节免疫、抗抑郁和抗肿瘤等多重功效[82],ZHANG等[83]对脊髓损伤小鼠模型应用胡椒碱同时使用3-甲基腺嘌呤(一种广泛使用的自噬抑制剂)抑制自噬[84],发现3-甲基腺嘌呤抑制了胡椒碱激活的神经元自噬-溶酶体通路,并部分逆转了胡椒碱对氧化应激、焦亡和炎症的抑制作用。他们的研究表明,胡椒碱通过诱导自噬抑制活性氧产生,降低氧化应激、减弱焦亡并抑制炎症,促进脊髓损伤的恢复。
综上,自噬作为细胞的天然保护机制,不仅促进受损细胞器和错误折叠蛋白质的回收与降解,在脊髓损伤后,其适度激活对神经元凋亡起到重要保护作用。紫檀芪和胡椒碱作为天然化合物,分别通过抑制哺乳动物雷帕霉素靶蛋白信号通路和直接诱导自噬,激活脊髓神经元自噬过程,进而减少活性氧产生、抑制细胞凋亡、降低氧化应激和炎症反应,展现出对脊髓损伤治疗的显著潜力,见表2。目前关于天然产物通过自噬抑制脊髓损伤后氧化应激的研究还处于初级阶段。尽管少数实验证明自噬的适度激活可以通过降低氧化应激发挥对神经元的保护作用,但还需要进一步深入研究和验证,未来的研究可以关注于筛选具有更强自噬激活作用的天然产物,并探究其具体的作用机制和最佳应用方式。
2.5 天然产物调控氧化应激应用于脊髓损伤领域的发展历史 详见图3。

| [1] LIN J, XIONG Z, GU J, et al. Sirtuins: Potential Therapeutic Targets for Defense against Oxidative Stress in Spinal Cord Injury. Oxid Med Cell Longev. 2021;2021:7207692. [2] 金元植,戎鑫,刘浩.外伤性SCI不同时期行干细胞移植治疗的研究进展[J].中国修复重建外科杂志,2023,37(6):721-726. [3] YANG CH, QUAN ZX, WANG GJ, et al. Elevated intraspinal pressure in traumatic spinal cord injury is a promising therapeutic target. Neural Regen Res. 2022;17(8):1703-1710. [4] COFANO F, BOIDO M, MONTICELLI M, et al. Mesenchymal Stem Cells for Spinal Cord Injury: Current Options, Limitations, and Future of Cell Therapy. Int J Mol Sci. 2019;20(11):2698. [5] AHUJA CS, NORI S, TETREAULT L, et al. Traumatic Spinal Cord Injury-Repair and Regeneration. Neurosurgery. 2017;80(3S):S9-S22. [6] ANJUM A, YAZID MD, FAUZI DAUD M, et al. Spinal Cord Injury: Pathophysiology, Multimolecular Interactions, and Underlying Recovery Mechanisms. Int J Mol Sci. 2020;21(20):7533. [7] LEE CY, CHOOI WH, NG SY, et al. Modulating neuroinflammation through molecular, cellular and biomaterial-based approaches to treat spinal cord injury. Bioeng Transl Med. 2022;8(2):e10389. [8] ISLAM F, BEPARY S, NAFADY MH, et al. Polyphenols Targeting Oxidative Stress in Spinal Cord Injury: Current Status and Future Vision. Oxid Med Cell Longev. 2022;2022:8741787. [9] SIES H. Oxidative Stress: Concept and Some Practical Aspects. Antioxidants (Basel). 2020;9(9):852. [10] SIES H, BELOUSOV VV, CHANDEL NS, et al. Defining roles of specific reactive oxygen species (ROS) in cell biology and physiology. Nat Rev Mol Cell Biol. 2022;23(7):499-515. [11] BRETHEAU F, CASTELLANOS-MOLINA A, BÉLANGER D, et al. The alarmin interleukin-1α triggers secondary degeneration through reactive astrocytes and endothelium after spinal cord injury. Nat Commun. 2022;13(1):5786. [12] BARIONI NO, DERAKHSHAN F, TENORIO LOPES L, et al. Novel oxygen sensing mechanism in the spinal cord involved in cardiorespiratory responses to hypoxia. Sci Adv. 2022;8(12):eabm1444. [13] HOLMSTRÖM KM, FINKEL T. Cellular mechanisms and physiological consequences of redox-dependent signalling. Nat Rev Mol Cell Biol. 2014; 15(6):411-421. [14] ZHANG C, ZHAI T, ZHU J, et al. Research Progress of Antioxidants in Oxidative Stress Therapy after Spinal Cord Injury. Neurochem Res. 2023;48(12): 3473-3484. [15] BRACKEN MB, SHEPARD MJ, HOLFORD TR, et al. Administration of methylprednisolone for 24 or 48 hours or tirilazad mesylate for 48 hours in the treatment of acute spinal cord injury. Results of the Third National Acute Spinal Cord Injury Randomized Controlled Trial. National Acute Spinal Cord Injury Study. JAMA. 1997;277(20):1597-1604. [16] BRACKEN MB, SHEPARD MJ, HOLFORD TR, et al. Methylprednisolone or tirilazad mesylate administration after acute spinal cord injury: 1-year follow up. Results of the third National Acute Spinal Cord Injury randomized controlled trial. J Neurosurg. 1998;89(5):699-706. [17] HALL ED. Antioxidant therapies for acute spinal cord injury. Neurotherapeutics. 2011;8(2):152-167. [18] FAKHRI S, ABBASZADEH F, MORADI SZ, et al. Effects of Polyphenols on Oxidative Stress, Inflammation, and Interconnected Pathways during Spinal Cord Injury. Oxid Med Cell Longev. 2022;2022:8100195. [19] KHALATBARY AR. Natural polyphenols and spinal cord injury. Iran Biomed J. 2014;18(3):120-129. [20] MA Q. Advances in mechanisms of anti-oxidation. Discov Med. 2014;17(93): 121-130. [21] POWERS SK, GOLDSTEIN E, SCHRAGER M, et al. Exercise Training and Skeletal Muscle Antioxidant Enzymes: An Update. Antioxidants (Basel). 2022;12(1):39. [22] LEI XG, ZHU JH, CHENG WH, et al. Paradoxical Roles of Antioxidant Enzymes: Basic Mechanisms and Health Implications. Physiol Rev. 2016;96(1):307-364. [23] SCHEIJEN EEM, HENDRIX S, WILSON DM 3RD. Oxidative DNA Damage in the Pathophysiology of Spinal Cord Injury: Seems Obvious, but Where Is the Evidence? Antioxidants (Basel). 2022;11(9):1728. [24] REHMAN MU, SEHAR N, DAR NJ, et al. Mitochondrial dysfunctions, oxidative stress and neuroinflammation as therapeutic targets for neurodegenerative diseases: An update on current advances and impediments. Neurosci Biobehav Rev. 2023;144:104961. [25] ANJUM A, YAZID MD, FAUZI DAUD M, et al. Spinal Cord Injury: Pathophysiology, Multimolecular Interactions, and Underlying Recovery Mechanisms. Int J Mol Sci. 2020;21(20):7533. [26] AK T, GÜLÇIN I. Antioxidant and radical scavenging properties of curcumin. Chem Biol Interact. 2008;174(1):27-37. [27] KIM JE, KIM AR, CHUNG HY, et al. In vitro peroxynitrite scavenging activity of diarylheptanoids from Curcuma longa. Phytother Res. 2003;17(5):481-484. [28] SUMANONT Y, MURAKAMI Y, TOHDA M, et al. Evaluation of the nitric oxide radical scavenging activity of manganese complexes of curcumin and its derivative. Biol Pharm Bull. 2004;27(2):170-173. [29] ALVARADO-SANCHEZ BG, SALGADO-CEBALLOS H, TORRES-CASTILLO S, et al. Electroacupuncture and Curcumin Promote Oxidative Balance and Motor Function Recovery in Rats Following Traumatic Spinal Cord Injury. Neurochem Res. 2019;44(2):498-506. [30] ANAND DAVID AV, ARULMOLI R, PARASURAMAN S. Overviews of Biological Importance of Quercetin: A Bioactive Flavonoid. Pharmacogn Rev. 2016; 10(20):84-89. [31] JUURLINK BH, PATERSON PG. Review of oxidative stress in brain and spinal cord injury: suggestions for pharmacological and nutritional management strategies. J Spinal Cord Med. 1998;21(4):309-334. [32] OSSOLA B, KÄÄRIÄINEN TM, MÄNNISTÖ PT. The multiple faces of quercetin in neuroprotection. Expert Opin Drug Saf. 2009;8(4):397-409. [33] ISHISAKA A, ICHIKAWA S, SAKAKIBARA H, et al. Accumulation of orally administered quercetin in brain tissue and its antioxidative effects in rats. Free Radic Biol Med. 2011;51(7):1329-1336. [34] SOHN EJ, KIM JM, KANG SH, et al. Restoring Effects of Natural Anti-Oxidant Quercetin on Cellular Senescent Human Dermal Fibroblasts. Am J Chin Med. 2018;46(4):853-873. [35] MISHRA MK, WANG J, MIRZAEI R, et al. A Distinct Hibiscus sabdariffa Extract Prevents Iron Neurotoxicity, a Driver of Multiple Sclerosis Pathology. Cells. 2022;11(3):440. [36] SUTHERLAND BA, RAHMAN RM, APPLETON I. Mechanisms of action of green tea catechins, with a focus on ischemia-induced neurodegeneration. J Nutr Biochem. 2006;17(5):291-306. [37] PINCEMAIL J, DUPUIS M, NASR C, et al. Superoxide anion scavenging effect and superoxide dismutase activity of Ginkgo biloba extract. Experientia. 1989;45(8):708-712. [38] SURAI PF. Silymarin as a Natural Antioxidant: An Overview of the Current Evidence and Perspectives. Antioxidants (Basel). 2015;4(1):204-247. [39] KHALATBARY AR. Olive oil phenols and neuroprotection. Nutr Neurosci. 2013;16(6):243-249. [40] COYOY-SALGADO A, SEGURA-URIBE JJ, GUERRA-ARAIZA C, et al. The Importance of Natural Antioxidants in the Treatment of Spinal Cord Injury in Animal Models: An Overview. Oxid Med Cell Longev. 2019;2019:3642491. [41] LI Y, ZOU Z, AN J, et al. Chitosan-modified hollow manganese dioxide nanoparticles loaded with resveratrol for the treatment of spinal cord injury. Drug Deliv. 2022;29(1):2498-2512. [42] LEI XG, ZHU JH, CHENG WH, et al. Paradoxical Roles of Antioxidant Enzymes: Basic Mechanisms and Health Implications. Physiol Rev. 2016;96(1):307-364. [43] WU AG, YONG YY, PAN YR, et al. Targeting Nrf2-Mediated Oxidative Stress Response in Traumatic Brain Injury: Therapeutic Perspectives of Phytochemicals. Oxid Med Cell Longev. 2022;2022:1015791. [44] SCHOTTLENDER N, GOTTFRIED I, ASHERY U. Hyperbaric Oxygen Treatment: Effects on Mitochondrial Function and Oxidative Stress. Biomolecules. 2021;11(12):1827. [45] TONELLI C, CHIO IIC, TUVESON DA. Transcriptional Regulation by Nrf2. Antioxid Redox Signal. 2018;29(17):1727-1745. [46] TONG KI, KATOH Y, KUSUNOKI H, et al. Keap1 recruits Neh2 through binding to ETGE and DLG motifs: characterization of the two-site molecular recognition model. Mol Cell Biol. 2006;26(8):2887-2900. [47] KASPAR JW, NITURE SK, JAISWAL AK. Nrf2:INrf2 (Keap1) signaling in oxidative stress. Free Radic Biol Med. 2009;47(9):1304-1309. [48] BAIRD L, YAMAMOTO M. The Molecular Mechanisms Regulating the KEAP1-NRF2 Pathway. Mol Cell Biol. 2020;40(13):e00099-20. [49] MAO L, WANG H, WANG X, et al. Transcription factor Nrf2 protects the spinal cord from inflammation produced by spinal cord injury. J Surg Res. 2011;170(1):e105-e115. [50] SHIN JW, CHUN KS, KIM DH, et al. Curcumin induces stabilization of Nrf2 protein through Keap1 cysteine modification. Biochem Pharmacol. 2020; 173:113820. [51] MEDINA-PIZAÑO MY, MEDINA-ROSALES MN, MARTÍNEZ-HERNÁNDEZ SL, et al. Protective Effect of Curcumin against Doxazosin- and Carvedilol-Induced Oxidative Stress in HepG2 Cells. Oxid Med Cell Longev. 2022;2022:6085515. [52] TU ZS, WANG Q, SUN DD, et al. Design, synthesis, and evaluation of curcumin derivatives as Nrf2 activators and cytoprotectors against oxidative death. Eur J Med Chem. 2017;134:72-85. [53] PARK JY, SOHN HY, KOH YH, et al. Curcumin activates Nrf2 through PKCδ-mediated p62 phosphorylation at Ser351. Sci Rep. 2021;11(1):8430. [54] DAVEREY A, AGRAWAL SK. Curcumin Protects against White Matter Injury through NF-κB and Nrf2 Cross Talk. J Neurotrauma. 2020;37(10):1255-1265. [55] MOORE J, YOUSEF M, TSIANI E. Anticancer Effects of Rosemary (Rosmarinus officinalis L.) Extract and Rosemary Extract Polyphenols. Nutrients. 2016; 8(11):731. [56] De Oliveira MR. The Dietary Components Carnosic Acid and Carnosol as Neuroprotective Agents: a Mechanistic View. Mol Neurobiol. 2016;53(9): 6155-6168. [57] BAHRI S, JAMELEDDINE S, SHLYONSKY V. Relevance of carnosic acid to the treatment of several health disorders: Molecular targets and mechanisms. Biomed Pharmacother. 2016;84:569-582. [58] SATOH T, KOSAKA K, ITOH K, et al. Carnosic acid, a catechol-type electrophilic compound, protects neurons both in vitro and in vivo through activation of the Keap1/Nrf2 pathway via S-alkylation of targeted cysteines on Keap1. J Neurochem. 2008;104(4):1116-1131. [59] MILLER DM, SINGH IN, WANG JA, et al. Nrf2-ARE activator carnosic acid decreases mitochondrial dysfunction, oxidative damage and neuronal cytoskeletal degradation following traumatic brain injury in mice. Exp Neurol. 2015;264:103-110. [60] MILLER DM, WANG JA, BUCHANAN AK, et al. Temporal and spatial dynamics of nrf2-antioxidant response elements mediated gene targets in cortex and hippocampus after controlled cortical impact traumatic brain injury in mice. J Neurotrauma. 2014;31(13):1194-1201. [61] ASHRAFIZADEH M, AHMADI Z, YARIBEYGI H, et al. Astaxanthin and Nrf2 Signaling Pathway: A Novel Target for New Therapeutic Approaches. Mini Rev Med Chem. 2022;22(2):312-321. [62] SZTRETYE M, DIENES B, GÖNCZI M, et al. Astaxanthin: A Potential Mitochondrial-Targeted Antioxidant Treatment in Diseases and with Aging. Oxid Med Cell Longev. 2019;2019:3849692. [63] BRASIL FB, BERTOLINI GOBBO RC, SOUZA DE ALMEIDA FJ, et al. The signaling pathway PI3K/Akt/Nrf2/HO-1 plays a role in the mitochondrial protection promoted by astaxanthin in the SH-SY5Y cells exposed to hydrogen peroxide. Neurochem Int. 2021;146:105024. [64] LIU Y, YANG G, CUI W, et al. Regulatory mechanisms of tetramethylpyrazine on central nervous system diseases: A review. Front Pharmacol. 2022;13: 948600. [65] WANG C, WANG P, ZENG W, et al. Tetramethylpyrazine improves the recovery of spinal cord injury via Akt/Nrf2/HO-1 pathway. Bioorg Med Chem Lett. 2016;26(4):1287-1291. [66] HEO SD, KIM J, CHOI Y, et al. Hesperidin improves motor disability in rat spinal cord injury through anti-inflammatory and antioxidant mechanism via Nrf-2/HO-1 pathway. Neurosci Lett. 2020;715:134619. [67] ZHANG Y, CHEN X, WANG X, et al. Hesperetin ameliorates spinal cord injury in rats through suppressing apoptosis, oxidative stress and inflammatory response. Eur J Pharmacol. 2024;971:176541. [68] WANG JF, WEN DT, WANG SJ, et al. Muscle-specific overexpression of Atg2 gene and endurance exercise delay age-related deteriorations of skeletal muscle and heart function via activating the AMPK/Sirt1/PGC-1α pathway in male Drosophila. FASEB J. 2023;37(11):e23214. [69] CHANDRASEKARAN K, ANJANEYULU M, CHOI J, et al. Role of mitochondria in diabetic peripheral neuropathy: Influencing the NAD+-dependent SIRT1-PGC-1α-TFAM pathway. Int Rev Neurobiol. 2019;145:177-209. [70] ZHANG Y, DENG Q, HONG H, et al. Caffeic acid phenethyl ester inhibits neuro-inflammation and oxidative stress following spinal cord injury by mitigating mitochondrial dysfunction via the SIRT1/PGC1α/DRP1 signaling pathway. J Transl Med. 2024;22(1):304. [71] NEZHAD SALARI AM, RASOULIZADEH Z, SHABGAH AG, et al. Exploring the mechanisms of kaempferol in neuroprotection: Implications for neurological disorders. Cell Biochem Funct. 2024;42(2):e3964. [72] LIU Z, YAO X, SUN B, et al. Pretreatment with kaempferol attenuates microglia-mediate neuroinflammation by inhibiting MAPKs-NF-κB signaling pathway and pyroptosis after secondary spinal cord injury. Free Radic Biol Med. 2021;168:142-154. [73] LIU Q, JIN Z, XU Z, et al. Antioxidant effects of ginkgolides and bilobalide against cerebral ischemia injury by activating the Akt/Nrf2 pathway in vitro and in vivo. Cell Stress Chaperones. 2019;24(2):441-452. [74] ZHU T, FANG BY, MENG XB, et al. Folium Ginkgo extract and tetramethylpyrazine sodium chloride injection (Xingxiong injection) protects against focal cerebral ischaemia/reperfusion injury via activating the Akt/Nrf2 pathway and inhibiting NLRP3 inflammasome activation. Pharm Biol. 2022;60(1):195-205. [75] WEN Z, HOU W, WU W, et al. 6’-O-Galloylpaeoniflorin Attenuates Cerebral Ischemia Reperfusion-Induced Neuroinflammation and Oxidative Stress via PI3K/Akt/Nrf2 Activation. Oxid Med Cell Longev. 2018;2018:8678267. [76] ALI T, KIM T, REHMAN SU, et al. Natural Dietary Supplementation of Anthocyanins via PI3K/Akt/Nrf2/HO-1 Pathways Mitigate Oxidative Stress, Neurodegeneration, and Memory Impairment in a Mouse Model of Alzheimer’s Disease. Mol Neurobiol. 2018;55(7):6076-6093. [77] ZHANG D, MA G, HOU M, et al. The Neuroprotective Effect of Puerarin in Acute Spinal Cord Injury Rats. Cell Physiol Biochem. 2016;39(3):1152-1164. [78] SHI Z, YUAN S, SHI L, et al. Programmed cell death in spinal cord injury pathogenesis and therapy. Cell Prolif. 2021;54(3):e12992. [79] TAGUCHI K, FUJIKAWA N, KOMATSU M, et al. Keap1 degradation by autophagy for the maintenance of redox homeostasis. Proc Natl Acad Sci U S A. 2012;109(34):13561-13566. [80] REMSBERG CM, YÁÑEZ JA, OHGAMI Y, et al. Pharmacometrics of pterostilbene: preclinical pharmacokinetics and metabolism, anticancer, antiinflammatory, antioxidant and analgesic activity. Phytother Res. 2008; 22(2):169-179. [81] HE JL, DONG XH, LI ZH, et al. Pterostilbene inhibits reactive oxygen species production and apoptosis in primary spinal cord neurons by activating autophagy via the mechanistic target of rapamycin signaling pathway. Mol Med Rep. 2018;17(3):4406-4414. [82] HAQ IU, IMRAN M, NADEEM M, et al. Piperine: A review of its biological effects. Phytother Res. 2021;35(2):680-700. [83] ZHANG H, WU C, YU DD, et al. Piperine attenuates the inflammation, oxidative stress, and pyroptosis to facilitate recovery from spinal cord injury via autophagy enhancement. Phytother Res. 2023;37(2):438-451. [84] WANG X, ZHOU G, LIU C, et al. Acanthopanax versus 3-Methyladenine Ameliorates Sodium Taurocholate-Induced Severe Acute Pancreatitis by Inhibiting the Autophagic Pathway in Rats. Mediators Inflamm. 2016; 2016:8369704. [85] DEMOPOULOS HB, FLAMM ES, SELIGMAN ML, et al. Further studies on free-radical pathology in the major central nervous system disorders: effect of very high doses of methylprednisolone on the functional outcome, morphology, and chemistry of experimental spinal cord impact injury. Can J Physiol Pharmacol. 1982;60(11):1415-1424. [86] ANDERSON DK, SAUNDERS RD, DEMEDIUK P, et al. Lipid hydrolysis and peroxidation in injured spinal cord: partial protection with methylprednisolone or vitamin E and selenium. Cent Nerv Syst Trauma. 1985;2(4):257-267. [87] COLES JC, AHMED SN, MEHTA HU, et al. Role of free radical scavenger in protection of spinal cord during ischemia. Ann Thorac Surg. 1986;41(5):551-556. [88] BRAUGHLER JM, HALL ED. Central nervous system trauma and stroke. I. Biochemical considerations for oxygen radical formation and lipid peroxidation. Free Radic Biol Med. 1989;6(3):289-301. [89] BRACKEN MB, SHEPARD MJ, COLLINS WF JR, et al. Methylprednisolone or naloxone treatment after acute spinal cord injury: 1-year follow-up data. Results of the second National Acute Spinal Cord Injury Study. J Neurosurg. 1992;76(1):23-31. [90] BRACKEN MB. Pharmacological treatment of acute spinal cord injury: current status and future projects. J Emerg Med. 1993;11 Suppl 1:43-48. [91] LI M, HONG X, WU Y. [Effect of antiserum against dynorphin A administered intrathecally on spinal cord injury (SCI) of rats and its significance]. Zhonghua Wai Ke Za Zhi. 1995;33(12):723-726. [92] KOÇ RK, AKDEMIR H, KURTSOY A, et al. Lipid peroxidation in experimental spinal cord injury. Comparison of treatment with Ginkgo biloba, TRH and methylprednisolone. Res Exp Med (Berl). 1995;195(2):117-123. [93] IMANAKA T, HUKUDA S, MAEDA T. The role of GM1-ganglioside in the injured spinal cord of rats: an immunohistochemical study using GM1-antisera. J Neurotrauma. 1996;13(3):163-170. [94] NI JD, DING RK, LÜ GH. [Protective effect of dansheng injection on experimental rabbits’ spinal cord injury]. Hunan Yi Ke Da Xue Xue Bao. 2002;27(6):507-508. [95] LI J, WEI J, WAN Y, et al. TAT-modified tetramethylpyrazine-loaded nanoparticles for targeted treatment of spinal cord injury. J Control Release. 2021;335:103-116. [96] DU Y, CAI X. Therapeutic potential of natural compounds from herbs and nutraceuticals in spinal cord injury: Regulation of the mTOR signaling pathway. Biomed Pharmacother. 2023;163:114905. [97] WIKLUND L, SHARMA A, MURESANU DF, et al. TiO2-Nanowired Delivery of Chinese Extract of Ginkgo biloba EGb-761 and Bilobalide BN-52021 Enhanced Neuroprotective Effects of Cerebrolysin Following Spinal Cord Injury at Cold Environment. Adv Neurobiol. 2023;32:353-384. |
| [1] | 赵济宇, 王少伟. 叉头框转录因子O1信号通路与骨代谢[J]. 中国组织工程研究, 2025, 29(9): 1923-1930. |
| [2] | 于经邦, 吴亚云. 非编码RNA在肺纤维化过程中的调控作用[J]. 中国组织工程研究, 2025, 29(8): 1659-1666. |
| [3] | 王秋月, 靳 攀, 蒲 锐. 运动干预与细胞焦亡在骨关节炎中的作用[J]. 中国组织工程研究, 2025, 29(8): 1667-1675. |
| [4] | 袁维勃, 刘 婵, 余丽梅. 肝脏类器官在肝脏疾病模型与移植治疗中的应用潜力[J]. 中国组织工程研究, 2025, 29(8): 1684-1692. |
| [5] | 赵瑞华, 陈思娴, 郭 杨, 石 磊, 吴承杰, 吴 毛, 杨光露, 张昊恒, 马 勇. 温肾通督方促进小鼠脊髓损伤的修复[J]. 中国组织工程研究, 2025, 29(6): 1118-1126. |
| [6] | 贺光辉, 原 杰, 柯燕琴, 丘小婷, 张晓玲. Hemin调控小鼠软骨细胞氧化应激的线粒体途径[J]. 中国组织工程研究, 2025, 29(6): 1183-1191. |
| [7] | 何 波, 陈 文, 马岁录, 何志军, 宋 渊, 李金鹏, 刘 涛, 魏晓涛, 王威威, 谢 婧. 皮瓣缺血再灌注损伤的发病机制及治疗进展[J]. 中国组织工程研究, 2025, 29(6): 1230-1238. |
| [8] | 逯冉冉, 周 旭, 张利杰, 杨新玲. 富马酸二甲酯减轻帕金森病模型鼠神经损伤的作用机制[J]. 中国组织工程研究, 2025, 29(5): 989-994. |
| [9] | 王自林, 牟秋菊, 刘宏杰, 申玉雪, 祝丽丽. 载富血小板血浆水凝胶对L929细胞氧化损伤的保护作用[J]. 中国组织工程研究, 2025, 29(4): 771-779. |
| [10] | 司马鑫利, 刘丹平, 綦 惠. 二甲双胍修饰骨髓间充质干细胞外泌体调节软骨细胞的作用及机制[J]. 中国组织工程研究, 2025, 29(36): 7728-7734. |
| [11] | 刘 璇, 丁雨晴, 夏若寒, 汪献旺, 胡淑娟. 运动防治胰岛素抵抗:Keap1/核因子E2相关因子2信号通路的作用与分子机制[J]. 中国组织工程研究, 2025, 29(35): 7578-7588. |
| [12] | 纪 龙, 陈子扬, 靳 攀, 孔祥魁, 蒲 锐, . 脂肪自噬、运动干预与非酒精性脂肪肝的防治[J]. 中国组织工程研究, 2025, 29(35): 7611-7619. |
| [13] | 张晓宇, 韦善文, 方佳炜, 倪 莉. 普鲁士蓝纳米粒子抗氧化恢复退变髓核细胞线粒体功能[J]. 中国组织工程研究, 2025, 29(34): 7318-7325. |
| [14] | 苏永昆, 孙 红, 刘 淼, 杨 华, 李青松. 开发纳米水凝胶系统搭载新型抗氧化剂与抗氧化剂联合治疗椎间盘退变[J]. 中国组织工程研究, 2025, 29(34): 7376-7384. |
| [15] | 吴青芸, 苏 强. 抗氧化纳米药物介导心肌缺血再灌注损伤的靶向治疗[J]. 中国组织工程研究, 2025, 29(34): 7431-7438. |
在脊髓继发性损伤中,其损伤部位将产生过量的活性氧并累积,引发氧化应激反应,持续对脊髓造成氧化损伤[9-13]。虽然目前对于抗氧化药物的研究较多,但真正应用于临床实践的药物少之又少,目前公认的唯一有效的治疗药物只有甲泼尼龙[14-16],并且因其大剂量冲击疗法会引起明显不良反应,它的临床治疗仍局限于实验阶段[17]。
近年来许多天然产物已被证明具有抗氧化、抗炎的作用,并能有效调节氧化应激反应,减轻脊髓损伤的程度[18-19],此文就天然产物在脊髓损伤后氧化应激过程中的调节作用作一综述,为进一步丰富发展脊髓损伤后的抗氧化治疗策略提供思路。作者通过检索自建库以来PubMed、Web of Science、Embase、Cochrane、维普、CBM、万方和中国知网数据库收录的有关天然产物通过调控氧化应激治疗脊髓损伤的相关研究,总结其主要机制包括天然产物直接清除脊髓损伤自由基、天然产物激活相关信号通路降低脊髓损伤氧化应激、天然产物激活自噬减弱脊髓损伤氧化应激等。此文就天然产物调控氧化应激治疗脊髓损伤的几种机制的研究进展展开综述。
中国组织工程研究杂志出版内容重点:组织构建;骨细胞;软骨细胞;细胞培养;成纤维细胞;血管内皮细胞;骨质疏松;组织工程
1.1 资料来源
1.1.1 检索人及检索时间 第一作者在2024-05-12进行检索。
1.1.2 检索文献时限 自各数据库建库至2024年5月。
1.1.3 检索数据库 PubMed、Web of Science、Embase、Cochrane、维普、CBM、万方和中国知网数据库。
1.1.4 检索词 中文检索词为“脊髓损伤,氧化应激,抗氧化,天然产物,天然化合物,多酚”;英文检索词为“spinal cord injury,oxidative stress,anti-oxidation,natural products,natural compounds,polyphenols”。
1.1.5 检索文献类型 研究原著、综述、临床试验、述评、病例报告和荟萃分析。
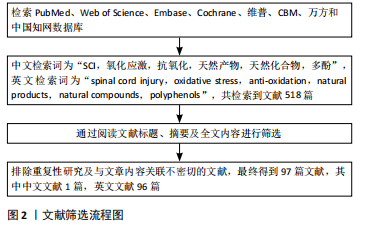
1.1.6 检索策略 通过上述数据库检索全文、检索主题词、检索关键词、检索摘要等。以PubMed数据库为例,文献检索策略见图1。
1.1.7 检索文献量 初步检索到文献518篇。
1.2 纳入标准 ①天然产物调控氧化应激治疗脊髓损伤的相关研究;②采用随机对照试验、临床前研究和动物实验等科学设计,具有较高的可靠性和可信度的文献;③同一研究领域内选择发表年份较新的文献。
1.3 排除标准 ①重复性研究的文献;②会议论文及短篇报道;③与该综述关系不密切的文献。
1.4 资料整合 共检索到518篇相关文献,通过阅读文献标题、摘要及全文内容进行筛选,排除重复性及与文章内容关联不密切的文献,最终纳入97篇相关文献,其中中文文献1篇,英文文献96篇。文献检索流程图见图2。
3.1 既往他人在该领域研究的贡献和存在的问题 近年来,已有不少研究致力于寻找天然产物作为减轻脊髓损伤后氧化应激的新方法和策略,这些研究让人们对天然产物的抗氧化特性、神经保护机制、疗效和安全性等有了大体的了解,为脊髓损伤的抗氧化治疗提供了新的思路和方向。然而,尽管许多天然产物被证明具有抗氧化和神经保护作用,它们在脊髓损伤中的确切作用机制如抗氧化与自由基清除机制、细胞信号通路的调控机制、抗炎与免疫调节机制、神经再生与修复机制、细胞凋亡与自噬的调节机制等仍不完全清楚,其具体的作用靶点、信号通路以及与其他治疗手段的协同作用等仍需进一步深入研究,这限制了它们的有效应用。尽管一些天然产物在动物实验和体外研究中表现出良好的治疗效果,但大多数研究仍停留在动物模型阶段,在临床试验中的应用仍面临诸多挑战,例如,临床试验的设计和实施需要考虑到患者的个体差异、疾病的严重程度以及治疗的安全性和有效性等因素;一些天然产物可能具有较差的生物利用度或药代动力学特性,这可能导致它们在体内的有效浓度不足或分布不均;脊髓损伤患者通常需要接受多种药物治疗,需要考虑天然产物与其他药物之间的潜在相互作用,以避免不良的药物反应等等。此外,天然产物的提纯和标准化也是一个挑战,由于天然产物的成分复杂,提取和纯化过程可能受到多种因素的影响,导致产物的质量和活性不稳定。
3.2 作者综述区别于他人他篇的特点 此篇综述不仅详细阐述了天然产物调控氧化应激的分子机制,还结合相关动物实验探讨了这些机制在脊髓损伤治疗中的具体作用。同时,此综述涵盖了黄酮类、生物碱类、多酚类、萜烯类、有机硫类等多种不同类型的天然产物,从而提供了一个更为全面的视角。在撰写时,作者特别关注并整合了最新的研究成果,包括新的天然产物、新的作用机制、新的治疗方法等,使得此综述能够更准确地反映当前的研究动态和趋势。此综述不仅是对已有研究的简单罗列,而且包含了深入的分析和讨论,针对某些关键问题如动物实验的具体流程、相关信号通路的具体作用机制进行了深入研究,总结出天然产物通过直接清除自由基、激活相关信号通路、激活自噬等3种主要途径发挥减轻脊髓损伤后氧化应激的作用,使其能够增加综述的学术价值,并激发读者的思考。此综述不仅关注了实验室研究的结果,还探讨了这些研究成果在临床应用中的前景,分析了当前治疗脊髓损伤的挑战和困难,并探讨了天然产物调控氧化应激治疗脊髓损伤的潜在优势和可能性,使其能够增加综述的实用性和指导意义。由于天然产物调控氧化应激治疗脊髓损伤涉及多个学科领域,如生物学、化学、医学等,此文采用了跨学科的视角来审视这个问题,从多个学科领域出发,综合分析了不同学科的研究成果和方法,从而提出了更为全面和深入的见解,增加了此综述的广度和深度,并促进不同学科之间的交流和合作。
3.3 综述的局限性
文献筛选的局限性:尽管作者努力涵盖了广泛的研究文献,但由于文献的数量庞大且增加迅速,很难确保所有相关的研究都被纳入其中。这可能导致某些重要的研究成果被遗漏,从而影响综述的全面性和准确性。
研究方法的局限性:综述的研究方法主要基于对现有文献的分析和整合,这种方法本身存在一定的局限性。例如,由于原始研究的质量、设计、样本大小等因素的差异,综述中的结果可能受到这些原始研究质量的限制。此外,无法直接进行实验研究来验证其提出的假设或结论。
通路分析的深度不足:尽管综述关注了氧化应激及相关通路在脊髓损伤中的作用,但可能对于某些特定通路的分析还不够深入。由于通路的复杂性和相互关联性,对于每个通路的详细作用机制和相互之间的调控关系可能需要更深入的研究和探讨。
临床应用的局限性:尽管综述探讨了天然产物在调控氧化应激治疗脊髓损伤方面的潜力,但目前大多数的研究还停留在实验室阶段,缺乏大规模、随机、双盲的临床试验来验证其疗效和安全性,将这些研究成果应用于临床治疗仍存在许多挑战。此外,临床治疗的复杂性使得将实验室研究成果直接转化为临床实践变得困难。
个人主观性的影响:综述的撰写过程中难免会受到作者个人主观性的影响。例如,在文献筛选、数据解读、结论推导等方面可能存在个人偏见或主观判断,这可能导致综述的客观性受到一定程度的影响。
综上所述,此综述在全面性和深度方面具有一定的优势,但也存在一些局限性。为了进一步提高综述的质量和准确性,需要在未来的研究中不断完善和优化。
3.4 综述的重要意义 天然产物作为一种具有丰富生物活性的物质来源,其中包含了许多具有抗氧化、抗炎以及神经保护作用的化合物,这些天然化合物在调控氧化应激过程中表现出多靶点作用的优势,这意味着它们可以同时作用于多个生物分子和信号通路,发挥多重治疗作用,从而更全面地抑制氧化应激反应,减轻脊髓损伤的程度。这种多靶点作用机制使得天然产物在治疗脊髓损伤方面显示出良好的治疗效果和潜力。同时,天然产物的不良反应相对较小,相比于一些合成药物,更有可能成为安全有效的治疗方案。此外,天然产物的来源广泛且可再生,如植物、动物和微生物等,这些资源的利用对环境影响较小,符合可持续发展的理念,因此,利用天然产物开发治疗脊髓损伤的药物,不仅有助于解决患者的病痛,还有助于推动医学和药物研发的可持续发展,为未来的医学研究和药物开发提供新的思路和方向。因此,研究和开发天然产物在脊髓损伤后氧化应激中的治疗作用,不仅有助于深入理解脊髓损伤的病理机制,还为寻找新的治疗方法提供了有力支持,这对于改善患者的生活质量、减轻社会负担以及推动医学进步都具有重要的意义。
中国组织工程研究杂志出版内容重点:组织构建;骨细胞;软骨细胞;细胞培养;成纤维细胞;血管内皮细胞;骨质疏松;组织工程
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||