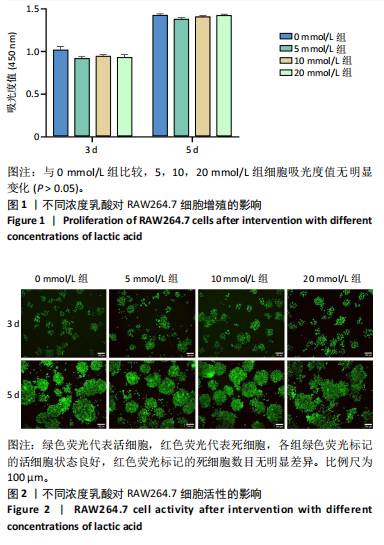
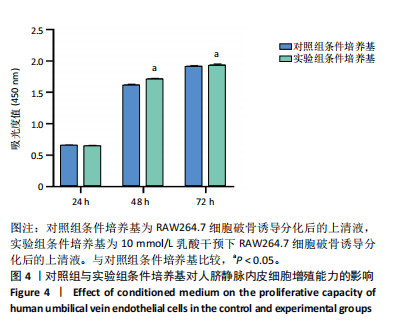
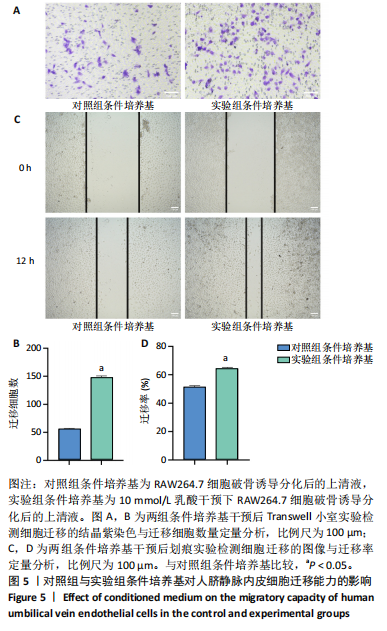
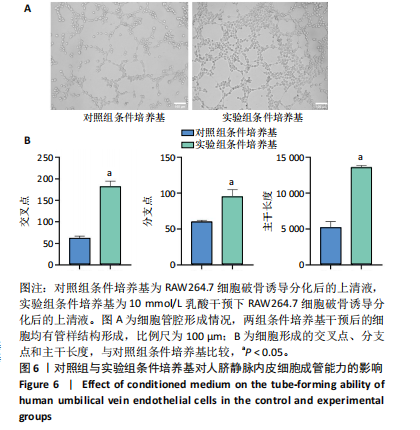
[1] 唐国柯,文根,刘彦斌,等.骨缺损修复生物材料的研究进展[J].中华骨与关节外科杂志,2023,16(2):185-192.
[2] BATTAFARANO G, ROSSI M, DE MARTINO V, et al. Strategies for Bone Regeneration: From Graft to Tissue Engineering. Int J Mol Sci. 2021;22(3): 1128.
[3] ZHAO R, YANG R, COOPER PR, et al. Bone Grafts and Substitutes in Dentistry: A Review of Current Trends and Developments. Molecules. 2021;26(10):3007.
[4] 宋颖,邓久鹏,张炜,等.聚乳酸支架材料复合改性的研究进展[J].生物医学工程研究,2020,39(4):431-434.
[5] OSMAN MA, VIRGILIO N, ROUABHIA M, et al. Development and Characterization of Functional Polylactic Acid/Chitosan Porous Scaffolds for Bone Tissue Engineering. Polymers (Basel). 2022;14(23):5079.
[6] HE J, HU X, CAO J, et al. Chitosan-coated hydroxyapatite and drug-loaded polytrimethylene carbonate/polylactic acid scaffold for enhancing bone regeneration. Carbohydr Polym. 2021;253:117198.
[7] BROOKS GA. The Science and Translation of Lactate Shuttle Theory . Cell Metab. 2018;27(4):757-785.
[8] BROOKS GA. Lactate as a fulcrum of metabolism. Redox Biol. 2020;35: 101454.
[9] FAN M, YANG K, WANG X, et al. Lactate promotes endothelial-to-mesenchymal transition via Snail1 lactylation after myocardial infarction. Sci Adv. 2023;9(5):eadc9465.
[10] YANG J, RUCHTI E, PETIT JM, et al. Lactate promotes plasticity gene expression by potentiating NMDA signaling in neurons. Proc Natl Acad Sci U S A. 2014;111(33):12228-12233.
[11] BROWN TP, GANAPATHY V. Lactate/GPR81 signaling and proton motive force in cancer: Role in angiogenesis, immune escape, nutrition, and Warburg phenomenon . Pharmacol Ther. 2020;206:107451.
[12] YI O, LIN Y, HU M, et al. Lactate metabolism in rheumatoid arthritis: Pathogenic mechanisms and therapeutic intervention with natural compounds. Phytomedicine. 2022;100:154048.
[13] IVASHKIV LB. The hypoxia-lactate axis tempers inflammation. Nat Rev Immunol. 2020;20(2):85-86.
[14] ZHOU HC, XIN-YAN Y, YU WW, et al. Lactic acid in macrophage polarization: The significant role in inflammation and cancer. Int Rev Immunol. 2022; 41(1):4-18.
[15] LUO Y, LI L, CHEN X, et al. Effects of lactate in immunosuppression and inflammation: Progress and prospects. Int Rev Immunol. 2022;41(1):19-29.
[16] CERTO M, TSAI CH, PUCINO V, et al. Lactate modulation of immune responses in inflammatory versus tumour microenvironments. Nat Rev Immunol. 2021;21(3):151-161.
[17] VEIS DJ, O’BRIEN CA. Osteoclasts, Master Sculptors of Bone. Annu Rev Pathol. 2023;18:257-281.
[18] TREBEC-REYNOLDS DP, VORONOV I, HEERSCHE JN, et al. VEGF-A expression in osteoclasts is regulated by NF-kappaB induction of HIF-1alpha. J Cell Biochem. 2010;110(2):343-351.
[19] GARIMELLA R, TAGUE SE, ZHANG J, et al. Expression and synthesis of bone morphogenetic proteins by osteoclasts: a possible path to anabolic bone remodeling. J Histochem Cytochem. 2008;56(6):569-577.
[20] YI T, LEE HL, CHA JH, et al. Epidermal growth factor receptor regulates osteoclast differentiation and survival through cross-talking with RANK signaling. J Cell Physiol. 2008;217(2):409-422.
[21] LEMMA S, DI POMPO G, PORPORATO PE, et al. MDA-MB-231 breast cancer cells fuel osteoclast metabolism and activity: A new rationale for the pathogenesis of osteolytic bone metastases. Biochim Biophys Acta Mol Basis Dis. 2017;1863(12):3254-3264.
[22] CERTO M, LLIBRE A, LEE W, et al. Understanding lactate sensing and signalling. Trends Endocrinol Metab. 2022;33(10):722-735.
[23] COLEGIO OR, CHU NQ, SZABO AL, et al. Functional polarization of tumour-associated macrophages by tumour-derived lactic acid. Nature. 2014;513(7519):559-563.
[24] RODRíGUEZ-COLMAN MJ, SCHEWE M, MEERLO M, et al. Interplay between metabolic identities in the intestinal crypt supports stem cell function. Nature. 2017;543(7645):424-427.
[25] WU Y, WANG M, ZHANG K, et al. Lactate enhanced the effect of parathyroid hormone on osteoblast differentiation via GPR81-PKC-Akt signaling. Biochem Biophys Res Commun. 2018;503(2):737-743.
[26] WU Y, WANG M, FENG H, et al. Lactate induces osteoblast differentiation by stabilization of HIF1α. Mol Cell Endocrinol. 2017;452:84-92.
[27] SELLERI S, BIFSHA P, CIVINI S, et al. Human mesenchymal stromal cell-secreted lactate induces M2-macrophage differentiation by metabolic reprogramming. Oncotarget. 2016;7(21):30193-30210.
[28] PATEL SD, PAPOUTSAKIS ET, WINTER JN, et al. The lactate issue revisited: novel feeding protocols to examine inhibition of cell proliferation and glucose metabolism in hematopoietic cell cultures. Biotechnol Prog. 2000;16(5):885-892.
[29] SCHOP D, JANSSEN FW, VAN RIJN LD, et al. Growth, metabolism, and growth inhibitors of mesenchymal stem cells. Tissue Eng Part A. 2009;15(8):1877-1886.
[30] HONG CY, LIN SK, WANG HW, et al. Metformin Reduces Bone Resorption in Apical Periodontitis Through Regulation of Osteoblast and Osteoclast Differentiation. J Endod. 2023;49(9):1129-1137.
[31] REN X, SHAN WH, WEI LL, et al. ACP5: Its Structure, Distribution, Regulation and Novel Functions. Anticancer Agents Med Chem. 2018;18(8):1082-1090.
[32] QIAN J, GONG ZC, ZHANG YN, et al. Lactic acid promotes metastatic niche formation in bone metastasis of colorectal cancer. Cell Commun Signal. 2021;19(1):9.
[33] CACKOWSKI FC, ANDERSON JL, PATRENE KD, et al. Osteoclasts are important for bone angiogenesis. Blood. 2010;115(1):140-149.
[34] HUANG J, HAN Q, CAI M, et al. Effect of Angiogenesis in Bone Tissue Engineering. Ann Biomed Eng. 2022;50(8):898-913.
[35] QIU J, WANG X, ZHOU H, et al. Enhancement of periodontal tissue regeneration by conditioned media from gingiva-derived or periodontal ligament-derived mesenchymal stem cells: a comparative study in rats. Stem Cell Res Ther. 2020;11(1):42.
[36] NOVELLO S, TRICOT-DOLEUX S, NOVELLA A, et al. Influence of Periodontal Ligament Stem Cell-Derived Conditioned Medium on Osteoblasts. Pharmaceutics. 2022;14(4):729.
[37] KATAGIRI W, KAWAI T, OSUGI M, et al. Angiogenesis in newly regenerated bone by secretomes of human mesenchymal stem cells. Maxillofac Plast Reconstr Surg. 2017;39(1):8.
[38] BURGER MG, GROSSO A, BRIQUEZ PS, et al. Robust coupling of angiogenesis and osteogenesis by VEGF-decorated matrices for bone regeneration. Acta Biomater. 2022;149:111-125.
[39] WENG T, YANG M, ZHANG W, et al. Dual gene-activated dermal scaffolds regulate angiogenesis and wound healing by mediating the coexpression of VEGF and angiopoietin-1. Bioeng Transl Med. 2023;8(5):e10562. |