中国组织工程研究 ›› 2018, Vol. 22 ›› Issue (18): 2890-2895.doi: 10.3969/j.issn.2095-4344.0887
• 药物控释材料 drug delivery materials • 上一篇 下一篇
雷帕霉素药物洗脱支架治疗老年退行性冠状动脉粥样硬化性心脏病无保护左主干病变:前瞻性、非随机、对照临床试验方案及预试验结果
陈 伟1,方志成1,郑 翔1,刘伯毅1,赵继先2,廖应英3
Rapamycin-eluting stents for unprotected left main coronary artery stenosis in coronary atherosclerosis in the older adults: study protocol for a prospective, non-randomized, controlled trial and preliminary results
Chen Wei1, Fang Zhi-cheng1, Zheng Xiang1, Liu Bo-yi1, Zhao Ji-xian2, Liao Ying-ying3
- 1Department of Critical Care Medicine, 2Department of Cardiovascular Medicine,3Department of Digestion, Taihe Hospital (Affiliated Hospital of Hubei University of Medicine), Shiyan 442000, Hubei Province, China
摘要:
文章快速阅读:
.jpg)
文题释义:
左主干病变:是指冠状动脉造影左主干狭窄程度> 50%的病变。
左主干病变分型:分为“有保护”和“无保护”,两种类型“有保护”左主干病变指左冠状动脉存在通畅的血管桥或其他来源的侧支循环;“无保护”左主干病变恰恰相反,不存在血管桥和侧支循环。
背景:冠脉旁路移植术常被认为是治疗冠状动脉粥样硬化性心脏病无保护左主干(unprotected left main coronary artery,ULMCA)病变的金标准疗法。近年来研究表明,药物洗脱支架在治疗ULMCA病变后的心血管不良事件发生率更低,甚至有研究者认为药物洗脱支架将取代冠脉旁路移植术成新的为治疗金标准,但国内外学者对此尚有一定争议。
目的:试验拟观察采用雷帕霉素药物洗脱支架植入治疗老年ULMCA病变的安全性和有效性,并与冠脉旁路移植术治疗进行比较。
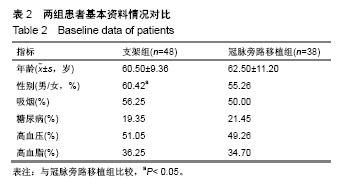
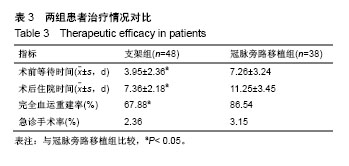
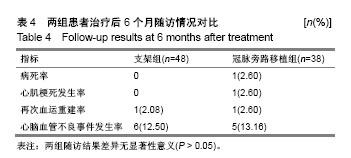
方法:纳入十堰市太和医院(湖北医药学院附属医院)心内科和十堰市人民医院(湖北医药学院附属医院)心内科收治的老年退行性ULMCA病变患者224例,按治疗方式的不同分为2组,支架组采用雷帕霉素药物洗脱支架植入治疗,冠脉旁路移植术采用冠脉旁路移植治疗,每组112例,术后随访9,12,24,36个月。研究中有效性的主要结局指标为术后36个月病变血管再次血运重建率。研究的次要结局指标为术后9,12,24个月病变血管再次血运重建率;术后9,12,24,36个月的血栓事件发生率、病变血管再狭窄率、病死率、死亡原因及生存时间。术前,术后9,12,24,36个月的病变血管的动脉造影形态。研究的安全性指标为术后9,12,24,36个月主要不良心脑血管事件发生率。课题组从2016年1月到2017年12月收集86例患者,分成支架组48例,冠脉旁路移植38例。预试验结果显示,与冠脉旁路移植组比较,支架组术前等待时间,术后住院时间及完全血运重建率均降低(P< 0.05)。术后6个月,两组病死率、心肌梗死发生率、再次血运重建率及心脑血管不良事件发生率差异无显著性意义(P > 0.05)。试验经十堰市太和医院和十堰市人民医院医学伦理委员会批准(审批单位:十堰市太和医院,审批时间2017年7月;审批号:TH005X;十堰市人民医院,审批时间2017年7月,审批号:RM011X)。研究符合世界医学会制定的《赫尔辛基宣言》的要求,研究对象均签署知情同意书。试验设计时间为2018年1月,试验计划于2018年8月开始进行患者招募,2019年8月招募结束,2022年10月进行结果指标分析,2022年12月试验完成。文章结果将以科学会议报道,或在同行评议的期刊上发表传播。试验已在中国临床试验注册中心注册(注册号:ChiCTR1800016413),方案版本号1.0。
讨论:试验希望观察,雷帕霉素药物洗脱支架植入治疗老年ULMCA病变中长期随访有效性及安全性方面的数据与冠脉旁路移植术在相比,明确哪种疗法的预后效果更好,以筛选出治疗ULMCA病变最佳的策略。
中图分类号:
.jpg)
.jpg)
.jpg)
.jpg)