Chinese Journal of Tissue Engineering Research ›› 2014, Vol. 18 ›› Issue (37): 6064-6068.doi: 10.3969/j.issn.2095-4344.2014.37.029
Cardiac stem cells in cardiac tissue engineering: present and future
Li Run-qin, Huang Chun
- Chongqing Three Gorges Medical College, Chongqing 404120, China
-
Revised:2014-08-03Online:2014-09-03Published:2014-09-03 -
Contact:Huang Chun, Master, Chief physician, Chongqing Three Gorges Medical College, Chongqing 404120, China -
About author:Li Run-qin, Studying for doctorate, Associate professor, Chongqing Three Gorges Medical College, Chongqing 404120, China -
Supported by:the Scientific and Technological Research Program of Chongqing Educational Committee, No. KJ101804
CLC Number:
Cite this article
Li Run-qin, Huang Chun. Cardiac stem cells in cardiac tissue engineering: present and future[J]. Chinese Journal of Tissue Engineering Research, 2014, 18(37): 6064-6068.
share this article
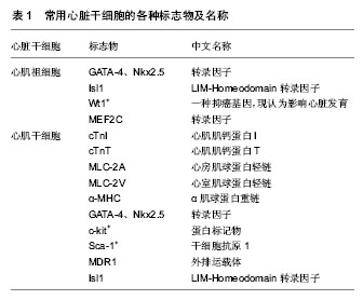
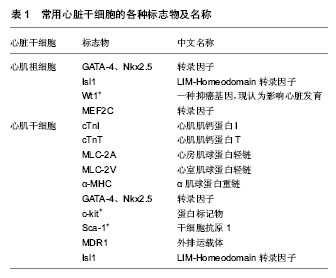
2.1 心肌纤维的特点 长期以来,人们都认为心肌细胞的特殊之处是:自动物出生之日起,心肌细胞就作为永久细胞只能通过体积的增大而代偿,不能通过数目的增多而再生。发表在2013年Pnas杂志的一篇论著[7]:美国波士顿儿童医院心脏研究实验室研究表明,动物的心肌细胞有增殖和再生的潜能。他们对左心室心肌细胞增殖的研究证实了从出生后到20岁心肌细胞有增殖和再生能力,但仅见于年轻人。通过刺激心肌细胞的增殖潜能可能被用于治疗很多心脏疾病。有研究显示,在成年哺乳动物的心肌细胞表面表达具有多种干细胞相关抗原,这些细胞具有心肌干细胞的潜能,能够自我复制[8]。心肌干细胞对心脏的修复与心脏的生长因子有关,这些表达干细胞相关抗原的心肌细胞在生长因子的作用下能重建室壁[9]。因此,虽然成人心肌细胞被称为永久细胞,但通过解除阻断仍具有再生潜能而不是一个终末分化的器官[10]。心肌干细胞的研究将为心肌组织工程研究开辟崭新的途径。 2.2 心脏损伤后的再生治疗 心肌受损后的再修复已经成为再生医学领域中发展最快的部分。把死亡或受损的心肌细胞替换为新的心肌细胞可能是一个逆转心脏疾病的理想方式。然而,成人心脏主要是晚期分化心肌细胞,没有明显的再生能力。最新研究表明干细胞有巨大的再生潜力,因此,当前心脏再生的研究专注于干细胞,使其作为替代受损心肌的来源用于心脏治疗。未来发展方向是努力改善当前干细胞的研究策略和方法[11]。干细胞疗法的临床意义是改善心肌梗死后心脏重构,诱导有功能心肌的再生和新构建血管的形成[12]。一旦心肌梗死导致心力衰竭,会有大量心肌纤维死亡,所以要达到心脏损伤后的再生治疗,需要有充足数目的自体心肌纤维和保证移植后进入受到损伤的宿主心脏的心肌纤维能够与宿主心肌纤维形成有效机械偶联,并且使心肌纤维能够同步收缩。除此之外,新移植的心肌干细胞是否能够接受神经内分泌的调节?及这些新生的心肌纤维是否具有接触性抑制?在局部致炎因子的作用下,移植的心肌干细胞是否会被清除等等问题仍然等待科学家继续研究[13]。 在以前的大量研究中,虽然已经证实干细胞治疗能够改善受损心肌的功能,但至今还没在已经梗死的心肌上建立与宿主心肌纤维同步收缩,并且可以复制的模型。以前的研究能够为现有心肌损伤后再修复的策略提供基础,并为有志于心肌纤维再生及其机制的探索提供研究思路。 2.3 心脏干细胞 表达转录因子Nkx2.5和Isl1的心脏祖细胞,能发育为心肌纤维,平滑肌纤维和血管内皮细胞[14]。正常小鼠心脏发育过程中,Wt1基因表达位于心外膜上,这些Wt1+的前体心肌细胞能向全功能的心肌细胞分化。Wt1+的细胞起源于表达转录因子Nkx2.5、Isl1的心脏祖细胞,这些结果确定Wt1+心外膜细胞是先前未确认的心肌细胞的祖细胞,为利用心肌祖细胞促进心肌的再生和修复提供有力的依据[15]。《自然》杂志也证实了Isl1心脏祖细胞的存在,2009年Bu研究小组对小鼠胚胎的研究,证实了多能心脏祖细胞的存在,心脏祖细胞可以发育为心脏中所有主要类型的细胞。对比小鼠的心脏发生发展,人类心脏细胞谱系有多元化扩张,具有不同的途径。这些结果为心血管疾病和人类心血管药物的再生治疗提供了新的研究思路[16]。 麻省总医院心血管研究中心的研究报告发表于《细胞》杂志。在这篇研究报告里,科学家记录了对大鼠祖细胞的鉴别和克隆单一胚胎干细胞的过程,以及表明这些克隆细胞可以分化成心肌细胞、平滑肌细胞和内皮细胞。大鼠胚胎试管研究发现原始心脏组织里有少量Isl1表达细胞和另外两种重要蛋白质Nkx2.5和flk1[17]。 《细胞》杂志刊载了另一篇研究方向相同的文章,来自于波士顿儿童医院司徒•沃金博士的实验室和哈佛干细胞研究所,他们发现表达蛋白质Nkx2.5的第一心区祖细胞能够产生心细胞和平滑肌细胞两种细胞。该文章的第一作者西恩•吴博士最近加盟麻省总医院心血管研究中心,他将与陈博士的研究小组对这些初步发现进行后续研究,包括弄清楚这两种祖细胞之间所存在的任何发展关系[18]。心脏干细胞是有可能修复受损伤心肌的“候选人”,应用这种方法进行心脏修复的基本原理是体外扩增心脏干细胞到临床实验以及后期进行活组织检查,输送心肌干细胞到受损伤的心肌区域,使心肌干细胞再生,以此来治疗心力衰竭[19]。 心肌梗死后的心脏是否拥有促进心肌再生的心肌干细胞池?为了这个目的,有人测定了20例急性心肌梗死患者的心脏、20例终末期梗死后心肌病的心脏和12例正常心脏的心肌干细胞生长和衰老的情况。结果发现急性心肌梗死心脏中的心肌干细胞数量明显增加,在终末期梗死后心肌病的心脏中心肌干细胞数量亦较少程度的增加。通过端粒酶活性成分来确定干细胞的生长:急性心肌梗死的心脏增加到28%、终末期梗死后心肌病的心脏增加到14%,而正常心脏为1.5%;来源于心肌干细胞的心肌细胞、平滑肌细胞和内皮谱系细胞在急性心肌梗死心脏中和终末期梗死后心肌病的心脏中分别增加了约85倍和25倍。然而,代表衰老的p16INK4a-p53阳性心肌干细胞也同样增多(急性心肌梗死:3.8%;终末期梗死后心肌病:9.6%;正常心脏:0.3%),有功能活性的心肌干细胞的数量明显减少(急性心肌梗死: 26 000/cm3;终末期梗死后心肌病:7 000/cm3)。在7个急性心肌梗死的心脏中,自发再生的心肌细胞相互聚集。总之,人类心脏拥有心肌干细胞池,并且心肌缺血损伤可诱导心肌干细胞的激活[20]。 2.4 心脏干细胞的标志和鉴定 2003年,Beltrami等首次从20-23个月龄的大鼠心肌内分离出一种in-c-kit+-CD45--CD38-细胞,其具有自我更新能力并能分化成心肌细胞、内皮细胞及平滑肌细胞。由于起源于心脏,缺乏血细胞系、心内皮祖细胞及骨髓造血干细胞的表面标记特征,因此判断此类细胞为心肌干细胞[21]。心肌干细胞是指存在于胚胎和成体心脏中提交心肌谱系的多能干细胞,在成熟心肌中,具有高度自我更新和特异性心肌分化、增殖潜能的未分化细胞。 心肌干细胞体积较小,呈圆形或杆状,多为单个细胞核,微丝束排列不规则或平行排列,呈现出肌微丝装配和新闰盘形成的特征,并表达心肌特异基因心肌肌钙蛋白I(cTnI)、心肌肌钙蛋白T(cTnT)、心房肌球蛋白轻链(MLC-2A)、心室肌球蛋白轻链(MLC-2V)和α肌球蛋白重链(α-MHC),也表达心肌转录因子GATA-4、Nkx2.5[22]。心脏祖细胞早期表达Nkx2.5,GATA-4,MEF2C[23]。心肌干细胞的研究采用c-kit、Sca-1、MDR1和Isl1等作为判断心肌干细胞的标志[24]。识别标志不同,心肌干细胞主要包括c-kit+细胞群、Sca-1+细胞群及侧群(SP)细胞等。c-kit+的心肌干细胞数量相对最多。有研究显示c-kit+的大鼠心肌干细胞表达CD29、CD90,不表达CD11bc、CD34和CD45[25]。 Hodgkiss-Geere取狗的心房组织培养并分离出成年心肌干细胞,这些细胞表达c-kit和GATA-4,心脏肌钙蛋白T和Nkx2.5表达水平低[26];干细胞向心脏的分化到一定程度后,心脏肌钙蛋白T和Nkx2.5表达增加,c-kit表达下调。Isl1基因的表达可以修复受损心肌,Isl1作为心肌祖细胞的标志之一受到广泛关注[27] (表1)。心肌干细胞常用正常情况下多处于G0期这个特性来鉴定,也就是LRC(label-retaining cell)法通过单细胞培养[28],它能分化为表面标志清楚的干细胞的特点及其具有无限扩增及其分化的潜能。鉴定时以此两点为主,再辅助表面标志物。"
| [1] Behfar A, Crespo-Diaz R, Terzic A,et al.Cell therapy for cardiac repair--lessons from clinical trials.Nat Rev Cardiol. 2014;11(4):232-246. [2] Martinez-Fernandez A, Nelson TJ, Ikeda Y,et al.c-MYC independent nuclear reprogramming favors cardiogenic potential of induced pluripotent stem cells.J Cardiovasc Transl Res. 2010;3(1):13-23. [3] Beltrami AP, Barlucchi L, Torella D,et al.Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114(6):763-776. [4] Hansen M, Nyby S, Eifer Møller J,et al.Intracoronary Injection of CD34-Cells in Chronic Ischemic Heart Failure: 7 Years Follow-Up of the DanCell Study.Cardiology. 2014;129(2): 69-74. [5] Canepa M, Coviello D, Chiarella F.Cardiac cell therapy: the puzzle is waiting to be solved.G Ital Cardiol (Rome). 2006; 7(4):252-265. [6] Wollert KC, Drexler H.Clinical applications of stem cells for the heart.Circ Res. 2005;96(2):151-163. [7] Mollova M, Bersell K, Walsh S,et al.Cardiomyocyte proliferation contributes to heart growth in young humans.Proc Natl Acad Sci U S A. 2013;110(4):1446-1451. [8] He JQ, Vu DM, Hunt G,et al.Human cardiac stem cells isolated from atrial appendages stably express c-kit.PLoS One. 2011;6(11):e27719. [9] Boucek Jr RJ, Steele J, Jacobs JP,et al.Ex vivo paracrine properties of cardiac tissue: Effects of chronic heart failure.J Heart Lung Transplant. 2014. [Epub ahead of print]. [10] Nadal-Ginard B, Anversa P, Kajstura J,et al.Cardiac stem cells and myocardial regeneration.Novartis Found Symp. 2005;265:142-154. [11] Cho GS, Fernandez L, Kwon C.Regenerative medicine for the heart: perspectives on stem-cell therapy.Antioxid Redox Signal. 2014 Aug 18. [Epub ahead of print]. [12] Ghadge SK, Mühlstedt S, Ozcelik C,et al. SDF-1α as a therapeutic stem cell homing factor in myocardial infarction.Pharmacol Ther. 2011;129(1):97-108. [13] Bergmann O, Jovinge S.Cardiac regeneration in vivo: Mending the heart from within?Stem Cell Res. 2014 Jul 16. [Epub ahead of print]. [14] Zhou B, von Gise A, Ma Q,et al.Nkx2-5- and Isl1-expressing cardiac progenitors contribute to proepicardium.Biochem Biophys Res Commun. 2008;375(3):450-453. [15] Zhou B, Ma Q, Rajagopal S,et al.Epicardial progenitors contribute to the cardiomyocyte lineage in the developing heart.Nature. 2008;454(7200):109-113. [16] Bu L, Jiang X, Martin-Puig S,et al.Human ISL1 heart progenitors generate diverse multipotent cardiovascular cell lineages.Nature. 2009;460(7251):113-117. [17] Moretti A, Caron L, Nakano A,et al.Multipotent embryonic isl1+ progenitor cells lead to cardiac, smooth muscle, and endothelial cell diversification.Cell. 2006;127(6):1151-1165. [18] Kattman SJ, Huber TL, Keller GM.Multipotent flk-1+ cardiovascular progenitor cells give rise to the cardiomyocyte, endothelial, and vascular smooth muscle lineages.Dev Cell. 2006;11(5):723-732. [19] Mayfield AE, Tilokee EL, Davis DR.Resident Cardiac Stem Cells and Their Role in Stem Cell Therapies for Myocardial Repair.Can J Cardiol. 2014 Mar 20. [Epub ahead of print] [20] Urbanek K, Torella D, Sheikh F, et al.Myocardial regeneration by activation of multipotent cardiac stem cells in ischemic heart failure.Proc Natl Acad Sci U S A. 2005;102(24): 8692-8697. [21] Linke A, Müller P, Nurzynska D,et al.Stem cells in the dog heart are self-renewing, clonogenic, and multipotent and regenerate infarcted myocardium, improving cardiac function.Proc Natl Acad Sci U S A. 2005;102(25):8966-8971. [22] Leri A, Kajstura J, Anversa P.Cardiac stem cells and mechanisms of myocardial regeneration.Physiol Rev. 2005; 85(4):1373-1416. [23] Barile L, Messina E, Giacomello A,et al.Endogenous cardiac stem cells.Prog Cardiovasc Dis. 2007;50(1):31-48. [24] 王彤,万智,黄辉,等.大鼠心肌干细胞的体外分离培养和生物学特性鉴定[J].岭南急诊医学杂志,2008,13(2) : 81-83. [25] Hodgkiss-Geere HM, Argyle DJ, Corcoran BM,et al.Characterisation and cardiac directed differentiation of canine adult cardiac stem cells.Vet J. 2012;191(2):176-182. [26] Dergilev KV, Rubina KA, Tsokolaeva ZI,et al.Left ventricular heart aneurism--a new source of resident cardiac stem cells.Tsitologiia. 2010;52(11):921-930. [27] Bolli R, Chugh AR, D'Amario D,et al.Cardiac stem cells in patients with ischaemic cardiomyopathy (SCIPIO): initial results of a randomised phase 1 trial.Lancet. 2011;378 (9806):1847-1857. [28] Chan RW, Gargett CE.Identification of label-retaining cells in mouse endometrium.Stem Cells. 2006;24(6):1529-1538. [29] Nagai T, Shiojima I, Matsuura K,et al.Promotion of cardiac regeneration by cardiac stem cells.Circ Res. 2005;97(7): 615-617. [30] Urbanek K, Rota M, Cascapera S,et al.Cardiac stem cells possess growth factor-receptor systems that after activation regenerate the infarcted myocardium, improving ventricular function and long-term survival.Circ Res. 2005;97(7):663-673. [31] Rosen MR, Myerburg RJ, Francis DP,et al.Translating Stem Cell Research to Cardiac Disease Therapies: Pitfalls and Prospects for Improvement.J Am Coll Cardiol. 2014;64(9): 922-937. [32] Chugh AR, Beache GM, Loughran JH,et al.Administration of cardiac stem cells in patients with ischemic cardiomyopathy: the SCIPIO trial: surgical aspects and interim analysis of myocardial function and viability by magnetic resonance. Circulation. 2012;126(11 Suppl 1):S54-64. [33] Chan HH, Meher Homji Z, Gomes RS,et al.Human cardiosphere-derived cells from patients with chronic ischaemic heart disease can be routinely expanded from atrial but not epicardial ventricular biopsies.J Cardiovasc Transl Res. 2012;5(5):678-687. [34] Ellison GM, Vicinanza C, Smith AJ,et al.Adult c-kit(pos) cardiac stem cells are necessary and sufficient for functional cardiac regeneration and repair.Cell. 2013;154(4):827-842. [35] Ellison GM, Torella D, Dellegrottaglie S,et al.Endogenous cardiac stem cell activation by insulin-like growth factor-1/hepatocyte growth factor intracoronary injection fosters survival and regeneration of the infarcted pig heart.J Am Coll Cardiol. 2011;58(9):977-986. [36] Tillmanns J, Rota M, Hosoda T,et al.Formation of large coronary arteries by cardiac progenitor cells.Proc Natl Acad Sci U S A. 2008;105(5):1668-1673. [37] Nadal-Ginard B, Ellison GM, Torella D.The cardiac stem cell compartment is indispensable for myocardial cell homeostasis, repair and regeneration in the adult.Stem Cell Res. 2014 Apr 29. [Epub ahead of print] [38] Xu XL, Yi F, Pan HZ,et al.Progress and prospects in stem cell therapy.Acta Pharmacol Sin. 2013;34(6):741-746. [39] Okano H, Nakamura M, Yoshida K,et al.Steps toward safe cell therapy using induced pluripotent stem cells.Circ Res. 2013; 112(3):523-533. [40] Fox IJ, Daley GQ, Goldman SA,et al.Stem cell therapy. Use of differentiated pluripotent stem cells as replacement therapy for treating disease.Science. 2014;345(6199):1247391. |
| [1] | Pu Rui, Chen Ziyang, Yuan Lingyan. Characteristics and effects of exosomes from different cell sources in cardioprotection [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(在线): 1-. |
| [2] | Lin Qingfan, Xie Yixin, Chen Wanqing, Ye Zhenzhong, Chen Youfang. Human placenta-derived mesenchymal stem cell conditioned medium can upregulate BeWo cell viability and zonula occludens expression under hypoxia [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(在线): 4970-4975. |
| [3] | Zhang Chao, Lü Xin. Heterotopic ossification after acetabular fracture fixation: risk factors, prevention and treatment progress [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1434-1439. |
| [4] | Zhou Jihui, Li Xinzhi, Zhou You, Huang Wei, Chen Wenyao. Multiple problems in the selection of implants for patellar fracture [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1440-1445. |
| [5] | Wang Debin, Bi Zhenggang. Related problems in anatomy mechanics, injury characteristics, fixed repair and three-dimensional technology application for olecranon fracture-dislocations [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1446-1451. |
| [6] | Zhang Xiumei, Zhai Yunkai, Zhao Jie, Zhao Meng. Research hotspots of organoid models in recent 10 years: a search in domestic and foreign databases [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(8): 1249-1255. |
| [7] | Ji Zhixiang, Lan Changgong. Polymorphism of urate transporter in gout and its correlation with gout treatment [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(8): 1290-1298. |
| [8] | Yuan Mei, Zhang Xinxin, Guo Yisha, Bi Xia. Diagnostic potential of circulating microRNA in vascular cognitive impairment [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(8): 1299-1304. |
| [9] | Hou Jingying, Yu Menglei, Guo Tianzhu, Long Huibao, Wu Hao. Hypoxia preconditioning promotes bone marrow mesenchymal stem cells survival and vascularization through the activation of HIF-1α/MALAT1/VEGFA pathway [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 985-990. |
| [10] | Shi Yangyang, Qin Yingfei, Wu Fuling, He Xiao, Zhang Xuejing. Pretreatment of placental mesenchymal stem cells to prevent bronchiolitis in mice [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 991-995. |
| [11] | Liang Xueqi, Guo Lijiao, Chen Hejie, Wu Jie, Sun Yaqi, Xing Zhikun, Zou Hailiang, Chen Xueling, Wu Xiangwei. Alveolar echinococcosis protoscolices inhibits the differentiation of bone marrow mesenchymal stem cells into fibroblasts [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 996-1001. |
| [12] | Fan Quanbao, Luo Huina, Wang Bingyun, Chen Shengfeng, Cui Lianxu, Jiang Wenkang, Zhao Mingming, Wang Jingjing, Luo Dongzhang, Chen Zhisheng, Bai Yinshan, Liu Canying, Zhang Hui. Biological characteristics of canine adipose-derived mesenchymal stem cells cultured in hypoxia [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1002-1007. |
| [13] | Geng Yao, Yin Zhiliang, Li Xingping, Xiao Dongqin, Hou Weiguang. Role of hsa-miRNA-223-3p in regulating osteogenic differentiation of human bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1008-1013. |
| [14] | Lun Zhigang, Jin Jing, Wang Tianyan, Li Aimin. Effect of peroxiredoxin 6 on proliferation and differentiation of bone marrow mesenchymal stem cells into neural lineage in vitro [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1014-1018. |
| [15] | Zhu Xuefen, Huang Cheng, Ding Jian, Dai Yongping, Liu Yuanbing, Le Lixiang, Wang Liangliang, Yang Jiandong. Mechanism of bone marrow mesenchymal stem cells differentiation into functional neurons induced by glial cell line derived neurotrophic factor [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1019-1025. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||