Chinese Journal of Tissue Engineering Research ›› 2014, Vol. 18 ›› Issue (10): 1539-1546.doi: 10.3969/j.issn.2095-4344.2014.10.010
Previous Articles Next Articles
Large-scale expansion of human umbilical cord mesenchymal stem cells in human platelet lysate as a substitute of fetal bovine serum
Li Bing-yao1, Wu Xiao-yun2, Wu Yan1
- 1 Department of Histology and Embryology, Inner Mongolia Medical University, Hohhot 010059, Inner Mongolia Autonomous Region, China; 2 Beijing Jingmeng Stem Cell High-Tech Co., Ltd., Beijing 100085, China
-
Online:2014-03-05Published:2014-03-05 -
Contact:Wu Yan, M.D., Professor, Master’s supervisor, Department of Histology and Embryology, Inner Mongolia Medical University, Hohhot 010059, Inner Mongolia Autonomous Region, China -
About author:Li Bing-yao, Studying for master’s degree, Attending physician, Department of Histology and Embryology, Inner Mongolia Medical University, Hohhot 010059, Inner Mongolia Autonomous Region, China -
Supported by:Stem Cell Technology Innovation Team Funded Projects in Inner Mongolia Autonomous Region, No. kjt0020947
CLC Number:
Cite this article
Li Bing-yao, Wu Xiao-yun, Wu Yan. Large-scale expansion of human umbilical cord mesenchymal stem cells in human platelet lysate as a substitute of fetal bovine serum[J]. Chinese Journal of Tissue Engineering Research, 2014, 18(10): 1539-1546.
share this article
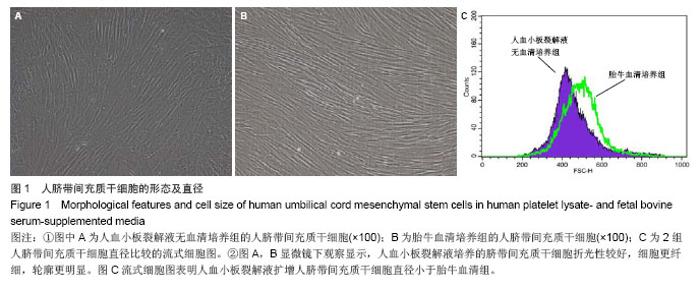
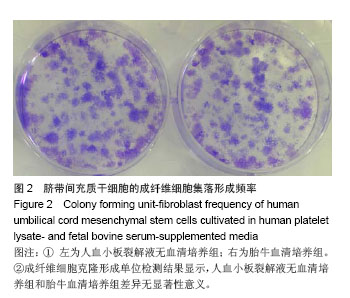
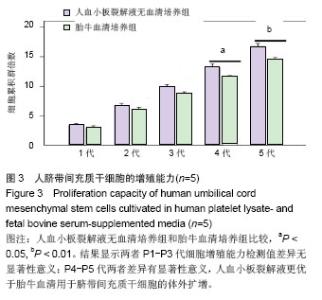
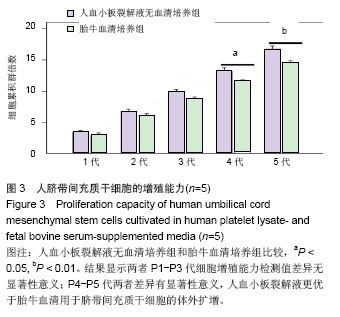
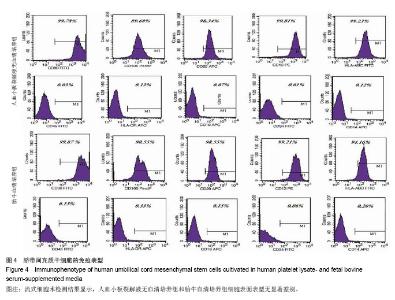
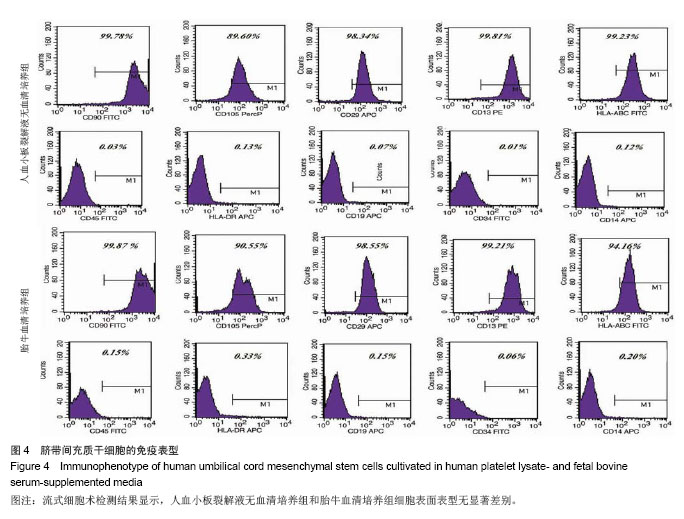
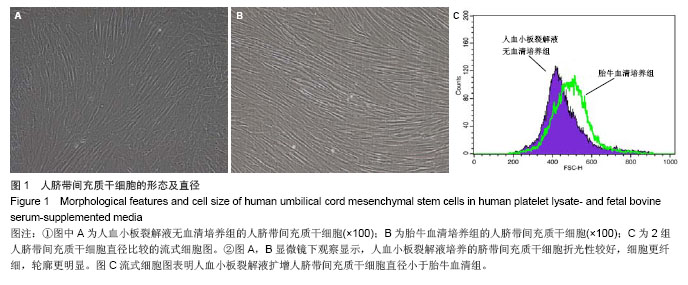
2.1 细胞形态观察 经酶消化后将脐带源有核细胞分别接种于含有人血小板裂解液无血清培养基和胎牛血清培养基进行细胞培养,人血小板裂解液无血清培养组细胞于12 h内可见少量细胞贴壁。在倒置显微镜下可见贴壁细胞单个散在或成簇生长,贴壁后增长速度较快,细胞体积增大,长梭形,多边形,细胞集落明显增大、增多。培养1周后细胞达80%-90% 融合,传代后呈成为形态相对均一的梭形细胞,呈平行排列生长或旋涡状生长。与体积分数10%胎牛血清培养基接种的间充质干细胞的细胞形态相比,二者形态相似(图1)。 2.2 细胞直径比较 流式细胞仪通过对细胞的前向角散射信号比较2种培养基扩增脐带间充质干细胞的细胞直径,结果显示人血小板裂解液培养基扩增的脐带间充质干细胞的细胞直径小于胎牛血清培养基扩增的脐带间充质干细胞(n=5,t=3.821,P < 0.05,图1)。"
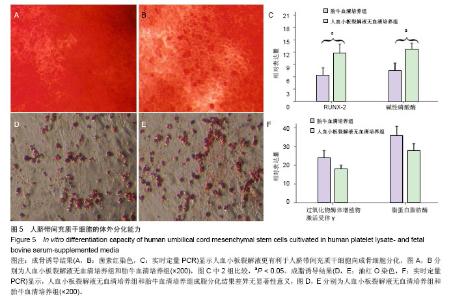
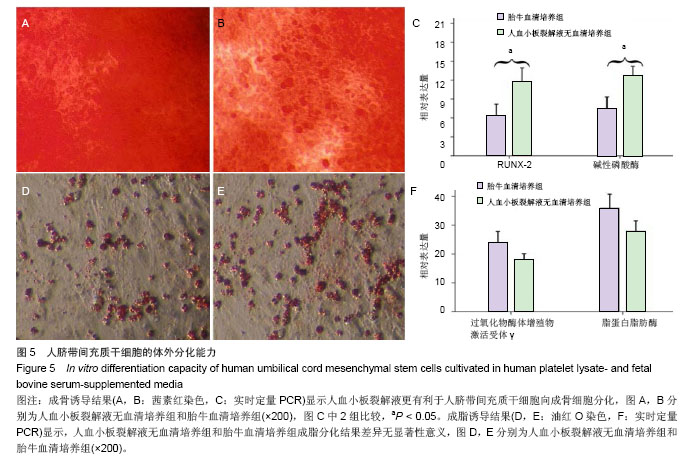
2.6 细胞分化能力检测结果 成骨诱导分化鉴定:人血小板裂解液无血清培养基扩增P5代脐带间充干细胞经成骨诱导3周后,茜素红染色结果结节呈橘红色,边界清晰,提示有矿化基质沉积,染色结果显示血小板裂解液无血清培养基扩增脐带间充干细胞的矿化结节(橘红色颗粒)数量高于胎牛血清培养基扩增的脐带间充质干细胞(图5A,B)。 实时定量PCR结果显示血小板裂解液无血清培养基扩增脐带间充干细胞的成骨分化特异性基因RUNX-2和碱性磷酸酶表达量均高于胎牛血清培养基扩增的脐带间充干细胞,差异有显著性意义(t =4.371,P < 0.05;t=3.358,P < 0.05,图5C)。 成脂诱导分化鉴定:人血小板裂解液无血清培养基扩增P5代脐带间充干细胞经成脂诱导定向诱导培养后1周,小部分细胞内已出现微小明亮的脂滴。随着诱导时间的延长,出现脂滴的细胞增多,细胞由梭形变成椭圆形或多角形。 培养3周后,油红O染色后镜下观察见大量的脂质沉积(图5D,E),提示细胞已分化成脂肪细胞,染色结果显示人血小板裂解液无血清培养基扩增脐带间充干细胞脂质沉积(红色颗粒)数量低于胎牛血清培养基扩增的脐带间充干细胞,实时定量PCR结果显示人血小板裂解液无血清培养基扩增脐带间充干细胞成脂分化特异性基因过氧化物酶体增殖物激活受体γ和脂蛋白脂肪酶表达量均低于胎牛血清培养基扩增的脐带间充干细胞,但两者之间比较差异无显著性意义(t=1.141,P > 0.05;t=1.256, P > 0.05,图5F)。"
| [1]Otto WR, Wright NA. Mesenchymal stem cells: from experiment to clinic. Fibrogenesis Tissue Repair. 2011; 4:20.[2]Spitkovsky D, Hescheler J. Adult mesenchymal stromal stem cells for therapeutic applications. Minim Invasive Ther Allied Technol. 2008;17(2):79-90.[3]Tantrawatpan C, Manochantr S, Kheolamai P, et al. Pluripotent gene expression in mesenchymal stem cells from human umbilical cord wharton's jelly and their differentiation potential to neural-like cells. J Med Assoc Thai. 2013;96: 1208-1217.[4]Luo X, Yao RS, Song H, et al. [Study on human umbilical cord mesenchymal stem cells transplantation in treatment of stress urinary incontinence in rats]. Zhonghua Fu Chan Ke Za Zhi. 2013;48:579-583.[5]Liu LY, Chai JK, Duan HJ, et al. [Comparison of different methods for the isolation of human umbilical cord mesenchymal stem cells]. Zhonghua Yi Xue Za Zhi. 2013;93:2592-2596.[6]Bai C, Gao Y, Li Q, et al. Differentiation of chicken umbilical cord mesenchymal stem cells into beta-like pancreatic islet cells. Artif Cells Nanomed Biotechnol. 2013.[7]van der Valk J, Brunner D, De Smet K, et al. Optimization of chemically defined cell culture media--replacing fetal bovine serum in mammalian in vitro methods. Toxicol In Vitro. 2010; 24(4):1053-1063.[8]Spees JL, Gregory CA, Singh H, et al. Internalized antigens must be removed to prepare hypoimmunogenic mesenchymal stem cells for cell and gene therapy. Mol Ther. 2004;9(5): 747-756.[9]Poloni A, Maurizi G, Serrani F, et al. Human AB serum for generation of mesenchymal stem cells from human chorionic villi: comparison with other source and other media including platelet lysate. Cell Prolif. 2012;45(1):66-75.[10]Gottipamula S, Sharma A, Krishnamurthy S, et al. Human platelet lysate is an alternative to fetal bovine serum for large-scale expansion of bone marrow-derived mesenchymal stromal cells. Biotechnol Lett. 2012;34(7):1367-1374.[11]Azouna NB, Jenhani F, Regaya Z, et al. Phenotypical and functional characteristics of mesenchymal stem cells from bone marrow: comparison of culture using different media supplemented with human platelet lysate or fetal bovine serum. Stem Cell Res Ther. 2012;3(1):6.[12]Ma HY, Yao L, Yu YQ, et al. An Effective and Safe Supplement for Stem Cells Expansion ex vivo - Cord Blood Serum. Cell Transplant. 2012;21(5):857-869.[13]Huang L, Critser PJ, Grimes BR, et al. Human umbilical cord blood plasma can replace fetal bovine serum for in vitro expansion of functional human endothelial colony-forming cells. Cytotherapy. 2011;13(6):712-721.[14]Tekkatte C, Vidyasekar P, Kapadia NK, et al. Enhancement of adipogenic and osteogenic differentiation of human bone-marrow-derived mesenchymal stem cells by supplementation with umbilical cord blood serum. Cell Tissue Res. 2012;347(2):383-395.[15]Wu KH, Tsai C, Wu HP, et al. Human application of ex vivo expanded umbilical cord-derived mesenchymal stem cells: Enhance hematopoiesis after cord blood transplantation. Cell Transplant. 2013;22:2041-2051.[16]Kishimoto S, Ishihar, M, Mori Y, et al. Effective expansion of human adipose-derived stromal cells and bone marrow-derived mesenchymal stem cells cultured on a fragmin/protamine nanoparticles-coated substratum with human platelet-rich plasma. J Tissue Eng Regen Med. 2012. [Epub ahead of print][17]Hartmann I, Hollweck T, Haffner S, et al. Umbilical cord tissue-derived mesenchymal stem cells grow best under GMP-compliant culture conditions and maintain their phenotypic and functional properties. J Immunol Methods. 2010;363(1):80-89.[18]Hou ZL, Liu Y, Mao XH, et al. Transplantation of umbilical cord and bone marrow-derived mesenchymal stem cells in a patient with relapsing-remitting multiple sclerosis. Cell Adh Migr. 2013.[19]Li L, Liu S, Xu Y, et al. Human umbilical cord-derived mesenchymal stem cells downregulate inflammatory responses by shifting the treg/th17 profile in experimental colitis. Pharmacology. 2013;92:257-264.[20]Li T, Ma Q, Ning M, et al. Cotransplantation of human umbilical cord-derived mesenchymal stem cells and umbilical cord blood-derived cd34 cells in a rabbit model of myocardial infarction. Mol Cell Biochem. 2013.[21]Meng HB, Gong J, Zhou B, et al. Therapeutic effect of human umbilical cord-derived mesenchymal stem cells in rat severe acute pancreatitis. Int J Clin Exp Pathol. 2013;6: 2703-2712.[22]Wang H, Qiu X, Ni P, et al. Immunological characteristics of human umbilical cord mesenchymal stem cells and the therapeutic effects of their transplantion on hyperglycemia in diabetic rats. Int J Mol Med. 2014;33:263-270.[23]Zhang X, Zhang Q, Li W, et al. Therapeutic effect of human umbilical cord mesenchymal stem cells on neonatal rat hypoxic-ischemic encephalopathy. J Neurosci Res. 2014; 92:35-45.[24]Liu L, Zhao X, Li P, et al. A novel way to isolate MSCs from umbilical cords. Eur J Immunol. 2012;42(8):2190-2193.[25]Nekanti U, Mohanty L, Venugopal P, et al. Optimization and scale-up of Wharton's jelly-derived mesenchymal stem cells for clinical applications. Stem Cell Res. 2010;5(3):244-254.[26]Schallmoser K, Rohde E, Reinisch A, et al. Rapid large-scale expansion of functional mesenchymal stem cells from unmanipulated bone marrow without animal serum. Tissue Eng Part C Methods. 2008;14(3):185-196.[27]Doucet C, Ernou I, Zhang Y, et al. Platelet lysates promote mesenchymal stem cell expansion: a safety substitute for animal serum in cell-based therapy applications. J Cell Physiol. 2005;205(2):228-236.[28]Weibrich G, Kleis WK, Hafner G, et al. Growth factor levels in platelet-rich plasma and correlations with donor age, sex, and platelet count. J Craniomaxillofac Surg. 2002;30(2):97-102.[29]Eppley BL, Woodell JE, Higgins J. Platelet quantification and growth factor analysis from platelet-rich plasma: implications for wound healing. Plast Reconstr Surg. 2004;114(6): 1502-1508.[30]Ng F, Boucher S, Koh S, et al. PDGF, TGF-beta, and FGF signaling is important for differentiation and growth of mesenchymal stem cells (MSCs): transcriptional profiling can identify markers and signaling pathways important in differentiation of MSCs into adipogenic, chondrogenic, and osteogenic lineages. Blood. 2008;112(2):295-307.[31]Chieregato K, Castegnaro S, Madeo D, et al. Epidermal growth factor, basic fibroblast growth factor and platelet-derived growth factor-bb can substitute for fetal bovine serum and compete with human platelet-rich plasma in the ex vivo expansion of mesenchymal stromal cells derived from adipose tissue. Cytotherapy. 2011;13(8): 933-943.[32]van Geemen D, Riem Vis PW, Soekhradj-Soechit S, et al. Decreased mechanical properties of heart valve tissue constructs cultured in platelet lysate as compared to fetal bovine serum. Tissue Eng Part C Methods. 2011;17(5): 607-617.[33]Rauch C, Feifel E, Amann EM, et al. Alternatives to the use of fetal bovine serum: human platelet lysates as a serum substitute in cell culture media. ALTEX. 2011;28(4):305-316.[34]Naaijkens BA, Niessen HW, Prins HJ, et al. Human platelet lysate as a fetal bovine serum substitute improves human adipose-derived stromal cell culture for future cardiac repair applications. Cell Tissue Res. 2012;348(1):119-130.[35]Xia W, Li H, Wang Z, et al. Human platelet lysate supports ex vivo expansion and enhances osteogenic differentiation of human bone marrow-derived mesenchymal stem cells. Cell Biol Int. 2011;35(6):639-643.[36]Gottipamula S, Sharma A, Krishnamurthy S, et al. Human platelet lysate is an alternative to fetal bovine serum for large-scale expansion of bone marrow-derived mesenchymal stromal cells. Biotechnol Lett. 2012;34:1367-1374.[37]Xia W, Li H, Wang Z, et al. Human platelet lysate supports ex vivo expansion and enhances osteogenic differentiation of human bone marrow-derived mesenchymal stem cells. Cell Biol Int. 2011;35:639-643.[38]Mimura S, Kimura N, Hirata M, et al. Growth factor-defined culture medium for human mesenchymal stem cells. Int J Dev Biol. 2011;55:181-187.[39]Maruyama T, Mirando AJ, Deng CX, et al. The balance of wnt and fgf signaling influences mesenchymal stem cell fate during skeletal development. Sci Signal. 2010;3:40.[40]吕江涛,田少奇,孙康,等. 血小板裂解液对人脐带间充质干细胞体外成骨分化的影响[J]. 中国组织工程研究,2012,16(41): 7637-7641.[41]冯学涛.血小板裂解液对人脐带间充质干细胞体外成软骨分化的影响[D].青岛大学,2012. [42]冯学涛,田少奇,孙康,等.血小板裂解液对人脐带间充质干细胞体外成软骨分化的影响[J].中国修复重建外科杂志,2011,25(10): 1250-1255.[43]孙伟雪,孙康,田少奇,等.血小板裂解液对脐带间充质干细胞增殖与分化的影响[J].中国组织工程研究与临床康复,2011, 15(27): 5100-5103.[44]陈博. 血小板裂解液对牙源性间充质干细胞的生物学影响[D]. 第四军医大学,2012.[45]宋会平,王志强,赵亚平,等.血小板裂解液对大鼠骨髓间充质干细胞生长的影响[J].中国组织工程研究与临床康复,2008, 12(21): 4031-4034.[46]陈璐.血小板血浆与血小板裂解液对骨髓基质干细胞增殖影响的比较[D].河北联合大学,2011.[47]董晶,聂李平,周宇,等.血小板裂解液快速扩增人羊膜来源间充质干细胞[J].微循环学杂志,2012,22(4):1-16.[48]许茹,夏文杰,戎霞,等.人血小板裂解液替代胎牛血清促进骨髓间质干细胞的增殖[J].南方医科大学学报,2011,31(8):1396-1400.[49]王贞,夏文杰,叶欣,等.人血小板裂解液对骨髓间质干细胞成骨成脂分化的影响[J].中国输血杂志,2011,24(10):853-857. |
| [1] | Lin Qingfan, Xie Yixin, Chen Wanqing, Ye Zhenzhong, Chen Youfang. Human placenta-derived mesenchymal stem cell conditioned medium can upregulate BeWo cell viability and zonula occludens expression under hypoxia [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(在线): 4970-4975. |
| [2] | Pu Rui, Chen Ziyang, Yuan Lingyan. Characteristics and effects of exosomes from different cell sources in cardioprotection [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(在线): 1-. |
| [3] | Zhang Xiumei, Zhai Yunkai, Zhao Jie, Zhao Meng. Research hotspots of organoid models in recent 10 years: a search in domestic and foreign databases [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(8): 1249-1255. |
| [4] | Hou Jingying, Yu Menglei, Guo Tianzhu, Long Huibao, Wu Hao. Hypoxia preconditioning promotes bone marrow mesenchymal stem cells survival and vascularization through the activation of HIF-1α/MALAT1/VEGFA pathway [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 985-990. |
| [5] | Shi Yangyang, Qin Yingfei, Wu Fuling, He Xiao, Zhang Xuejing. Pretreatment of placental mesenchymal stem cells to prevent bronchiolitis in mice [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 991-995. |
| [6] | Liang Xueqi, Guo Lijiao, Chen Hejie, Wu Jie, Sun Yaqi, Xing Zhikun, Zou Hailiang, Chen Xueling, Wu Xiangwei. Alveolar echinococcosis protoscolices inhibits the differentiation of bone marrow mesenchymal stem cells into fibroblasts [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 996-1001. |
| [7] | Fan Quanbao, Luo Huina, Wang Bingyun, Chen Shengfeng, Cui Lianxu, Jiang Wenkang, Zhao Mingming, Wang Jingjing, Luo Dongzhang, Chen Zhisheng, Bai Yinshan, Liu Canying, Zhang Hui. Biological characteristics of canine adipose-derived mesenchymal stem cells cultured in hypoxia [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1002-1007. |
| [8] | Geng Yao, Yin Zhiliang, Li Xingping, Xiao Dongqin, Hou Weiguang. Role of hsa-miRNA-223-3p in regulating osteogenic differentiation of human bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1008-1013. |
| [9] | Lun Zhigang, Jin Jing, Wang Tianyan, Li Aimin. Effect of peroxiredoxin 6 on proliferation and differentiation of bone marrow mesenchymal stem cells into neural lineage in vitro [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1014-1018. |
| [10] | Zhu Xuefen, Huang Cheng, Ding Jian, Dai Yongping, Liu Yuanbing, Le Lixiang, Wang Liangliang, Yang Jiandong. Mechanism of bone marrow mesenchymal stem cells differentiation into functional neurons induced by glial cell line derived neurotrophic factor [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1019-1025. |
| [11] | Duan Liyun, Cao Xiaocang. Human placenta mesenchymal stem cells-derived extracellular vesicles regulate collagen deposition in intestinal mucosa of mice with colitis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1026-1031. |
| [12] | Pei Lili, Sun Guicai, Wang Di. Salvianolic acid B inhibits oxidative damage of bone marrow mesenchymal stem cells and promotes differentiation into cardiomyocytes [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1032-1036. |
| [13] | Guan Qian, Luan Zuo, Ye Dou, Yang Yinxiang, Wang Zhaoyan, Wang Qian, Yao Ruiqin. Morphological changes in human oligodendrocyte progenitor cells during passage [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1045-1049. |
| [14] | Wang Zhengdong, Huang Na, Chen Jingxian, Zheng Zuobing, Hu Xinyu, Li Mei, Su Xiao, Su Xuesen, Yan Nan. Inhibitory effects of sodium butyrate on microglial activation and expression of inflammatory factors induced by fluorosis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1075-1080. |
| [15] | Wang Xianyao, Guan Yalin, Liu Zhongshan. Strategies for improving the therapeutic efficacy of mesenchymal stem cells in the treatment of nonhealing wounds [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1081-1087. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||