Chinese Journal of Tissue Engineering Research ›› 2014, Vol. 18 ›› Issue (10): 1514-1520.doi: 10.3969/j.issn.2095-4344.2014.10.007
Previous Articles Next Articles
Histone deacetylases mRNA profile in mesenchymal stem cells derived from ovariectomized mice
Liu Da-yong1, Tai Yong 1, 2, Wang Mei-rui 1, 2, Cui Ting1, Liu Ping1, Zhao Meng-ming1, Jia Zhi1
- 1 Department of Endodontics, Tianjin Medical University School of Stomatology, Tianjin 300070, China; 2 Department of Stomatology, the Second Hospital of Tianjin Medical University, Tianjin 300211, China
-
Online:2014-03-05Published:2014-03-05 -
Contact:Jia Zhi, Associate professor, Department of Endodontics, Tianjin Medical University School of Stomatology, Tianjin 300070, China -
About author:Liu Da-yong, M.D., Attending physician, Department of Endodontics, Tianjin Medical University School of Stomatology, Tianjin 300070, China -
Supported by:the National Natural Science Foundation of China, No. 81371109
CLC Number:
Cite this article
Liu Da-yong, Tai Yong, Wang Mei-rui, Cui Ting, Liu Ping, Zhao Meng-ming, Jia Zhi. Histone deacetylases mRNA profile in mesenchymal stem cells derived from ovariectomized mice[J]. Chinese Journal of Tissue Engineering Research, 2014, 18(10): 1514-1520.
share this article
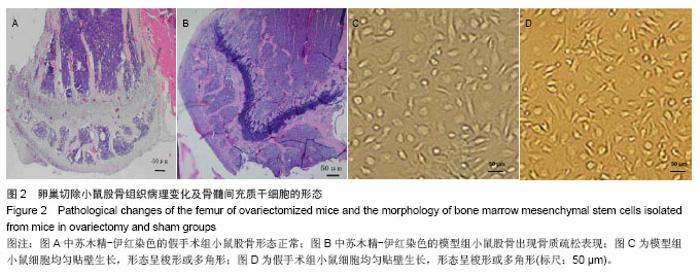
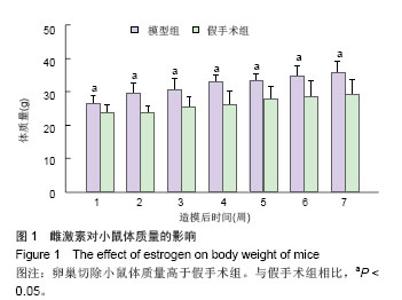
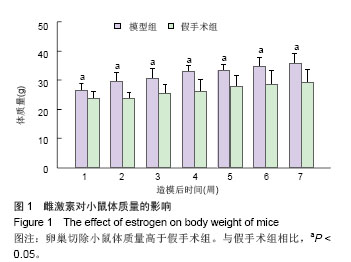
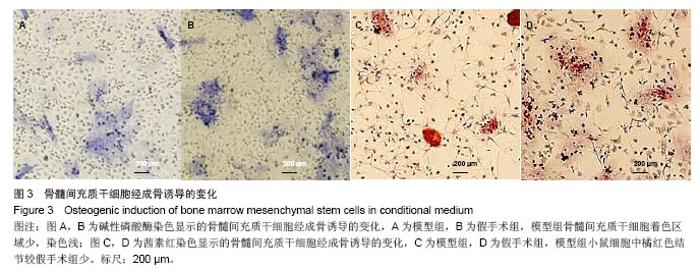
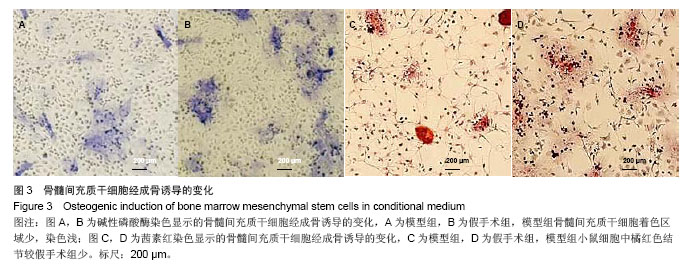
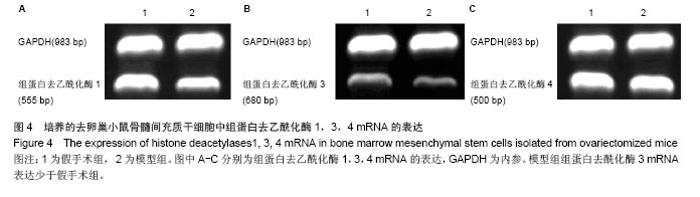
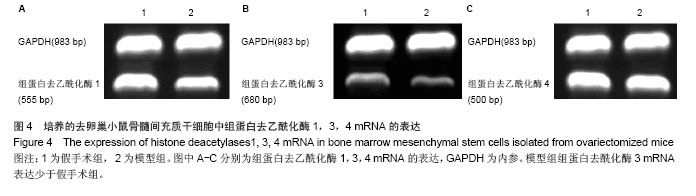
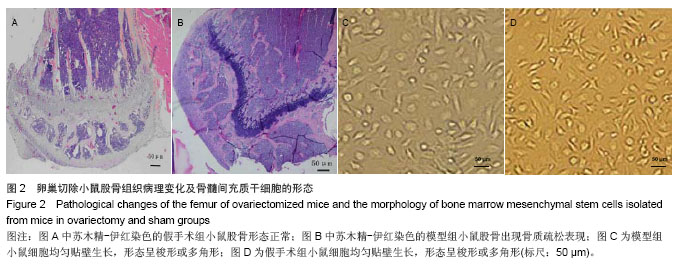
2.3 卵巢切除小鼠股骨组织病理变化 光镜下观察可见,卵巢切除小鼠股骨出现骨吸收,骨皮质变薄,骨髓腔扩大,骨小梁稀疏或断裂,骨小梁宽度变窄,骨小梁间距变宽,骨小梁占视野面积降低;假手术组股骨干骺端小梁丰富,骨皮质致密,骨小梁排列以纵向为主,骨小梁间距较小(图2A,B)。 2.4 卵巢切除小鼠股骨骨髓间充质干细胞的形态 倒置显微镜下,骨髓间充质干细胞培养传代后细胞呈均匀分布贴壁生长,形态为长梭形(图2C,D)。 卵巢切除小鼠和假手术小鼠骨髓间充质干细胞经成骨诱导7 d后,行碱性磷酸酶染色与假手术组相比,模型组小鼠细胞蓝色着色区域少,染色浅(图3A,B)。 卵巢切除小鼠和假手术小鼠骨髓间充质干细胞经成骨诱导14 d后,行茜素红染色,模型组小鼠细胞中橘红色结节较假手术组少(图3C,D)。"
| [1]Deal C. Potential new drug targets for osteoporosis. Nat Clin Pract Rheumatol. 2009;5(1):20-27. [2]Harada S, Rodan GA. Control of osteoblast function and regulation of bone mass. Nature. 2003;423(6937):349-355.[3]Gao Y, Yang G, Weng T, et al. Disruption of Smad4 in odontoblasts causes multiple keratocystic odontogenic tumors and tooth malformation in mice. Mol Cell Biol. 2009; 29(21):5941-5951. [4]Ho JH, Ma WH, Su Y, et al. Thymosin beta-4 directs cell fate determination of human mesenchymal stem cells through biophysical effects. J Orthop Res. 2010;28(1):131-138.[5]Leskelä HV, Olkku A, Lehtonen S, et al. Estrogen receptor alpha genotype confers interindividual variability of response to estrogen and testosterone in mesenchymal-stem -cell-derived osteoblasts. Bone. 2006;39(5):1026-1034. [6]Liu Y, Wang L, Kikuiri T, et al. Mesenchymal stem cell-based tissue regeneration is governed by recipient T lymphocytes via IFN-γ and TNF-α. Nat Med. 2011;17(12):1594-1601.[7]Scheller EL, Song J, Dishowitz MI, et al. Leptin functions peripherally to regulate differentiation of mesenchymal progenitor cells. Stem Cells. 2010;28(6):1071-1080. [8]Zhang X, Yang M, Lin L, et al. Runx2 overexpression enhances osteoblastic differentiation and mineralization in adipose--derived stem cells in vitro and in vivo. Calcif Tissue Int. 2006;79(3):169-178. [9]Wolffe AP, Matzke MA. Epigenetics: regulation through repression. Science. 1999;286(5439):481-486.[10]Calarco JP, Borges F, Donoghue MT, et al. Reprogramming of DNA methylation in pollen guides epigenetic inheritance via small RNA. Cell. 2012;151(1):194-205. [11]Du J, Zhong X, Bernatavichute YV, et al. Dual binding of chromomethylase domains to H3K9me2-containing nucleosomes directs DNA methylation in plants. Cell. 2012; 151(1):167-180. [12]Johnson LM, Du J, Hale CJ, et al. SRA- and SET-domain-containing proteins link RNA polymerase V occupancy to DNA methylation. Nature. 2014. in press.[13]Xie W, Barr CL, Kim A, et al. Base-resolution analyses of sequence and parent-of-origin dependent DNA methylation in the mouse genome. Cell. 2012;148(4):816-831. [14]Black JC, Manning AL, Van Rechem C, et al. KDM4A lysine demethylase induces site-specific copy gain and rereplication of regions amplified in tumors. Cell. 2013;154(3):541-555. [15]Liu W, Ma Q, Wong K, et al. Brd4 and JMJD6-associated anti-pause enhancers in regulation of transcriptional pause release. Cell. 2013;155(7):1581-1595. [16]Lyons DB, Allen WE, Goh T, et al. An epigenetic trap stabilizes singular olfactory receptor expression. Cell. 2013; 154(2):325-336. [17]Rickels R, Shilatifard A. A histone modifier's ill-gotten copy gains. Cell. 2013;154(3):477-479. [18]Zhao W, Li Q, Ayers S, et al. Jmjd3 inhibits reprogramming by upregulating expression of INK4a/Arf and targeting PHF20 for ubiquitination. Cell. 2013;152(5):1037-1050. [19]Hakim O, Misteli T. SnapShot: Chromosome confirmation capture. Cell. 2012;148(5):1068.e1-2. [20]Zhong FL, Batista LF, Freund A, et al. TPP1 OB-fold domain controls telomere maintenance by recruiting telomerase to chromosome ends. Cell. 2012;150(3):481-494. [21]Chou J, Lin JH, Brenot A, et al. GATA3 suppresses metastasis and modulates the tumour microenvironment by regulating microRNA-29b expression. Nat Cell Biol. 2013; 15(2):201-213. [22]Pencheva N, Tavazoie SF. Control of metastatic progression by microRNA regulatory networks. Nat Cell Biol. 2013;15(6): 546-554. [23]Wang D, Zhang Z, O'Loughlin E, et al. MicroRNA-205 controls neonatal expansion of skin stem cells by modulating the PI(3)K pathway. Nat Cell Biol. 2013;15(10):1153-1163. [24]Minoda A, Saitoh S, Takahashi K, et al. BAF53/Arp4 homolog Alp5 in fission yeast is required for histone H4 acetylation, kinetochore-spindle attachment, and gene silencing at centromere. Mol Biol Cell. 2005;16(1):316-327. [25]Vega RB, Matsuda K, Oh J, et al. Histone deacetylase 4 controls chondrocyte hypertrophy during skeletogenesis. Cell. 2004;119(4):555-566.[26]Schroeder TM, Kahler RA, Li X, et al. Histone deacetylase 3 interacts with runx2 to repress the osteocalcin promoter and regulate osteoblast differentiation. J Biol Chem. 2004;279(40): 41998-42007.[27]Westendorf JJ. Histone deacetylases in control of skeletogenesis. J Cell Biochem. 2007;102(2):332-340.[28]Westendorf JJ, Zaidi SK, Cascino JE, et al. Runx2 (Cbfa1, AML-3) interacts with histone deacetylase 6 and represses the p21(CIP1/WAF1) promoter. Mol Cell Biol. 2002;22(22): 7982-7992.[29]Kang JS, Alliston T, Delston R, et al. Repression of Runx2 function by TGF-beta through recruitment of class II histone deacetylases by Smad3. EMBO J. 2005;24(14):2543-2555.[30]Westendorf JJ. Transcriptional co-repressors of Runx2. J Cell Biochem. 2006;98(1):54-64.[31]Lee HW, Suh JH, Kim AY, et al. Histone deacetylase 1-mediated histone modification regulates osteoblast differentiation. Mol Endocrinol. 2006;20(10):2432-2443. [32]Thompson DD, Simmons HA, Pirie CM, et al. FDA Guidelines and animal models for osteoporosis. Bone. 1995;17(4 Suppl): 125S-133S.[33]Kim JL, Kim YH, Kang MK, et al. Antiosteoclastic activity of milk thistle extract after ovariectomy to suppress estrogen deficiency-induced osteoporosis. Biomed Res Int. 2013; 2013: 919374. [34]Ma B, Li X, Zhang Q, et al. Metabonomic profiling in studying anti-osteoporosis effects of strontium fructose 1,6-diphosphate on estrogen deficiency-induced osteoporosis in rats by GC/TOF-MS. Eur J Pharmacol. 2013;718(1-3): 524-532. [35]Miyauchi Y, Sato Y, Kobayashi T, et al. HIF1α is required for osteoclast activation by estrogen deficiency in postmenopausal osteoporosis. Proc Natl Acad Sci U S A. 2013;110(41):16568-16573. [36]Rossini M, Lello S, Sblendorio I, et al. Profile of bazedoxifene/conjugated estrogens for the treatment of estrogen deficiency symptoms and osteoporosis in women at risk of fracture. Drug Des Devel Ther. 2013;7:601-610. [37]Yang N, Wang G, Hu C, et al. Tumor necrosis factor α suppresses the mesenchymal stem cell osteogenesis promoter miR-21 in estrogen deficiency-induced osteoporosis. J Bone Miner Res. 2013;28(3):559-573. [38]Almeida M, Iyer S, Martin-Millan M, et al. Estrogen receptor-α signaling in osteoblast progenitors stimulates cortical bone accrual. J Clin Invest. 2013;123(1):394-404. [39]Galea GL, Meakin LB, Sugiyama T, et al. Estrogen receptor α mediates proliferation of osteoblastic cells stimulated by estrogen and mechanical strain, but their acute down-regulation of the Wnt antagonist Sost is mediated by estrogen receptor β. J Biol Chem. 2013;288(13):9035-9048.[40]Sapir-Koren R, Livshits G. Is interaction between age-dependent decline in mechanical stimulation and osteocyte-estrogen receptor levels the culprit for postmenopausal-impaired bone formation? Osteoporos Int. 2013;24(6):1771-1789. [41]Song L, Zhao J, Zhang X, et al. Icariin induces osteoblast proliferation, differentiation and mineralization through estrogen receptor-mediated ERK and JNK signal activation. Eur J Pharmacol. 2013;714(1-3):15-22. [42]Zirngibl RA, Chan JS, Aubin JE. Divergent regulation of the Osteopontin promoter by the estrogen receptor-related receptors is isoform- and cell context dependent. J Cell Biochem. 2013;114(10):2356-2362. [43]Binder NB, Niederreiter B, Hoffmann O, et al. Estrogen-dependent and C-C chemokine receptor-2- dependent pathways determine osteoclast behavior in osteoporosis. Nat Med. 2009;15(4):417-424. [44]Bonnelye E, Saltel F, Chabadel A, et al. Involvement of the orphan nuclear estrogen receptor-related receptor α in osteoclast adhesion and transmigration. J Mol Endocrinol. 2010;45(6):365-377. [45]Crusodé de Souza M, Sasso-Cerri E, Cerri PS. Immunohistochemical detection of estrogen receptor beta in alveolar bone cells of estradiol-treated female rats: possible direct action of estrogen on osteoclast life span. J Anat. 2009;215(6):673-681. [46]Pederson L, Kremer M, Foged NT, et al. Evidence of a correlation of estrogen receptor level and avian osteoclast estrogen responsiveness. J Bone Miner Res. 1997;12(5): 742-752.[47]Taranta A, Brama M, Teti A, et al. The selective estrogen receptor modulator raloxifene regulates osteoclast and osteoblast activity in vitro. Bone. 2002;30(2):368-376.[48]Almeida M, Iyer S, Martin-Millan M, et al. Estrogen receptor-α signaling in osteoblast progenitors stimulates cortical bone accrual. J Clin Invest. 2013;123(1):394-404. [49]Bonnelye E, Aubin JE. An energetic orphan in an endocrine tissue: a revised perspective of the function of estrogen receptor-related receptor alpha in bone and cartilage. J Bone Miner Res. 2013;28(2):225-233. [50]Ming LG, Chen KM, Xian CJ. Functions and action mechanisms of flavonoids genistein and icariin in regulating bone remodeling. J Cell Physiol. 2013;228(3):513-521. [51]Chen JD, Evans RM. A transcriptional co-repressor that interacts with nuclear hormone receptors. Nature. 1995;377 (6548):454-457.[52]Sevetson B, Taylor S, Pan Y. Cbfa1/RUNX2 directs specific expression of the sclerosteosis gene (SOST). J Biol Chem. 2004;279(14):13849-13858.[53]Lamour V, Detry C, Sanchez C, et al. Runx2- and histone deacetylase 3-mediated repression is relieved in differentiating human osteoblast cells to allow high bone sialoprotein expression. J Biol Chem. 2007;282(50):36240- 36249. [54]Nam HK, Liu J, Li Y, et al. Ectonucleotide pyrophosphatase/ phosphodiesterase-1 (ENPP1) protein regulates osteoblast differentiation. J Biol Chem. 2011;286(45):39059-39071.[55]Marulanda J, Gao C, Roman H, et al. Prevention of arterial calcification corrects the low bone mass phenotype in MGP-deficient mice. Bone. 2013;57(2):499-508. [56]Lie DC, Colamarino SA, Song HJ, et al. Wnt signalling regulates adult hippocampal neurogenesis. Nature. 2005; 437(7063):1370-1375.[57]Anastas JN, Moon RT. WNT signalling pathways as therapeutic targets in cancer. Nat Rev Cancer. 2013;13(1): 11-26. [58]Baron R, Kneissel M. WNT signaling in bone homeostasis and disease: from human mutations to treatments. Nat Med. 2013;19(2):179-192. [59]Bennett CN, Longo KA, Wright WS, et al. Regulation of osteoblastogenesis and bone mass by Wnt10b. Proc Natl Acad Sci U S A. 2005;102(9):3324-3329.[60]Wahli W, Michalik L. PPARs at the crossroads of lipid signaling and inflammation. Trends Endocrinol Metab. 2012;23(7):351-363. [61]Maroni P, Brini AT, Arrigoni E, et al. Chemical and genetic blockade of HDACs enhances osteogenic differentiation of human adipose tissue-derived stem cells by oppositely affecting osteogenic and adipogenic transcription factors. Biochem Biophys Res Commun. 2012;428(2):271-277. |
| [1] | Pu Rui, Chen Ziyang, Yuan Lingyan. Characteristics and effects of exosomes from different cell sources in cardioprotection [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(在线): 1-. |
| [2] | Lin Qingfan, Xie Yixin, Chen Wanqing, Ye Zhenzhong, Chen Youfang. Human placenta-derived mesenchymal stem cell conditioned medium can upregulate BeWo cell viability and zonula occludens expression under hypoxia [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(在线): 4970-4975. |
| [3] | Tang Hui, Yao Zhihao, Luo Daowen, Peng Shuanglin, Yang Shuanglin, Wang Lang, Xiao Jingang. High fat and high sugar diet combined with streptozotocin to establish a rat model of type 2 diabetic osteoporosis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(8): 1207-1211. |
| [4] | Li Zhongfeng, Chen Minghai, Fan Yinuo, Wei Qiushi, He Wei, Chen Zhenqiu. Mechanism of Yougui Yin for steroid-induced femoral head necrosis based on network pharmacology [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(8): 1256-1263. |
| [5] | Hou Jingying, Yu Menglei, Guo Tianzhu, Long Huibao, Wu Hao. Hypoxia preconditioning promotes bone marrow mesenchymal stem cells survival and vascularization through the activation of HIF-1α/MALAT1/VEGFA pathway [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 985-990. |
| [6] | Shi Yangyang, Qin Yingfei, Wu Fuling, He Xiao, Zhang Xuejing. Pretreatment of placental mesenchymal stem cells to prevent bronchiolitis in mice [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 991-995. |
| [7] | Liang Xueqi, Guo Lijiao, Chen Hejie, Wu Jie, Sun Yaqi, Xing Zhikun, Zou Hailiang, Chen Xueling, Wu Xiangwei. Alveolar echinococcosis protoscolices inhibits the differentiation of bone marrow mesenchymal stem cells into fibroblasts [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 996-1001. |
| [8] | Fan Quanbao, Luo Huina, Wang Bingyun, Chen Shengfeng, Cui Lianxu, Jiang Wenkang, Zhao Mingming, Wang Jingjing, Luo Dongzhang, Chen Zhisheng, Bai Yinshan, Liu Canying, Zhang Hui. Biological characteristics of canine adipose-derived mesenchymal stem cells cultured in hypoxia [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1002-1007. |
| [9] | Geng Yao, Yin Zhiliang, Li Xingping, Xiao Dongqin, Hou Weiguang. Role of hsa-miRNA-223-3p in regulating osteogenic differentiation of human bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1008-1013. |
| [10] | Lun Zhigang, Jin Jing, Wang Tianyan, Li Aimin. Effect of peroxiredoxin 6 on proliferation and differentiation of bone marrow mesenchymal stem cells into neural lineage in vitro [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1014-1018. |
| [11] | Zhu Xuefen, Huang Cheng, Ding Jian, Dai Yongping, Liu Yuanbing, Le Lixiang, Wang Liangliang, Yang Jiandong. Mechanism of bone marrow mesenchymal stem cells differentiation into functional neurons induced by glial cell line derived neurotrophic factor [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1019-1025. |
| [12] | Duan Liyun, Cao Xiaocang. Human placenta mesenchymal stem cells-derived extracellular vesicles regulate collagen deposition in intestinal mucosa of mice with colitis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1026-1031. |
| [13] | Pei Lili, Sun Guicai, Wang Di. Salvianolic acid B inhibits oxidative damage of bone marrow mesenchymal stem cells and promotes differentiation into cardiomyocytes [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1032-1036. |
| [14] | Li Cai, Zhao Ting, Tan Ge, Zheng Yulin, Zhang Ruonan, Wu Yan, Tang Junming. Platelet-derived growth factor-BB promotes proliferation, differentiation and migration of skeletal muscle myoblast [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1050-1055. |
| [15] | Wang Xianyao, Guan Yalin, Liu Zhongshan. Strategies for improving the therapeutic efficacy of mesenchymal stem cells in the treatment of nonhealing wounds [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1081-1087. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||