Chinese Journal of Tissue Engineering Research ›› 2025, Vol. 29 ›› Issue (22): 4702-4709.doi: 10.12307/2025.438
Previous Articles Next Articles
Key role of calcium ion in sodium alginate based composite hydrogel for breast cancer organoid culture
Lin Zhiguang1, 2, 3, Rao Qi1, 2, 3, Liang Shanshan1, 2, 3, Wang Ruoyu1, 2, Yu Weiting1, 2, 3
- 1Department of Oncology, Affiliated Zhongshan Hospital of Dalian University, Dalian 116001, Liaoning Province, China; 2Zhongshan Clinical College of Dalian University, Dalian 116001, Liaoning Province, China; 3Dalian Key Laboratory of Translational Medicine of Marine Polysaccharide Biomaterials, Dalian 116001, Liaoning Province, China
-
Received:2024-03-01Accepted:2024-05-17Online:2025-08-08Published:2024-12-06 -
Contact:Yu Weiting, PhD, Researcher, Master’s supervisor, Department of Oncology, Affiliated Zhongshan Hospital of Dalian University, Dalian 116001, Liaoning Province, China; Zhongshan Clinical College of Dalian University, Dalian 116001, Liaoning Province, China; Dalian Key Laboratory of Translational Medicine of Marine Polysaccharide Biomaterials, Dalian 116001, Liaoning Province, China Liang Shanshan, PhD, Associate researcher, Master’s supervisor, Department of Oncology, Affiliated Zhongshan Hospital of Dalian University, Dalian 116001, Liaoning Province, China; Zhongshan Clinical College of Dalian University, Dalian 116001, Liaoning Province, China; Dalian Key Laboratory of Translational Medicine of Marine Polysaccharide Biomaterials, Dalian 116001, Liaoning Province, China -
About author:Lin Zhiguang, Master candidate, Department of Oncology, Affiliated Zhongshan Hospital of Dalian University, Dalian 116001, Liaoning Province, China; Zhongshan Clinical College of Dalian University, Dalian 116001, Liaoning Province, China; Dalian Key Laboratory of Translational Medicine of Marine Polysaccharide Biomaterials, Dalian 116001, Liaoning Province, China -
Supported by:National Key Research and Development Plan “Stem Cell and Translational Research” Special Topic, No. 2018YFA0108203 (to YWT); 2021 Dalian City High-level Talent Innovation, Science and Technology Talent Entrepreneurship and Key Field Innovation Team Support Plan Project, No. 2021RD02 (to WRY)
CLC Number:
Cite this article
Lin Zhiguang, Rao Qi, Liang Shanshan, Wang Ruoyu, Yu Weiting. Key role of calcium ion in sodium alginate based composite hydrogel for breast cancer organoid culture[J]. Chinese Journal of Tissue Engineering Research, 2025, 29(22): 4702-4709.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
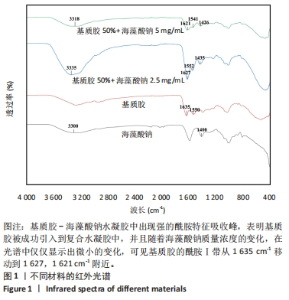
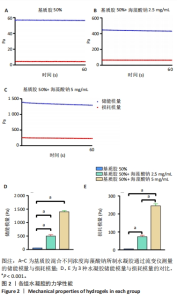
2.1 水凝胶的制备与表征 采用红外光谱模式对干燥的基质胶、海藻酸钠水凝胶及基质胶-海藻酸钠水凝胶表面进行扫描,并以单纯的基质胶和海藻酸钠水凝胶为对照,考察两者之间可能存在的化学作用。 吸收峰在3 300 cm?1和1 400 cm?1附近归因于海藻酸钠上羟基和羧基的特征吸收峰[32]。在基质胶-海藻酸钠水凝胶中可以观察到该特征吸收峰,表明在混合水凝胶表面存在海藻酸钠分子。此外,纯基质胶具有酰胺吸收峰(酰胺Ⅰ在约1 635 cm-1和酰胺Ⅱ在约1 550 cm -1)处,这与a螺旋和?折叠直接相关[33]。从谱图中可见在基质胶-海藻酸钠水凝胶中出现强的酰胺特征吸收峰,这表明基质胶被成功引入到复合水凝胶中,并且随着海藻酸钠质量浓度的变化,在光谱中仅仅显示出微小的变化,可见基质胶的酰胺Ⅰ带从1 635 cm-1移动到1 627,1 621 cm-1附近,见图1。"
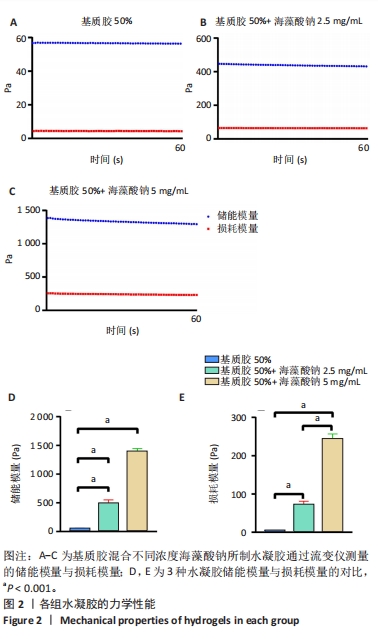
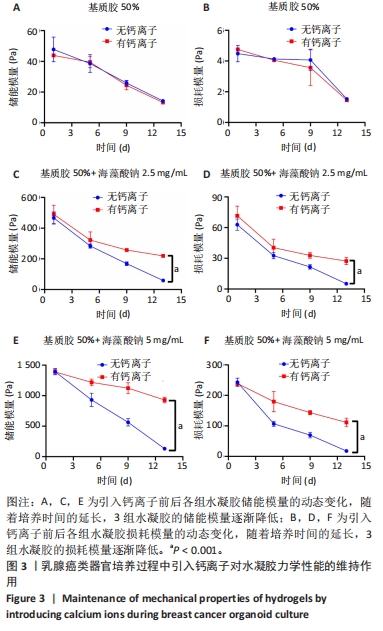
2.2 乳腺癌类器官培养过程中钙离子维持水凝胶力学性能的长期稳定 在乳腺癌类器官培养过程中,3组水凝胶的力学强度都随培养时间的延长而显著下降。乳腺癌类器官培养第13天,在未引入钙离子条件下,低刚度水凝胶的储能模量降低至(14.1±0.9) Pa,损耗模量也显著降低;在引入钙离子条件下,低刚度水凝胶的储能模量、损耗模量与未引入钙离子条件下的储能模量、损耗模量相比未发生明显变化,见图3A,B。乳腺癌类器官培养第13天,在未引入钙离子条件下,中刚度水凝胶的储能模量降低至(64.1±1.0) Pa,损耗模量降低至10 Pa以下;在引入钙离子条件下,中刚度水凝胶的储能模量可维持在(219.4±9.1) Pa,损耗模量可维持在(27.3±3.5) Pa,较未引入钙离子条件下的储能模量、损耗模量均提升近4倍(P < 0.001),见图3C,D。乳腺癌类器官培养第13天,在未引入钙离子条件下,高刚度水凝胶的储能模量降低至于(164.8±1.6) Pa,损耗模量降低至10 Pa以下;在引入钙离子条件下,高刚度水凝胶的储能模量可维持在(957.6±45.0) Pa,较未引入钙离子条件下的储能模量提升近7倍(P < 0.001),损耗模量可维持在(111.2±14.1) Pa,较未引入钙离子条件下的损耗模量提升近6倍(P < 0.001),见图3E,F。"
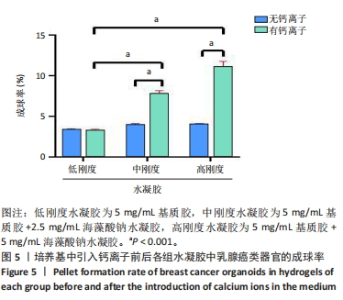
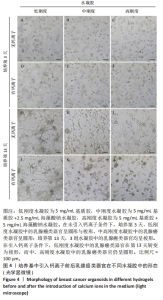
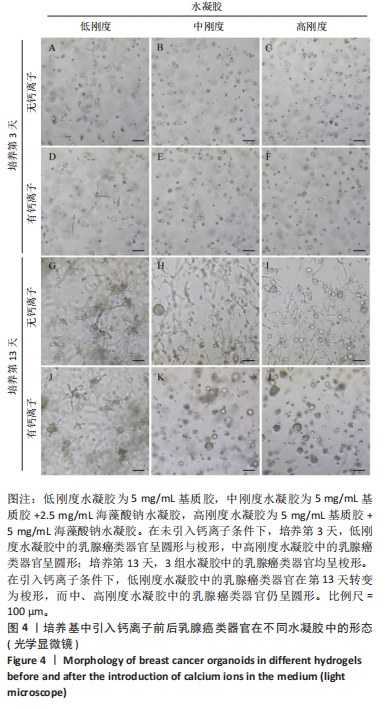
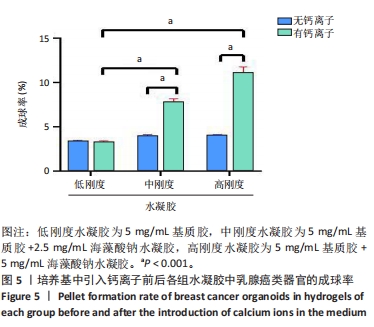
培养第3天,由于低刚度水凝胶的力学强度较低,水凝胶中的乳腺癌类器官显示出圆形与梭形2种形态,见图4A,至第13天乳腺癌类器官均转变为梭形;引入低浓度钙离子后未见明显差异。培养第3天,中、高刚度水凝胶中的乳腺癌类器官均显示为圆形,见图4B,C,但随培养时间延长,中、高刚度水凝胶中的乳腺癌类器官也开始变成梭形,见图4H,I;而引入低浓度钙离子后,两组水凝胶中的乳腺癌类器官呈圆形,直到第13天仍为圆形,见图4K,L。 2.4 钙离子对水凝胶中乳腺癌类器官成球率的影响 培养第13天统计钙离子引入对乳腺癌类器官成球率的影响,结果显示:低刚度水凝胶组引入钙离子前的乳腺癌类器官成球率为(3.34±0.15)%,引入钙离子后为(3.28±0.11)%,引入钙离子前后的乳腺癌类器官成球率比较差异无显著性意义(P > 0.05),初步证明钙离子对细胞的生物学行为没有显著影响。中、高刚度水凝胶组引入钙离子前的乳腺癌类器官成球率分别为(3.96±0.10)%,(4.32±0.08)%,高刚度水凝胶组乳腺癌类器官成球率高于低刚度水凝胶组(P < 0.05);引入钙离子后,中、高刚度水凝胶组乳腺癌类器官成球率分别为(7.81±0.33)%,(11.09±0.71)%,较同组引入钙离子前的乳腺癌类器官成球率分别提升近2,3倍(P < 0.001),也显著高于引入钙离后低刚度水凝胶组乳腺癌类器官成球率(P < 0.001),见图5。"
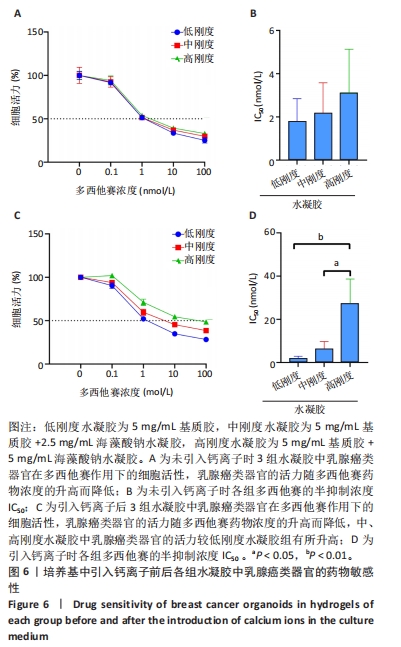
2.5 水凝胶刚度调控乳腺癌类器官的药物敏感性 在未引入钙离子条件下,3组水凝胶中肿瘤类器官的活力随多西他赛药物浓度的升高而降低,见图6A;低、中、高水凝胶组多西他赛的IC50分别为(1.7±0.8),(2.2±1.4),(3.1±2.0) nmol/L,3组间多西他赛的IC50比较差异无显著性意义(P > 0.05),见图6B,表明在该条件下3种水凝胶的力学性能对乳腺癌类器官的化疗敏感性无明显影响。 在引入钙离条件下,3组水凝胶中乳腺癌类器官的活力随多西他赛药物浓度的升高而降低,但是从曲线整体看,中、高刚度水凝胶中乳腺癌类器官的活力升高,对药物的敏感性降低,见图6C;低、中、高水凝胶组多西他赛的IC50分别为(1.7±1.2),(6.2±3.5),(27.3±11.2) nmol/L,高刚度水凝胶组多西他赛的IC50高于低、中刚度水凝胶组(P < 0.01,P < 0.05),见图6D。"
| [1] SIEGEL R, MILLER K, WAGLE N, et al. Cancer statistics, 2023. CA Cancer J Clin. 2023;73(1):17-48. [2] PLODINEC M, LOPARIC M, MONNIER C, et al. The nanomechanical signature of breast cancer. Nat Nanotechnol. 2012;7(11):757-765. [3] PICKUP M, MOUW J, WEAVER V. The extracellular matrix modulates the hallmarks of cancer. EMBO Rep. 2014;15(12):1243-1253. [4] LIU C, LI M, DONG Z, et al. Heterogeneous microenvironmental stiffness regulates pro-metastatic functions of breast cancer cells. Acta Biomater. 2021;131:326-340. [5] YUAN Z, LI Y, ZHANG S, et al. Extracellular matrix remodeling in tumor progression and immune escape: from mechanisms to treatments. Mol Cancer. 2023;22(1):48. [6] SAXENA N, CHAKRABORTY S, DUTTA S, et al. Stiffness-dependent MSC homing and differentiation into CAFs - implications for breast cancer invasion. J Cell Sci. 2024;137(1):jcs261145. [7] CHAUDHURI O, KOSHY S, BRANCO DA CUNHA C, et al. Extracellular matrix stiffness and composition jointly regulate the induction of malignant phenotypes in mammary epithelium. Nat Mater. 2014; 13(10):970-978. [8] LEVENTAL K, YU H, KASS L, et al. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell. 2009;139(5):891-906. [9] MALIK R, LELKES P, CUKIERMAN E. Biomechanical and biochemical remodeling of stromal extracellular matrix in cancer. Trends Biotechnol. 2015;33(4):230-236. [10] HUIJBERS I, IRAVANI M, POPOV S, et al. A role for fibrillar collagen deposition and the collagen internalization receptor endo180 in glioma invasion. PLoS One. 2010;5(3):e9808. [11] BONNANS C, CHOU J, WERB Z. Remodelling the extracellular matrix in development and disease. Nat Rev Mol Cell Biol. 2014;15(12):786-801. [12] COX T, BIRD D, BAKER A, et al. LOX-mediated collagen crosslinking is responsible for fibrosis-enhanced metastasis. Cancer Res. 2013;73(6): 1721-1732. [13] PARK J, KIM J, YANG G, et al. Lysyl oxidase-responsive anchoring nanoparticles for modulation of the tumor immune microenvironment. J Control Release. 2023;360:376-391. [14] NILAND S, RISCANEVO A, EBLE J. Matrix Metalloproteinases Shape the Tumor Microenvironment in Cancer Progression. Int J Mol Sci. 2021;23(1):146. [15] QUAIL D, JOYCE J. Microenvironmental regulation of tumor progression and metastasis. Nat Med. 2013;19(11):1423-1437. [16] LU Y, CHU T, HALL M, et al. Physical confinement induces malignant transformation in mammary epithelial cells. Biomaterials. 2019;217: 119307. [17] YU T, ZHANG G, CHAI X, et al. Recent progress on the effect of extracellular matrix on occurrence and progression of breast cancer. Life Sci. 2023;332:122084. [18] GE H, TIAN M, PEI Q, et al. Extracellular Matrix Stiffness: New Areas Affecting Cell Metabolism. Front Oncol. 2021;11:631991. [19] KIM M, EUN N, AHN S, et al. Elasticity Values as a Predictive Modality for Response to Neoadjuvant Chemotherapy in Breast Cancer. Cancers (Basel). 2024;16(2):377. [20] MATOSSIAN M, CHANG T, WRIGHT M, et al. In-depth characterization of a new patient-derived xenograft model for metaplastic breast carcinoma to identify viable biologic targets and patterns of matrix evolution within rare tumor types. Clin Transl Oncol. 2022;24(1):127-144. [21] JABBARI E, SARVESTANI S, DANESHIAN L, et al. Optimum 3D Matrix Stiffness for Maintenance of Cancer Stem Cells Is Dependent on Tissue Origin of Cancer Cells. PLoS One. 2015;10(7):e0132377. [22] XU H, JIAO D, LIU A, et al. Tumor organoids: applications in cancer modeling and potentials in precision medicine. J Hematol Oncol. 2022; 15(1):58. [23] CHRISNANDY A, BLONDEL D, REZAKHANI S, et al. Synthetic dynamic hydrogels promote degradation-independent in vitro organogenesis. Nat Mater. 2022;21(4):479-487. [24] NATHALIE B, FARZANEH F, LUKE H, et al. GelMA, Click-Chemistry Gelatin and Bioprinted Polyethylene Glycol-Based Hydrogels as 3D Ex Vivo Drug Testing Platforms for Patient-Derived Breast Cancer Organoids. Pharmaceutics. 2023;15:261. [25] MELIS I, BABATUNDE OO, CEMIL CAN E, et al. Bioactive and chemically defined hydrogels with tunable stiffness guide cerebral organoid formation and modulate multi-omics plasticity in cerebral organoids. Acta Biomater. 2023;171:223-238. [26] DONGZHI W, YIBING G, JIACHENG Z, et al. Hyaluronic acid methacrylate/pancreatic extracellular matrix as a potential 3D printing bioink for constructing islet organoids. Acta Biomater. 2022;165:86-101. [27] ZI-YAN X, JIN-JIAN H, YE L, et al. Extracellular matrix bioink boosts stemness and facilitates transplantation of intestinal organoids as a biosafe Matrigel alternative. Bioeng Transl Med. 2023;8:e10327. [28] AN Q, REN J, JIA X, et al. Anisotropic materials based on carbohydrate polymers: A review of fabrication strategies, properties, and applications. Carbohydr Polym. 2024;330:121801. [29] LEE K, MOONEY D. Alginate: properties and biomedical applications. Prog Polym Sci. 2012;37(1):106-126. [30] CASSEL DE CAMPS C, ASLANI S, STYLIANESIS N, et al. Hydrogel Mechanics Influence the Growth and Development of Embedded Brain Organoids. ACS Appl Bio Mater. 2022;5(1):214-224. [31] LI Y, KHUU N, PRINCE E, et al. Matrix Stiffness-Regulated Growth of Breast Tumor Spheroids and Their Response to Chemotherapy. Biomacromolecules. 2021;22(2):419-429. [32] KACZMAREK B, SIONKOWSKA A, STOJKOVSKA J. Characterization of scaffolds based on chitosan and collagen with glycosaminoglycans and sodium alginate addition. Polym Test. 2018;68:229-232. [33] TIAN H, CHEN Y, DING C, et al. Interaction study in homogeneous collagen/chondroitin sulfate blends by two-dimensional infrared spectroscopy. Carbohydr Polym. 2012;89(2):542-550. [34] TZENG YT, HSIAO JH, TSENG LM, et al. Breast cancer organoids derived from patients: A platform for tailored drug screening. Biochem Pharmacol. 2023;217:115803. [35] LIU X, YE Y, ZHU L, et al. Niche stiffness sustains cancer stemness via TAZ and NANOG phase separation. Nat Commun. 2023;14(1):238. [36] YANG X, SARVESTANI S, MOEINZADEH S, et al. Three-dimensional-engineered matrix to study cancer stem cells and tumorsphere formation: effect of matrix modulus. Tissue Eng Part A. 2013;19:669-684. [37] THONGROM B, TANG P, ARORA S, et al. Polyglycerol-Based Hydrogel as Versatile Support Matrix for 3D Multicellular Tumor Spheroid Formation. Gels. 2023;9(12):938. [38] LEE D, CHA C. Cell subtype-dependent formation of breast tumor spheroids and their variable responses to chemotherapeutics within microfluidics-generated 3D microgels with tunable mechanics. Mater Sci Eng C Mater Biol Appl. 2020;112:110932. [39] SO J, OHM J, LIPKOWITZ S, et al. Triple negative breast cancer (TNBC): Non-genetic tumor heterogeneity and immune microenvironment: Emerging treatment options. Pharmacol Ther. 2022;237:108253. [40] ORT C, CHEN Y, GHAGRE A, et al. Bioprintable, Stiffness-Tunable Collagen-Alginate Microgels for Increased Throughput 3D Cell Culture Studies. ACS Biomater Sci Eng. 2021;7(6):2814-2822. [41] JANG M, KOH I, LEE J, et al. Increased extracellular matrix density disrupts E-cadherin/β-catenin complex in gastric cancer cells. Biomater Sci. 2018;6(10):2704-2713. |
| [1] | Zhao Zengbo, Li Chenxi, Dou Chenlei, Ma Na, Zhou Guanjun. Anti-inflammatory and osteogenic effects of chitosan/sodium glycerophosphate/sodium alginate/leonurine hydrogel [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(4): 678-685. |
| [2] | Hu Chen, Jiang Ying, Chen Jia, Qiao Guangwei, Dong Wen, Ma Jian. Preparation and characterization of alendronate/chitosan/polyvinyl alcohol composite hydrogel films [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(22): 4720-4730. |
| [3] | Wu Lijuan, Wang Zhenfei, Tan Xiaohui, Wu Yingcai, Zheng Yanling, Dai Fengxue. Effect of extracellular matrix stiffness on tumor progression and treatment strategies [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(20): 4286-4294. |
| [4] | Chen Mingwei, Yu Wenli, Xia Suhang, Chen Bin, Chen Wenzhong, Li Fengzhen, Zhou Yu, Si Wenteng. rticular cartilage injury repaired with microRNA-140 exosomes/sodium alginate/collagen hydrogel [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(16): 3326-3334. |
| [5] | Wang Renzhi, Chen Yuanfen, Li Jinwei. 3D printed hollow pipe double-crosslinked hydrogel tissue engineering scaffold [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(16): 3432-3439. |
| [6] | Chen Xiaofang, Zheng Guoshuang, Li Maoyuan, Yu Weiting. Preparation and application of injectable sodium alginate hydrogels [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(5): 789-794. |
| [7] | Dai Jing, Liu Shasha, Shen Mingjing. Exosome-loaded injectable hydrogel for repairing bone defects around implants [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(3): 347-354. |
| [8] | Tian Linling, Guo Hairui, Du Xiaoming, Feng Jie, Zhang Xianzhe, Zhang Wenbin, Sun Haoran, Zhang Xiaobin, Wang Jingxia, Hu Yimei, Wang Yi. Advantages and features of nanocomposite hydrogel in treatment of osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(15): 2410-2415. |
| [9] | Su Kunyang, Chen Bineng, Chen Yiliang, Jin Shaofeng. Strontium ranelate-loaded sodium alginate/collagen hydrogel promotes bone defect repair in osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(10): 1568-1574. |
| [10] | Pan Yujun, Shi Changjiang, Cao Sheng, Mu Huaizhao, Zhang Yilong, Wang Kaihua. An injectable dextran/gelatin composite hydrogel loaded with exosomes for repair of rat intervertebral disc degeneration [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(34): 5477-5482. |
| [11] | Yuan Jialu, Liu Mei, Qin Xiaolong, Qiu Yang, Li Jing. Preparation and release property of norfloxacin sustained-release microspheres [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(30): 4790-4795. |
| [12] | Yang Yong, Xu Shunen, Gu Xianyang, Hua Dawei, Teng Jianxiang, Wang Zhen, Ye Chuan. Preparation and property analysis of electrospinning composite sustained-release antibacterial microspheres [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(16): 2534-2541. |
| [13] | Tan Guozhong, Tu Xinran, Guo Liyang, Zhong Jialin, Zhang Yang, Jiang Qianzhou. Biosafety evaluation of three-dimensional printed gelatin/sodium alginate/58S bioactive glass scaffolds for bone defect repair [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 521-527. |
| [14] | Liu Yin, Liu Qin, Chen Jiao, Gu Xianyang, Chen Jiawen, Ma Minxian. Properties of injectable gluconolactone-sodium alginate/beta-tricalcium phosphate/polyethylene glycol composite hydrogel scaffold [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(27): 4308-4313. |
| [15] | Liu Yingsong, Guo Xiaopeng, Wei Mingzhu. Transforming growth factor beta 3 and alginate hydrogel complex on the repair of articular cartilage defects of the knee [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(16): 2504-2509. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||