Chinese Journal of Tissue Engineering Research ›› 2025, Vol. 29 ›› Issue (20): 4286-4294.doi: 10.12307/2025.698
Previous Articles Next Articles
Effect of extracellular matrix stiffness on tumor progression and treatment strategies
Wu Lijuan1, Wang Zhenfei2, Tan Xiaohui1, Wu Yingcai2, Zheng Yanling1, 2, Dai Fengxue1, 2
- 1Inner Mongolia Medical University, Hohhot 010059, Inner Mongolia Autonomous Region, China; 2Laboratory of Molecular Tumor Diagnosis, Inner Mongolia Hospital, Peking University Cancer Hospital/Affiliated Cancer Hospital of Inner Mongolia Medical University, Hohhot 010020, Inner Mongolia Autonomous Region, China
-
Received:2024-07-08Accepted:2024-09-07Online:2025-07-18Published:2024-12-21 -
Contact:Wang Zhenfei, MD, Researcher, Laboratory of Molecular Tumor Diagnosis, Inner Mongolia Hospital, Peking University Cancer Hospital, Hohhot 010020, Inner Mongolia Autonomous Region, China -
About author:Wu Lijuan, Master’s candidate, Inner Mongolia Medical University, Hohhot 010059, Inner Mongolia Autonomous Region, China -
Supported by:the National Natural Science Foundation of China, No. 82074144 (to WZF); Key R&D and Achievement Transformation Project of Inner Mongolia Autonomous Region, No. 2023KJHZ0023 (to WZF); Science and Technology Innovation Team of Inner Mongolia Medical University, No. YKD2023TD010 (to WZF); Science and Technology Innovation Team of Higher Education Institutions in Inner Mongolia Autonomous Region, No. NMGIRT2327 (to WZF); Scientific Research Project of Higher Education Institutions in Inner Mongolia Autonomous Region, No. NJZY22636 (to TXH)
CLC Number:
Cite this article
Wu Lijuan, Wang Zhenfei, Tan Xiaohui, Wu Yingcai, Zheng Yanling, Dai Fengxue. Effect of extracellular matrix stiffness on tumor progression and treatment strategies[J]. Chinese Journal of Tissue Engineering Research, 2025, 29(20): 4286-4294.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
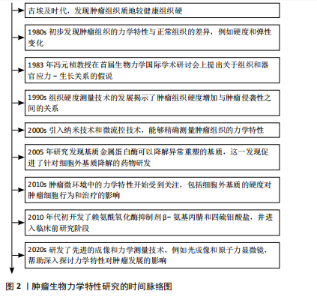
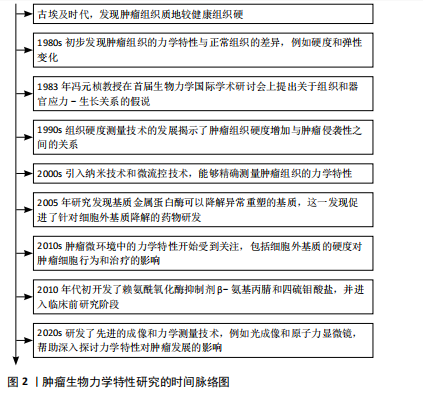
2.1 肿瘤生物力学特性研究的时间历程 自古埃及时期起,人们就注意到肿瘤组织的硬度显著高于周围的健康组织。20世纪80年代的研究初步揭示了肿瘤组织与正常组织在力学特性上的差异。1983年,冯元桢教授在首届生物力学国际学术研讨会上提出了组织和器官应力-生长关系的假说,此后肿瘤组织的力学特性研究逐渐深入。目前,该领域已有一定的研究成果。详见图2。 2.2 肿瘤基质硬度增加的机制 2.2.1 胶原沉积 胶原蛋白是人体最丰富的蛋白质,也是细胞外基质的主要成分,在生理情况下,原胶原蛋白被分泌到细胞外基质后,经蛋白酶的修饰转变为成熟的胶原单体,胶原单体通过聚集形成不溶性胶原纤维,并在赖氨酰氧化酶的作用下,胶原纤维相互交联,形成适宜的网状结构,赋予细胞外基质一定的硬度和韧性[13]。在肿瘤发生期间,胶原基因过度表达,现有的细胞外基质不断降解并被肿瘤特异性细胞外基质所取代,从而具有更高的胶原蛋白含量[14]。例如,Ⅰ型胶原蛋白在乳腺癌、结直肠癌、膀胱癌等肿瘤组织中过度表达[15-17],Ⅵ型、Ⅹ型胶原蛋白分别在肺癌、胃癌中过度表达[18-19]。经研究发现,巨噬细胞分泌的转化生长因子β可以诱导Ⅰ型胶原蛋白和热休克蛋白Hsp47(一种促进胶原折叠和加工的分子伴侣)的过表达,合成大量胶原蛋白[20],衰老的间充"
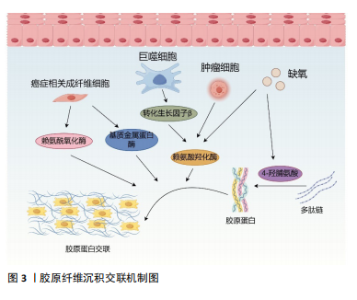
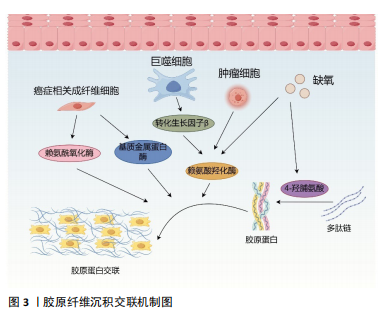
质干细胞和癌细胞也会分泌大量胶原蛋白,增加细胞外基质胶原的沉积,胶原蛋白沉积在基质中,形成致密且形态稳定的纤维结构,导致肿瘤基质硬化[21-22]。目前已经发现了28种具有不同结构、功能和分布的胶原蛋白类型,每种蛋白质都包含由重复序列GXY形成的独特三螺旋结构(其中G是甘氨酸,X通常是脯氨酸或赖氨酸,Y通常是羟脯氨酸),胶原蛋白的形成涉及广泛的翻译后修饰,特别是脯氨酰和赖氨酰残基的羟基化。在癌症进展过程中,瘤内缺氧会诱导脯氨酰羟化酶的表达,催化脯氨酸残基形成4-羟脯氨酸,而4-羟脯氨酸残基有助于胶原蛋白多肽链折叠成紧密的三螺旋分子,此结构对蛋白水解酶具有很强的抵抗力,从而使胶原纤维稳定的沉积于基质中,增加基质的硬度,促进肿瘤进展[23-24]。此外,胶原蛋白的降解对于结缔组织的正常周转和病理性破坏也至关重要。基质金属蛋白酶是细胞外基质中的一种蛋白水解酶,具有降解细胞外基质几乎所有成分的能力, 正常情况下基质金属蛋白酶与基质金属蛋白酶组织抑制剂之间的动态平衡可以维持健康组织的生物学活性,参与细胞修复和组织重塑[25]。但在癌症进展过程中,转化生长因子β抑制了基质金属蛋白酶的生成并诱导基质金属蛋白酶组织抑制剂的表达,减少了基质蛋白的降解[7,26];活化的成纤维细胞也通过下调基质金属蛋白酶9的表达以及上调基质金属蛋白酶组织抑制剂1的表达分泌产生更多的基质蛋白,重新调节基质蛋白的含量与分布,导致基质重塑[27]。而逐渐硬化的基质又会影响细胞的收缩力,增加基质中胶原纤维的收缩性和排列,相对于无序基质,胶原基质的有序排列可以显著提高整个基质的强度和硬度[7,11]。 2.2.2 胶原交联 成熟后的胶原蛋白通过分子间相互交联,形成三维网络结构,从而增强基质的硬度和稳定性。研究表明,赖氨酰氧化酶的过度表达可以增加乳腺肿瘤的基质硬度,并促进癌细胞的迁移[28],同时它也是胃癌预后不良的独立因素,可以增加胶原蛋白和弹性蛋白的交联,触发多种信号通路,重塑胃癌的基质微环境,促进癌症进展[29]。赖氨酰氧化酶是一种铜依赖性单胺氧化酶,包括赖氨酰氧化酶、赖氨酰氧化酶样蛋白1、赖氨酰氧化酶样蛋白2、赖氨酰氧化酶样蛋白3和赖氨酰氧化酶样蛋白4共5个成员,赖氨酰氧化酶和赖氨酰氧化酶样蛋白1涉及蛋白水解切割过程,而赖氨酰氧化酶样蛋白2-4参与细胞外基质中蛋白质间的相互作用[30],它们催化胶原蛋白和弹性蛋白的赖氨酸和羟赖氨酸的ε-氨基集团氧化脱氨,形成高活性醛,这些高活性醛与其他氧化基团或未发生改变的赖氨酸残基形成各种交联,使基质蛋白间相互缠绕,调节细胞外基质的拉伸强度和弹性特征[31]。赖氨酸羟化酶2作为一种胶原修饰酶,是目前唯一确认可以改变胶原纤维交联模式的赖氨酸羟化酶,肿瘤组织的乏氧环境和转化生长因子β都能够刺激赖氨酸羟化酶2的表达,它能够催化胶原端肽区的赖氨酸残基羟基化,使胶原纤维分泌出细胞后,形成结构稳定的赖氨酸吡啶交联胶原纤维,这种交联形态不易被基质酶降解,进一步增加细胞外基质硬度。且随着赖氨酸羟化酶2的表达升高,胶原交联的模式发生改变,胶原纤维由包绕肿瘤细胞的卷曲状态变为线性状态,肿瘤细胞可以沿着这种线性的胶原纤维快速转移,加速了恶性肿瘤的进展[32],见图3。"
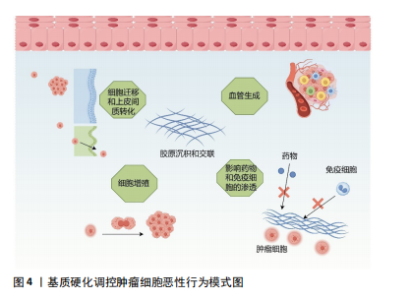
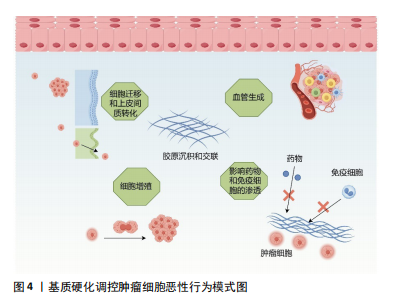
2.2.3 癌症相关成纤维细胞及其异质性 成纤维细胞是一种纺锤形细胞,分泌胶原蛋白,合成结缔组织的细胞外基质,在肿瘤组织中,活化的成纤维细胞被称为癌症相关成纤维细胞,是肿瘤进展过程中的关键物质,可以产生多种蛋白成分,如Ⅰ、Ⅲ、Ⅳ、Ⅴ型胶原蛋白、层粘连蛋白和纤连蛋白等。在肿瘤进展过程中,癌症相关成纤维细胞过度表达胶原蛋白,导致胶原蛋白的沉积及病理性交联,相比于静止的成纤维细胞,癌症相关成纤维细胞的代谢更加活跃,产生的纤维基质更加坚韧[7,33],同时癌症相关成纤维细胞还可以通过表达赖氨酰氧化酶重新修饰肿瘤微环境,增加基质硬度。反过来病理性僵硬的细胞外基质使周围组织施加的固体应力增加,毛细血管压力增大,导致局部组织缺血缺氧,进一步激活癌症相关成纤维细胞[34-35]。僵硬的基质可以维持癌症相关成纤维细胞的功能,并诱导间充质干细胞分化为癌症相关成纤维细胞[33],肿瘤细胞分泌的细胞外囊泡也可以促进成纤维细胞分化为癌症相关成纤维细胞[7],而癌症相关成纤维细胞需要转录因子YAP的激活来促进基质硬化,基质硬化又进一步增强了YAP活化[36]。由此可见,基质硬化和癌症相关成纤维细胞之间可能存在多条正反馈回路,使肿瘤组织的硬度逐渐增加。 然而,癌症相关成纤维细胞对肿瘤的影响很复杂,并不只是简单地促进或抑制,不同的癌症相关成纤维细胞亚群具有不同的来源、标记物、功能甚至机制,研究认为,α-平滑肌肌动蛋白是癌症相关成纤维细胞活化的标志物,但并非所有的癌症相关成纤维细胞都表达经典标志物α-平滑肌肌动蛋白[37]。例如,胰腺导管腺癌中3种不同的癌症相关成纤维细胞亚型,即表达MHC Ⅱ类家族基因的抗原呈递癌症相关成纤维细胞;具有高水平α-平滑肌肌动蛋白表达的转化生长因子β 依赖性癌症相关成纤维细胞,以及具有低水平α-平滑肌肌动蛋白和高水平白细胞介素6表达的炎性癌症相关成纤维细胞[38]。转化生长因子β 依赖性癌症相关成纤维细胞可以对细胞外基质和其他细胞施加收缩力,利用这种收缩力使基质中的胶原蛋白和纤连蛋白有序排列,重塑细胞外基质的结构,并增加基质硬度。白细胞介素1可以通过诱导炎症相关因子的表达和下游JAK/STAT激活以产生炎性癌症相关成纤维细胞,而转化生长因子β可以通过下调白细胞介素1受体1的表达来拮抗这一过程,增加转化生长因子β 依赖性癌症相关成纤维细胞的分化,促进胰腺导管腺癌的恶性进展[39]。除此以外,LI等[40]在胃癌组织中发现了4个主要的成纤维细胞亚群:肌成纤维细胞、周细胞、细胞外基质癌症相关成纤维细胞和炎性癌症相关成纤维细胞,其中肌成纤维细胞、周细胞和炎性癌症相关成纤维细胞分别调节内皮细胞分化、肿瘤血管生成以及免疫炎症反应,而细胞外基质癌症相关成纤维细胞高表达与细胞外基质重塑相关的基因,包括编码胶原蛋白和胶原代谢酶的基因,增加基质硬度,同时也高表达基质金属蛋白酶14、POSTN等与肿瘤侵袭相关的基因,是促进细胞外基质硬化和肿瘤转移的重要组成部分。 近些年尽管学者们对癌症相关成纤维细胞的异质性做了大量的研究,但仍有癌症相关成纤维细胞亚型有待被发现,不同群体的癌症相关成纤维细胞对肿瘤进展的作用方式及影响在很大程度上仍然未知,因此深入研究癌症相关成纤维细胞异质性对于制定和完善基于癌症相关成纤维细胞的治疗策略至关重要。 2.3 基质硬度对肿瘤生物学行为的影响 2.3.1 基质硬度和癌细胞增殖 有研究于软质载体(1 kPa)和高硬度载体(12 kPa)上培养肝癌细胞,通过Ki67染色评估发现,与软质载体相比,Huh7和HepG2细胞在高硬度载体上的增殖指数分别高出2.7倍和12.2倍;同时用肝细胞生长因子作用于肝癌细胞,观察到在2种载体上培养的细胞中细胞周期蛋白D1表达均上调,但在高硬度载体上培养的细胞其细胞周期蛋白D1上调幅度要高得多,且在高硬度载体上培养的细胞中,胞外调节激酶(extracellular regulated kinase,ERK)和转录激活因子3(activator of transcription 3,STAT3)的活化增加,说明基质硬度增加可以通过ERK、STAT3以及细胞周期蛋白D1多种信号通路调节肝癌细胞的增殖能力[41]。另外刘秋萍等[42]发现肝癌细胞HepG2的增殖能力、葡萄糖摄取能力以及葡萄糖转运蛋白1的表达都与基质硬度呈正相关,说明基质硬化也可以通过糖酵解途径促进肝癌细胞的增殖。且随着基质硬度增加,癌细胞的形状、细胞的骨架结构、细胞内张力都发生了变化,细胞通过拉动细胞外基质和相邻细胞来感知其所处微环境的硬度,基质硬度变化产生的牵引力可以通过Rho/ROCK信号通路改变癌细胞的形态和细胞骨架结构,并影响细胞-细胞外基质黏附,上调增殖相关基因,促进癌细胞的增殖[43-44]。 2.3.2 基质硬度和癌细胞转移 肿瘤转移往往是肿瘤治疗失败的一个主要原因,基质硬度增加使得癌细胞更容易迁移和扩散。例如,在一系列硬度为1-13 kPa的底物中培养乳腺癌细胞系MDA-MB-231细胞,发现癌细胞的运动能力随着底物硬度的增加而不断增加,迁移速度从软基质上的0.7 μm/min增加到硬基质上的1 μm/min,且细胞在僵硬的基质上运动持续的时间更长,这表明细胞外基质硬化不仅促进了癌细胞的迁移能力,而且通过增加定向运动的持久性来促进乳腺癌细胞向远处部位的转移[45]。非肌肉Ⅱ型肌球蛋白可以为细胞骨架重构提供收缩应力,在转移过程中,僵硬的基质可以通过整合素β1-FAK传递力学信号,促进非肌肉Ⅱ型肌球蛋白磷酸化,进而调控细胞骨架重构,调整细胞的形态及伪足形成,促进乳腺癌细胞的迁移[46]。在转移后期,远处的靶器官和组织为肿瘤细胞的沉降准备了肥沃的土壤,基质硬化诱导赖氨酰氧化酶样蛋白2表达上调,分泌的赖氨酰氧化酶样蛋白2促进纤连蛋白的产生,同时上调基质金属蛋白酶9和趋化因子CXCL12的表达,重塑细胞外基质,为原发性肿瘤在继发器官和组织的转移创造了有利的微环境,以帮助转移前生态位的形成[47]。这些研究均提示了基质硬度与肿瘤的侵袭和转移密切相关。 2.3.3 基质硬度和上皮间质转化 基质硬度可以调控肿瘤细胞的上皮间质转化,细胞表现出上皮基因(如E-钙黏蛋白)表达水平的降低以及间充质基因(如N-钙黏蛋白、vimentin)表达水平的升高,细胞的形态发生变化、黏附减弱,有利于肿瘤细胞的侵袭和转移[48-49]。使用硬度分别为16 kPa、10 kPa、6 kPa的载体模拟肝硬化、纤维化和正常肝脏的硬度并培养肝癌细胞,发现与正常硬度组相比,在高硬度基质中生长的肝癌细胞E-钙黏蛋白表达均明显降低,N-钙黏蛋白和vimentin的表达均明显升高,同时Smad2/Smad3磷酸化水平也明显升高。整合素β1和整合素α5可以向肝癌细胞传递基质僵硬的信号,这2个整合素亚基的敲低显著抑制了高硬度组肝癌细胞中N-钙黏蛋白、vimentin、p-Smad2和p-Smad3的表达,但上调了E-钙黏蛋白的表达,这表明较高的基质硬度通过整合素β1和整合素α5驱动了肝癌细胞的上皮间质转化[50]。此外基质硬化还会激活乳腺癌细胞中转录因子TWIST1的核易位,而TWIST1则会抑制E-钙黏蛋白的表达以促进间充质样肿瘤细胞的侵袭和转移[51]。高硬度基质还可以通过NEAT1/WNT/β-连环蛋白机械转导途径[52]、Pin/YAP[53]、赖氨酰氧化酶样蛋白2等通路调控肝癌、宫颈癌等不同肿瘤细胞的上皮间质转化[54]。 2.3.4 基质硬度和癌细胞干性相关标志物的表达 肿瘤细胞具有干性特征,具有自我更新能力、高增殖能力、多分化潜能以及具有更强的耐药性等特点,在癌症的进展、转移和复发中起着重要的作用。研究表明,基质硬化可以调节肿瘤细胞干性相关标志物的表达。例如:在胶质瘤组织中,基质硬化通过激活BCL9L及其下游信号Wnt/β-连环蛋白增强了胶质瘤细胞的干性特征[55];在胰腺癌组织中,基质硬化通过激活YAP/TAZ、丝裂原活化蛋白激酶(MAPK)和磷脂酰肌醇3-激酶(PI3K)信号通路增强胰腺癌细胞干性相关标志物的表达[56];在乳腺癌组织中,僵硬和缺氧的微环境通过调节与整合素连接的整合素相关激酶促进乳腺癌症干细胞的发育[57]。但基质硬度增加并非对所有肿瘤细胞的干性特征都有促进作用,在肝脏软组织中,为了更好地生存,肝癌细胞表达高水平的癌症干细胞相关标志物以维持细胞干性,并进入休眠状态,随着组织基质硬度的增加,休眠细胞被激活并分化为癌细胞,进一步促进肝癌的进展[58];在骨肉瘤组织中,随着基质硬度的增加,miR-29b-5p的表达上调,通过抑制PI3K/Akt/Stat3/Sox2信号通路降低了骨肉瘤细胞的干细胞特性[59],说明不同类型肿瘤细胞的干性特征会受到不同基质硬度的调节。 2.3.5 基质硬度和肿瘤细胞代谢 如上所述,基质硬化会促进癌细胞的增殖,而细胞增殖需要不断地汲取能量,因此,癌细胞在组织变硬的情况下会相应的调整其代谢以满足细胞增殖的能量需求。有研究发现基质硬化上调了YAP/TAZ的表达,激活的YAP/TAZ通过3种方式参与葡萄糖代谢。首先,它增加了葡萄糖转运蛋白(如葡萄糖转运蛋白1和葡萄糖转运蛋白3)的表达,促进了细胞对葡萄糖的摄取;其次,活化的YAP/TAZ通过增加糖酵解途径关键酶(例如己糖激酶、乳酸脱氢酶、丙酮酸激酶、果糖-2,6-二磷酸酶)的表达促进细胞的糖酵解,为细胞活动提供更多的能量;最后,上调的YAP/TAZ可以通过调节糖异生途径中的关键酶,增强细胞的葡萄糖代谢。然而仅增加糖酵解不足以满足增殖细胞的能量代谢需求,基质硬化还可以通过上调参与细胞脂质代谢的甾醇调控元件结合蛋白SREBP的活性,调节癌细胞的脂质代谢;上调的YAP/TAZ也可以增加氨基酸合成酶、氨基酸转运蛋白的表达,调节癌细胞的氨基酸代谢。除此以外,基质硬化激活的癌症相关成纤维细胞可以分泌大量的天冬氨酸,癌症相关成纤维细胞衍生的天冬氨酸对于维持癌细胞的增殖至关重要,癌细胞能够根据肿瘤基质硬度调整其对天冬氨酸的摄取,以满足细胞增殖所需的能量需求[60-61]。 2.3.6 基质硬度和肿瘤的耐药性 基质硬度增加不仅加速了肿瘤的进展,也影响治疗药物的渗透性以及药物在肿瘤内的分布。在肿瘤组织中,基质硬度的增加导致肿瘤微环境内缺氧,在缺氧环境下生成的血管并不成熟,其周细胞覆盖减少,基底膜脱离或丢失,内皮细胞之间开口较大,导致血管通透性增加,增加药物外渗;同时大量液体从血管转运到肿瘤的细胞外间隙,进而导致间质液压力升高,将药物从肿瘤中冲洗出来,降低药物疗效[62];此外,肿瘤中的药物分布还受到压力梯度的影响,在肿瘤中,特别是在肿瘤的中心区域,其间质液压力可能与微血管压力相似,药物的渗透压力梯度几乎为零,导致药物分布不均,不能以致死浓度到达肿瘤中的所有活细胞,从而诱导细胞耐药[63]。此外,基质硬度的变化也会直接激活耐药相关蛋白的表达。例如,在不同硬度的底物中分别培养乳腺癌细胞,经阿霉素处理后,在低硬度和高硬度底物中培养的细胞增殖明显减少,整合素相关激酶作为组织力学的传感器,在中等硬度培养的癌细胞中表达显著增加,且通过诱导YAP的活化和核易位,上调耐药基因P-gp的表达,以调节乳腺癌细胞的耐药性[64]。 2.3.7 基质硬度和血管生成 实体瘤具有很强的诱导新生血管生成的能力,新生血管不仅为肿瘤组织提供更多的氧气和营养物质,使其无限生长,肿瘤内微血管系统高通透性、曲折性的形态,也促进了实体瘤的侵袭和转移,并影响药物递送而降低疗效[65]。血管内皮尖端细胞的生长标志着血管生成的开始,在血管内皮生长因子等促血管生成信号的引导下,内皮细胞发生表型变化,转变为尖端细胞,变得具有运动性和侵袭性,探测无血管环境以寻找目标,当尖端细胞形成后,邻近的茎细胞分裂增殖以建立管腔并传达其附近的空间信息,从而使茎伸长,最后尖端细胞与血管芽的细胞吻合以构建血管环[66]。基质硬化通过整合素αVβ5/Akt/Sp1途径上调了血管内皮生长因子受体2的表达[67],通过p-PXN-Rac1-YAP信号轴调节尖端细胞的形成,并使其定向迁移到无血管区域[68],同时活化的YAP可以诱导血管内皮细胞增殖和迁移,以促进血管管腔的生成[69]。此外,Piezo1作为一种机械传感器,可以通过加速Ca2+流动将物理刺激转化为电和化学信号,基质硬度的增加在细胞和组织水平上显著上调了Piezo1的表达及其活化水平,激活的Piezo1可以抑制缺氧诱导因子1α泛素化,随后增强其下游促血管生成因子的表达,进一步加速肿瘤组织血管生成[65]。在新血管形成后,周细胞和血管平滑肌细胞等壁细胞的募集有利于维持血管稳定,保持血管的完整性,而硬化的基质会导致周细胞丢失,因此肿瘤僵硬常伴有血管扭曲和渗漏[70]。 尽管目前已经研发出了多种抗血管生成药物,但在治疗过程中,很多肿瘤细胞依然表现出了不同程度的耐药性,机械敏感性离子通道在基质力学对肿瘤进展的影响中发挥了很大的作用,更多地了解这些机械传感器可能为肿瘤的治疗提供新思路。 2.3.8 基质硬度和免疫逃逸 肿瘤免疫微环境中的免疫细胞主要包括树突状细胞、细胞毒性T细胞、调节性T细胞、肿瘤相关巨噬细胞、自然杀伤细胞、髓源性抑制细胞等,其中效应T细胞、自然杀伤细胞、树突状细胞和M1型巨噬细胞主要发挥抗肿瘤作用,调节性T细胞、髓源性抑制细胞以及M2型巨噬细胞则会促进肿瘤的发展[71]。随着基质硬化,癌细胞内某些基因的表达发生改变,越来越有利于其逃避CD8+ T细胞的识别。例如SWI/SNF参与肿瘤发生过程中机械信号的传导,其许多亚基的表达水平都受到肿瘤基质硬度的调控,SNF5是SWI/SNF复合物的核心亚基之一,在黑色素瘤中,基质硬度增加显著上调了SNF5的表达,SNF5的过表达通过STAT3/p-STAT3信号通路使FAS、IDO1、PD-L1等免疫逃逸相关基因的表达增加,并与T细胞上的受体结合,避免了CD8+ T细胞的攻击,促进了黑色素瘤的免疫逃逸[72]。随着胶原蛋白的过度沉积及基质硬度的增加,肿瘤微环境呈现高度免疫抑制的状态,高密度的胶原蛋白使T细胞的迁移速度降低,限制了T细胞在肿瘤组织中的浸润,且减少了T细胞的活化以及T细胞与抗原呈递细胞间的相互作用,导致T细胞清除癌细胞的能力受到抑制,从而诱导癌细胞的免疫逃逸[14]。此外,基质硬化激活的癌症相关成纤维细胞能够通过分泌不同的趋化因子来限制CD8+ T细胞等免疫细胞募集到肿瘤组织中,而使M2型巨噬细胞、调节性T细胞、髓源性抑制细胞等免疫抑制细胞在肿瘤免疫微环境中的比例显著增加,从而促进肿瘤免疫抑制。癌症相关成纤维细胞也可以通过多种调节因子促进单核细胞募集且分化为M2型巨噬细胞,抑制自然杀伤细胞、 T细胞、中性粒细胞等多种免疫细胞的活性,帮助肿瘤细胞逃避免疫清除[73],见图4。"
| [1] CHEN F, ZHUANG X, LIN L, et al. New horizons in tumor microenvironment biology: challenges and opportunities. BMC Med. 2015;13:45. [2] KARAMANOS NK, THEOCHARIS AD, PIPERIGKOU Z, et al. A guide to the composition and functions of the extracellular matrix. Febs J. 2021; 288(24):6850-6912. [3] BONNANS C, CHOU J, WERB Z. Remodelling the extracellular matrix in development and disease. Nat Rev Mol Cell Biol. 2014;15(12):786-801. [4] BOYD DF, THOMAS PG. Towards integrating extracellular matrix and immunological pathways. Cytokine. 2017;98:79-86. [5] WALKER C, MOJARES E, DEL RíO HERNÁNDEZ A. Role of Extracellular Matrix in Development and Cancer Progression. Int J Mol Sci. 2018; 19(10):3028. [6] NAGELKERKE A, BUSSINK J, ROWAN AE, et al. The mechanical microenvironment in cancer: How physics affects tumours. Semin Cancer Biol. 2015;35:62-70. [7] WEI J, YAO J, YAN M, et al. The role of matrix stiffness in cancer stromal cell fate and targeting therapeutic strategies. Acta Biomater. 2022;150: 34-47. [8] NIA HT, MUNN LL, JAIN RK. Physical traits of cancer. Science. 2020; 370(6516):eaaz0868. [9] JAIN RK, MARTIN JD, STYLIANOPOULOS T. The role of mechanical forces in tumor growth and therapy. Annu Rev Biomed Eng. 2014;16: 321-346. [10] ULRICH TA, DE JUAN PARDO EM, KUMAR S. The mechanical rigidity of the extracellular matrix regulates the structure, motility, and proliferation of glioma cells. Cancer Res. 2009;69(10):4167-4174. [11] CHEN G, XIA B, FU Q, et al. Matrix Mechanics as Regulatory Factors and Therapeutic Targets in Hepatic Fibrosis. Int J Biol Sci. 2019;15(12): 2509-2521. [12] JIANG Y, ZHANG H, WANG J, et al. Targeting extracellular matrix stiffness and mechanotransducers to improve cancer therapy. J Hematol Oncol. 2022;15(1):34. [13] XU S, XU H, WANG W, et al. The role of collagen in cancer: from bench to bedside. J Transl Med. 2019;17(1):309. [14] RØMER AMA, THORSETH ML, MADSEN DH. Immune Modulatory Properties of Collagen in Cancer. Front Immunol. 2021;12:791453. [15] LIU J, SHEN JX, WU HT, et al. Collagen 1A1 (COL1A1) promotes metastasis of breast cancer and is a potential therapeutic target. Discov Med. 2018;25(139):211-223. [16] LIANG Y, LV Z, HUANG G, et al. Prognostic significance of abnormal matrix collagen remodeling in colorectal cancer based on histologic and bioinformatics analysis. Oncol Rep. 2020;44(4):1671-1685. [17] BROOKS M, MO Q, KRASNOW R, et al. Positive association of collagen type I with non-muscle invasive bladder cancer progression. Oncotarget. 2016;7(50):82609-82619. [18] VOILES L, LEWIS DE, HAN L, et al. Overexpression of type VI collagen in neoplastic lung tissues. Oncol Rep. 2014;32(5):1897-1904. [19] CHEN S, WEI Y, LIU H, et al. Analysis of Collagen type X alpha 1 (COL10A1) expression and prognostic significance in gastric cancer based on bioinformatics. Bioengineered. 2021;12(1):127-137. [20] HISATOMI K, MUKAE H, SAKAMOTO N, et al. Pirfenidone inhibits TGF-β1-induced over-expression of collagen type I and heat shock protein 47 in A549 cells. BMC Pulm Med. 2012;12:24. [21] GHOSH D, MEJIA PENA C, QUACH N, et al. Senescent mesenchymal stem cells remodel extracellular matrix driving breast cancer cells to a more-invasive phenotype. J Cell Sci. 2020;133(2):jcs232470. [22] PANKOVA D, JIANG Y, CHATZIFRANGKESKOU M, et al. RASSF1A controls tissue stiffness and cancer stem-like cells in lung adenocarcinoma. Embo J. 2019;38(13):e100532. [23] NECULA L, MATEI L, DRAGU D, et al. Collagen Family as Promising Biomarkers and Therapeutic Targets in Cancer. Int J Mol Sci. 2022; 23(20):12415. [24] GILKES DM, BAJPAI S, CHATURVEDI P, et al. Hypoxia-inducible factor 1 (HIF-1) promotes extracellular matrix remodeling under hypoxic conditions by inducing P4HA1, P4HA2, and PLOD2 expression in fibroblasts. J Biol Chem. 2013;288(15):10819-10829. [25] CABRAL-PACHECO GA, GARZA-VELOZ I, CASTRUITA-DE LA ROSA C, et al. The Roles of Matrix Metalloproteinases and Their Inhibitors in Human Diseases. J Mol Sci. 2020;21(24):9739. [26] ACERBI I, CASSEREAU L, DEAN I, et al. Human breast cancer invasion and aggression correlates with ECM stiffening and immune cell infiltration. Integr Biol (Camb). 2015;7(10):1120-1134. [27] LACHOWSKI D, CORTES E, RICE A, et al. Matrix stiffness modulates the activity of MMP-9 and TIMP-1 in hepatic stellate cells to perpetuate fibrosis. Sci Rep. 2019;9(1):7299. [28] RAMOS S, FERREIRA S, FERNANDES AS, et al. Lysyl Oxidases Expression and Breast Cancer Progression: A Bioinformatic Analysis. Front Pharmacol. 2022;13:883998. [29] ZHU J, LUO C, ZHAO J, et al. Expression of LOX Suggests Poor Prognosis in Gastric Cancer. Front Med (Lausanne). 2021;8:718986. [30] KIM YM, KIM EC, KIM Y. The human lysyl oxidase-like 2 protein functions as an amine oxidase toward collagen and elastin. Mol Biol Rep. 2011;38(1):145-149. [31] SCHMELZER CEH, HEINZ A, TROILO H, et al. Lysyl oxidase-like 2 (LOXL2)-mediated cross-linking of tropoelastin. Faseb J. 2019;33(4):5468-5481. [32] 唐晓男, 康小红. 赖氨酸羟化酶2在肿瘤转移中的研究进展[J]. 医学研究生学报,2019,32(10):1099-1103. [33] ISHIHARA S, HAGA H. Matrix Stiffness Contributes to Cancer Progression by Regulating Transcription Factors. Cancers (Basel). 2022;14(4):1049. [34] DENG B, ZHAO Z, KONG W, et al. Biological role of matrix stiffness in tumor growth and treatment. J Transl Med. 2022;20(1):540. [35] LIU C, PEI H, TAN F. Matrix Stiffness and Colorectal Cancer. Onco Targets Ther. 2020;13:2747-2755. [36] CALVO F, EGE N, GRANDE-GARCIA A, et al. Mechanotransduction and YAP-dependent matrix remodelling is required for the generation and maintenance of cancer-associated fibroblasts. Nat Cell Biol. 2013; 15(6):637-646. [37] PIERSMA B, HAYWARD MK, WEAVER VM. Fibrosis and cancer: A strained relationship. Biochim Biophys Acta Rev Cancer. 2020;1873(2):188356. [38] GENG X, CHEN H, ZHAO L, et al. Cancer-Associated Fibroblast (CAF) Heterogeneity and Targeting Therapy of CAFs in Pancreatic Cancer. Front Cell Dev Biol. 2021;9:655152. [39] BATES ME, LIBRING S, REINHART-KING CA. Forces exerted and transduced by cancer-associated fibroblasts during cancer progression. Biol Cell. 2023;115(8):e2200104. [40] LI X, SUN Z, PENG G, et al. Single-cell RNA sequencing reveals a pro-invasive cancer-associated fibroblast subgroup associated with poor clinical outcomes in patients with gastric cancer. Theranostics. 2022; 12(2):620-638.
[41] SCHRADER J, GORDON-WALKER TT, AUCOTT RL, et al. Matrix stiffness modulates proliferation, chemotherapeutic response, and dormancy in hepatocellular carcinoma cells. Hepatology. 2011;53(4):1192-1205. [42] 刘秋萍, 田博仁, 罗庆, 等. 基质刚度对肝癌细胞增殖及糖代谢的影响[J]. 医用生物力学,2019,34(2):133-138. [43] KHALIL K, EON A, JANODY F. Cell Architecture-Dependent Constraints: Critical Safeguards to Carcinogenesis. Int J Mol Sci. 2022;23(15):8622. [44] PROVENZANO PP, KEELY PJ. Mechanical signaling through the cytoskeleton regulates cell proliferation by coordinated focal adhesion and Rho GTPase signaling. J Cell Sci. 2011;124(Pt 8):1195-1205. [45] PANZETTA V, VERDE G, PUGLIESE M, et al. Adhesion and Migration Response to Radiation Therapy of Mammary Epithelial and Adenocarcinoma Cells Interacting with Different Stiffness Substrates. Cancers (Basel). 2020;12(5):1170. [46] 彭悦婷. 基质刚度通过ROCK及NMII亚型差异调控乳腺癌细胞迁移的机制研究[D]. 成都: 电子科技大学,2021. [47] WU S, ZHENG Q, XING X, et al. Matrix stiffness-upregulated LOXL2 promotes fibronectin production, MMP9 and CXCL12 expression and BMDCs recruitment to assist pre-metastatic niche formation. J Exp Clin Cancer Res. 2018;37(1):99. [48] LAMOUILLE S, XU J, DERYNCK R. Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol. 2014;15(3):178-196. [49] XU J, LAMOUILLE S, DERYNCK R. TGF-beta-induced epithelial to mesenchymal transition. Cell Res. 2009;19(2):156-172. [50] DONG Y, ZHENG Q, WANG Z, et al. Higher matrix stiffness as an independent initiator triggers epithelial-mesenchymal transition and facilitates HCC metastasis. J Hematol Oncol. 2019;12(1):112. [51] WEI SC, FATTET L, TSAI JH, et al. Matrix stiffness drives epithelial-mesenchymal transition and tumour metastasis through a TWIST1-G3BP2 mechanotransduction pathway. Nat Cell Biol. 2015;17(5):678-688. [52] XU X, ZHANG Y, WANG X, et al. Substrate Stiffness Drives Epithelial to Mesenchymal Transition and Proliferation through the NEAT1-Wnt/β-Catenin Pathway in Liver Cancer. Int J Mol Sci. 2021;22(21):12066. [53] YANG L, LI J, ZANG G, et al. Pin1/YAP pathway mediates matrix stiffness-induced epithelial-mesenchymal transition driving cervical cancer metastasis via a non-Hippo mechanism. Bioeng Transl Med. 2023;8(1): e10375. [54] DENG Y, CHAKRABORTY P, JOLLY MK, et al. A Theoretical Approach to Coupling the Epithelial-Mesenchymal Transition (EMT) to Extracellular Matrix (ECM) Stiffness via LOXL2. Cancers (Basel). 2021;13(7):1609. [55] TAO B, SONG Y, WU Y, et al. Matrix stiffness promotes glioma cell stemness by activating BCL9L/Wnt/β-catenin signaling. Aging (Albany NY). 2021;13(4):5284-5296. [56] SAFAEI S, SAJED R, SHARIFTABRIZI A, et al. Tumor matrix stiffness provides fertile soil for cancer stem cells. Cancer Cell Int. 2023;23(1): 143. [57] PANG M F, SIEDLIK MJ, HAN S, et al. Tissue Stiffness and Hypoxia Modulate the Integrin-Linked Kinase ILK to Control Breast Cancer Stem-like Cells. Cancer Res. 2016;76(18):5277-5287. [58] TIAN B, LUO Q, JU Y, et al. A Soft Matrix Enhances the Cancer Stem Cell Phenotype of HCC Cells. Int J Mol Sci. 2019;20(11):2831. [59] 陈瑜, 白红霞, 彭悦婷, 等. 胞外基质刚度对骨肉瘤干细胞自我更新的调控机制研究[J]. 医用生物力学,2019,34(S1):66-67. [60] BERTERO T, OLDHAM WM, GRASSET EM, et al. Tumor-Stroma Mechanics Coordinate Amino Acid Availability to Sustain Tumor Growth and Malignancy. Cell Metab. 2019;29(1):124-140.e110. [61] GE H, TIAN M, PEI Q, et al. Extracellular Matrix Stiffness: New Areas Affecting Cell Metabolism. Front Oncol. 2021;11:631991. [62] KALLI M, POSKUS MD, STYLIANOPOULOS T, et al. Beyond matrix stiffness: targeting force-induced cancer drug resistance. Trends Cancer. 2023;9(11):937-954. [63] TRÉDAN O, GALMARINI CM, PATEL K, et al. Drug resistance and the solid tumor microenvironment. J Natl Cancer Inst. 2007;99(19):1441-1454. [64] QIN X, LV X, LI P, et al. Matrix stiffness modulates ILK-mediated YAP activation to control the drug resistance of breast cancer cells. Biochim Biophys Acta Mol Basis Dis. 2020;1866(3):165625. [65] LI M, ZHANG X, WANG M, et al. Activation of Piezo1 contributes to matrix stiffness-induced angiogenesis in hepatocellular carcinoma. Cancer Commun (Lond). 2022;42(11):1162-1184. [66] CARMELIET P, JAIN RK. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;473(7347):298-307. [67] WANG Y, ZHANG X, WANG W, et al. Integrin αVβ5/Akt/Sp1 pathway participates in matrix stiffness-mediated effects on VEGFR2 upregulation in vascular endothelial cells. Am J Cancer Res. 2020; 10(8):2635-2648. [68] GUO Y, MEI F, HUANG Y, et al. Matrix stiffness modulates tip cell formation through the p-PXN-Rac1-YAP signaling axis. Bioact Mater. 2022;7:364-376. [69] JIANG X, HU J, WU Z, et al. Protein Phosphatase 2A Mediates YAP Activation in Endothelial Cells Upon VEGF Stimulation and Matrix Stiffness. Front Cell Dev Biol. 2021;9:675562. [70] KRETSCHMER M, RüDIGER D, ZAHLER S. Mechanical Aspects of Angiogenesis. Cancers (Basel). 2021;13(19):4987. [71] LV B, WANG Y, MA D, et al. Immunotherapy: Reshape the Tumor Immune Microenvironment. Front Immunol. 2022;13:844142. [72] CHEN Y, ZHAO M, ZHANG L, et al. SNF5, a core subunit of SWI/SNF complex, regulates melanoma cancer cell growth, metastasis, and immune escape in response to matrix stiffness. Transl Oncol. 2022;17:101335. [73] MAO X, XU J, WANG W, et al. Crosstalk between cancer-associated fibroblasts and immune cells in the tumor microenvironment: new findings and future perspectives. Mol Cancer. 2021;20(1):131. [74] EDWARDS DR, MURPHY G, REYNOLDS JJ, et al. Transforming growth factor beta modulates the expression of collagenase and metalloproteinase inhibitor. Embo j. 1987;6(7):1899-1904. [75] LIU YL, BAGER CL, WILLUMSEN N, et al. Tetrathiomolybdate (TM)-associated copper depletion influences collagen remodeling and immune response in the pre-metastatic niche of breast cancer. NPJ Breast Cancer. 2021;7(1):108. [76] SCHILTER H, FINDLAY AD, PERRYMAN L, et al. The lysyl oxidase like 2/3 enzymatic inhibitor, PXS-5153A, reduces crosslinks and ameliorates fibrosis. J Cell Mol Med. 2019;23(3):1759-1770. [77] KANEDA A, SEIKE T, DANJO T, et al. The novel potent TEAD inhibitor, K-975, inhibits YAP1/TAZ-TEAD protein-protein interactions and exerts an anti-tumor effect on malignant pleural mesothelioma. Am J Cancer Res. 2020;10(12):4399-4415. [78] LI X, SONG Y. Proteolysis-targeting chimera (PROTAC) for targeted protein degradation and cancer therapy. J Hematol Oncol. 2020;13(1): 50. [79] MAI Z, LIN Y, LIN P, et al. Modulating extracellular matrix stiffness: a strategic approach to boost cancer immunotherapy. Cell Death Dis. 2024;15(5):307. [80] JOHNSON LA, MORGAN RA, DUDLEY ME, et al. Gene therapy with human and mouse T-cell receptors mediates cancer regression and targets normal tissues expressing cognate antigen. Blood. 2009;114(3): 535-546. |
| [1] | Lai Pengyu, Liang Ran, Shen Shan. Tissue engineering technology for repairing temporomandibular joint: problems and challenges [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(在线): 1-9. |
| [2] | Shui Jing, He Yu, Jiang Nan, Xu Kun, Song Lijuan, Ding Zhibin, Ma Cungen, Li Xinyi. Astrocytes regulate remyelination in central nervous system [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(36): 7889-7897. |
| [3] | Zhang Xiaoyu, Wei Shanwen, Fang Jiawei, Ni Li. Prussian blue nanoparticles restore mitochondrial function in nucleus pulposus cells through antioxidation [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(34): 7318-7325. |
| [4] | Yu Qinghe, Cai Ziming, Wu Jintao, Ma Pengfei, Zhang Xin, Zhou Longqian, Wang Yakun, Lin Xiaoqin, Lin Wenping. Vanillic acid inhibits inflammatory response and extracellular matrix degradation of endplate chondrocytes [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(30): 6391-9397. |
| [5] | Fan Jiaxin, Jia Xiang, Xu Tianjie, Liu Kainan, Guo Xiaoling, Zhang Hui, Wang Qian . Metformin inhibits ferroptosis and improves cartilage damage in osteoarthritis model rats [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(30): 6398-6408. |
| [6] | Zhou Ying, Tian Yong, Zhong Zhimei, Gu Yongxiang, Fang Hao. Inhibition of tumor necrosis factor receptor associated factor 6 regulates mTORC1/ULK1 signaling and promotes autophagy to improve myocardial injury in sepsis mice [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(30): 6434-6440. |
| [7] | Wang Wanchun, , Yi Jun, Yan Zhangren, Yang Yue, Dong Degang, Li Yumei. 717 Jiedu Decoction remodels homeostasis of extracellular matrix and promotes repair of local injured tissues in rats after Agkistrodon halys bite [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(30): 6457-6465. |
| [8] | Zhang Xin, Guo Baojuan, Xu Huixin, Shen Yuzhen, Yang Xiaofan, Yang Xufang, Chen Pei. Protective effects and mechanisms of 3-N-butylphthalide in Parkinson’s disease cell models [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(30): 6466-6473. |
| [9] | Zhang Songjiang, Li Longyang, Zhou Chunguang, Gao Jianfeng. Central anti-inflammatory effect and mechanism of tea polyphenols in exercise fatigue model mice [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(30): 6474-6481. |
| [10] | Hu Shujuan, Liu Dang, Ding Yiting, Liu Xuan, Xia Ruohan, Wang Xianwang. Ameliorative effect of walnut oil and peanut oil on atherosclerosis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(30): 6482-6488. |
| [11] | Zhang Jian, Cai Feng, Li Tingwen, Ren Pengbo. Fatigue gait recognition of athletes based on fish swarm algorithm [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(30): 6489-6498. |
| [12] | Zhang Zihan¹, Wang Jiaxin¹, Yang Wenyi², Zhu Lei¹. Regulatory mechanism of exercise promoting mitochondrial biogenesis in skeletal muscle [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(30): 6499-6508. |
| [13] | Wang Jianxu, Dong Zihao, Huang Zishuai, Li Siying, Yang Guang. Interaction between immune microenvironment and bone aging and treatment strategies [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(30): 6509-6519. |
| [14] | Zhang Bochun, Li Wei, Li Guangzheng, Ding Haoqin, Li Gang, Liang Xuezhen, . Association between neuroimaging changes and osteonecrosis: a large sample analysis from UK Biobank and FinnGen databases [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(30): 6574-6582. |
| [15] | Xu Zhi, Chen Yundong, Sun Yujie, Gong Xiaonan, Li Yuwan. Data of spinal osteosarcoma patients in United States based on SEER database: construction and validation of a prediction model for treatment outcomes and prognosis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(30): 6583-6590. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||