Chinese Journal of Tissue Engineering Research ›› 2024, Vol. 28 ›› Issue (15): 2410-2415.doi: 10.12307/2024.411
Previous Articles Next Articles
Advantages and features of nanocomposite hydrogel in treatment of osteoarthritis
Tian Linling1, Guo Hairui1, Du Xiaoming1, Feng Jie1, Zhang Xianzhe1, Zhang Wenbin1, Sun Haoran1, Zhang Xiaobin2, Wang Jingxia2, Hu Yimei1, 3, Wang Yi4
- 1Chengdu University of Traditional Chinese Medicine, Chengdu 610041, Sichuan Province, China; 2Sichuan Atomic Energy Research Institute, Chengdu 610199, Sichuan Province, China; 3Affiliated Hospital of Chengdu University of Traditional Chinese Medicine, Chengdu 610041, Sichuan Province, China; 4Southwest Jiaotong University, Chengdu 610031, Sichuan Province, China
-
Received:2023-05-30Accepted:2023-07-17Online:2024-05-28Published:2023-09-23 -
Contact:Hu Yimei, PhD, Professor, Master’s supervisor, Chengdu University of Traditional Chinese Medicine, Chengdu 610041, Sichuan Province, China; Affiliated Hospital of Chengdu University of Traditional Chinese Medicine, Chengdu 610041, Sichuan Province, China Wang Yi, PhD, Professor, Master’s supervisor, Southwest Jiaotong University, Chengdu 610031, Sichuan Province, China -
About author:Tian Linling, Master candidate, Chengdu University of Traditional Chinese Medicine, Chengdu 610041, Sichuan Province, China -
Supported by:National Natural Science Foundation of China, No. 325021015 (to HYM); Science and Technology Department of Chengdu University of Traditional Chinese Medicine, Nos. 030058047, 030038164 (to HYM); “Hundred Talents Plan” for Improving the Scientific Research Ability of the Affiliated Hospital of Chengdu University of Traditional Chinese Medicine, No. 22-B07 (to HYM); International Cooperation Project of Sichuan Provincial Department of Science and Technology, No. 2020YFH0052 (to WY); Central University Basic Research Business Fee Project, No. 2682022ZTPY038 (to WY)
CLC Number:
Cite this article
Tian Linling, Guo Hairui, Du Xiaoming, Feng Jie, Zhang Xianzhe, Zhang Wenbin, Sun Haoran, Zhang Xiaobin, Wang Jingxia, Hu Yimei, Wang Yi. Advantages and features of nanocomposite hydrogel in treatment of osteoarthritis[J]. Chinese Journal of Tissue Engineering Research, 2024, 28(15): 2410-2415.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
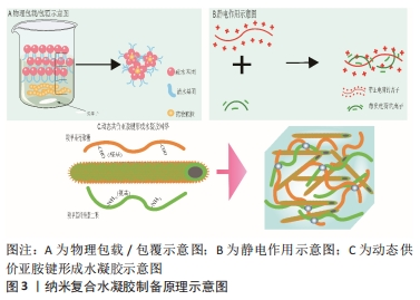
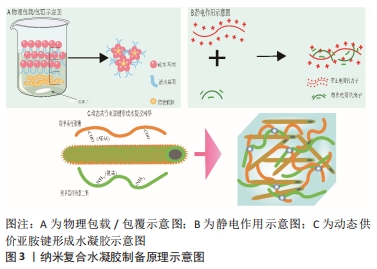
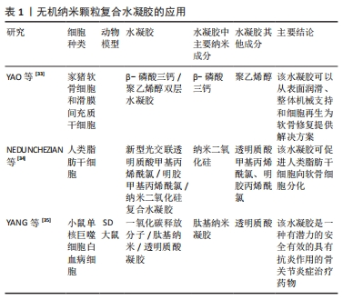
2.1 骨关节炎关节内环境 骨关节炎的病程进展过程中促炎因子大量分泌,关节周围微环境改变,软骨细胞代谢发生紊乱,滑膜细胞以多种不同的方式被激活[9]。有研究表明,胶原蛋白、糖胺聚糖、血管内皮生长因子和血小板衍生生长因子、转化生长因子β等在其中发挥着重要作用,它们参与调控滑膜成纤维细胞、巨噬细胞、干细胞的极化等,对软骨细胞死亡和肥大、细胞外基质分解、异位骨形成至关重要[10]。而随着炎症进展,关节软骨结构遭到破坏、关节失稳,最终导致关节不可逆的损伤。因此,抑制滑膜炎症反应、调控滑膜细胞极化、清除衰老细胞(滑膜成纤维细胞、软骨细胞等)、促进新生软骨细胞分化、增加关节润滑等可能是调控骨关节炎炎症微环境的关键因素。 2.2 可用于骨关节炎治疗的纳米复合凝胶研究进展 纳米复合水凝胶是指将纳米颗粒引入水凝胶基体,使纳米颗粒和凝胶基体高分子链间发生协同作用形成网络结构,从而达到改善传统水凝胶较脆、易碎、力学性能差[11]、生物相容性较差的目的。纳米复合水凝胶颗粒的含水率较高,通常能到达70%以上,具有较高的比表面积、装载效率、较好的稳定性,可根据需求制备成环境响应(如离子强度、光照、酸碱度和温度等)的智能水凝胶,被广泛应用于药物靶向输送与可控释放[12-13]、基因转染、医学诊断、生物传感器和生物物质的分离与纯化等[14-15]。 2.2.1 纳米复合水凝胶制备原理 纳米复合水凝胶的交联方式分为物理交联和化学交联,其形成原理主要有简单物理包覆、正负电荷作用、共价键交联等。 物理包载/包覆:是目前制备纳米复合水凝胶最常用的方式,将药物通过物理包裹的方式直接嵌入聚合物纳米颗粒内,制备成纳米复合水凝胶。这种纳米粒子属于典型的核壳结构,其中亲水性的聚合物在外壳充当保护层,疏水性的药物在内核作为有效成分,既提高了药物生物利用度降低了药物毒副作用,还改善了疏水药物在水体系中的稳定性,其中聚合物常用的材料包括聚乙烯醇、聚己内酯、海藻酸盐、壳聚糖等。ZHANG等[16]将人脐带间充质干细胞外泌体包覆在聚乙烯醇/海藻酸钠纳米水凝胶中,这种水凝胶能够通过血管内皮生长因子/细胞外调节蛋白激酶1/2通路促进糖尿病大鼠新生血管的形成,促进人脐静脉内皮细胞的增殖、迁移和血管生成,还可以避免人脐带间充质干细胞外泌体快速清除,见图3A。 静电作用:静电作用形成纳米复合水凝胶的原理即通常由带正电荷的金离子、铜离子等金属粒子与其他带负电荷的离子,在静电作用下形成具有稳定化学键的纳米复合颗粒,然后将纳米复合颗粒引入水凝胶三维网络中以制备出具有导电、温敏等特殊性质的材料。在QUAZI等[17]的研究中,带正电荷的蟾蜍抗菌肽(Buforin IIb)和带负电荷的DNA通过静电作用形成的纳米复合水凝胶,可以增强抗菌肽在水凝胶体系中的稳定性,见图3B。 共价键交联:将表面具有活性化学基团的纳米胶束与活性基团修饰的聚合物链发生化学反应形成共价键连接,然后使其在水溶液中分散成纳米尺寸的水凝胶颗粒,最终获得纳米复合水凝胶材料。THOMAS等[18]将羧甲基纤维素二醛和羧甲基壳聚糖共价交联得到动态碳氧双键的多糖网络,再加入提供动力学控制的自组装淀粉样肽两亲性分子纳米纤维,最后合成可为软骨细胞再生提供稳定三维力学的微结构,见图3C。"
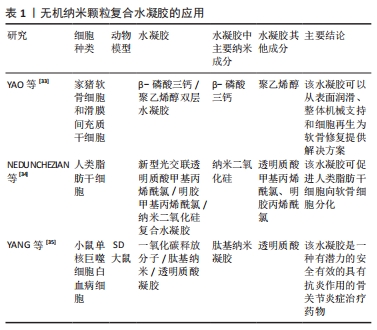
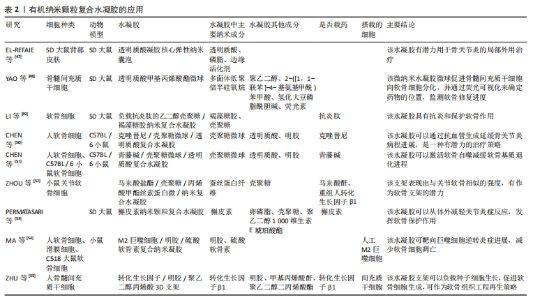
其他原理:除了几种上述常见的纳米复合水凝胶形成原理,还有很多其他方法来实现纳米复合水凝胶的制作,比如微流控技术、配位自组装、超分子组装与氢键作用组装等。微流控技术是通过将化学反应(包括进样、混合、反应、分离、检测等)集成至一个微小芯片以高效合成纳米复合材料,所制备的纳米颗粒形态可控、粒径分布均一、制备过程绿色环保且低耗。HSU等[19]通过液滴微流控技术制备的多级微凝胶/聚(乳酸-羟基乙酸)纳米粒子复合材料显示出尺寸可调性和单分散的尺寸分布,与裸聚乳酸羟基乙酸纳米颗粒相比,负载药物的释放动力学得到了改善。配位自组装是一种利用具有配位基团的有机分子或无机离子作为构建块,通过分子之间的化学键和非共价相互作用,将分子自发地组装成特定结构的方法,构建具有特定形状、大小和功能的纳米结构,可实现纳米结构的精确控制。SUN等[20]开发了一种利用儿茶酚接枝壳聚糖和姜黄素/3价铁离子配位纳米粒子组成的儿茶酚接枝壳聚糖配位3价铁离子纳米复合水凝胶,用于促进感染伤口的愈合。超分子组装通常是指由两种或两种以上分子依靠分子间相互作用结合在一起,组成保持一定的完整性、有组织、复杂的聚集体,使其具有明确的微观结构和宏观特性。CORREA等[21]利用脂质纳米技术和超分子自组装原理开发了一种可注射的脂质体纳米复合水凝胶平台,并通过编程实现多种蛋白质药物的精确共释放。氢键作用自组装是指利用电负性较大的氮、氧、氟原子之间形成具有饱和性和方向性的极性共价键,得到易于修饰的水凝胶结构。MA等[22]通过疏水相互作用和氢键自组装获得具有高吸附容量和抗黏附性能的聚丙烯膜,用于在海水中提取铀。 2.2.2 用于骨关节炎治疗的纳米复合水凝胶主要类型 根据纳米颗粒的物质性质,可将纳米复合水凝胶分为无机纳米颗粒复合水凝胶、有机纳米颗粒复合水凝胶、纳米纤维复合水凝胶,该文将分别从这三类材料来进行论述。 (1)无机纳米颗粒复合水凝胶:目前研究中关于骨关节炎治疗的无机纳米颗粒材料有β-磷酸三钙、二氧化硅、一氧化碳释放分子401。β-磷酸三钙具有良好的生物降解性、生物相容性、生物无毒性和新生骨诱导性[23],是一种常用于骨组织工程研究的材料[24]。二氧化硅最早是一种陶瓷材料,因其高稳定性被用于骨和软骨的再生研究[25-27],一些文献还报道了纳米二氧化硅可以通过激活细胞外调节蛋白激酶1/2磷酸化来增强细胞生长、改善成骨细胞功能、抑制破骨细胞功能、促进骨矿化[28]。大量研究证实,骨关节炎的炎症反应与过度活化的巨噬细胞相关[29-32],而过度活化的巨噬细胞会在关节内产生大量的活性氧,因此靶向巨噬细胞抑制其过度活化、清除关节内的活性氧也是减轻骨关节炎症状的一种思路。其中一氧化碳释放分子401被发现可以清除关节内的活性氧成分,将其制备为纳米复合水凝胶体系也成为治疗骨关节炎的潜在途径。 YAO等[33]使用纳米球磨工艺将β-磷酸三钙制备成纳米直径大小的颗粒,然后与聚乙烯醇混合构建了一种新型的集成双层水凝胶骨软骨置换体,其上层采用致密结构模拟软骨层,依靠高亲水性和高弹性来维持水合膜在流体动力学效应中的稳定性与界面微观形貌,以实现良好的边界润滑作用,使其具有与天然软骨相似的摩擦和压缩性能;下层采用多孔结构(空隙控制在100-250 μm)模拟软骨下层和松质骨结构,有利于细胞的生长和侵袭。进一步实验将材料与猪软骨细胞和滑膜间充质干细胞共培养,其结果显示这种材料可以促进软骨细胞在上层密度层的黏附,多孔下层也具有更好的成骨性能。NEDUNCHEZIAN等[34]的研究利用透明质酸甲基丙烯酰氯/明胶甲基丙烯酰氯水凝胶加上丙烯酸酯功能化纳米二氧化硅制作的新型光交联杂化水凝胶,可以负载人类脂肪衍生干细胞并促进其向软骨分化。YANG等[35]将一氧化碳释放分子401表面包裹上叶酸修饰后的透明质酸,构建了一种基于多肽树突状分子纳米水凝胶的多功能抗炎药物,这种抗炎纳米复合水凝胶能够靶向滑膜巨噬细胞抑制细胞增殖、消耗骨关节内活性氧成分,有效抑制白细胞介素1β、白细胞介素6和肿瘤坏死因子α的分泌,诱导血红素加氧酶的活化、下调P38丝裂原活化蛋白激酶、核因子激活的B细胞的κ-轻链增强和Toll样受体2的表达。无机纳米颗粒复合水凝胶的应用见表1。"
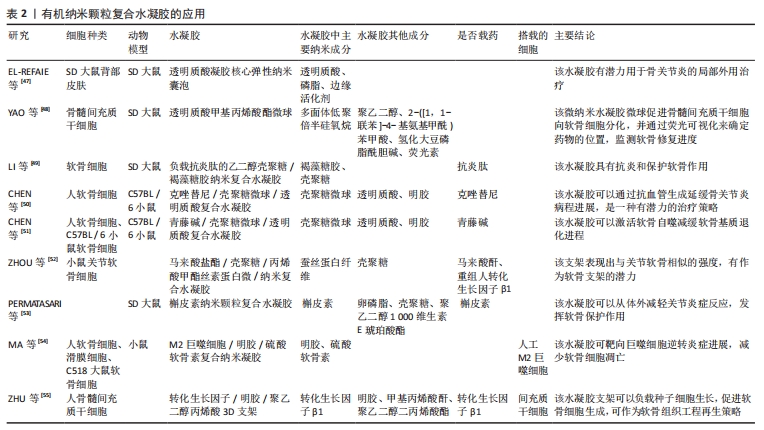
(2)有机纳米颗粒复合水凝胶:现有基于骨关节炎研究的有机纳米复合水凝胶材料包括透明质酸、壳聚糖、槲皮素纳米颗粒、人工巨噬细胞、转化生长因子等。透明质酸是一种大量存在于组织和体液中的多糖类物质,携带有大量的水分,具有保湿、润滑、调节渗透压、促进细胞修复等重要功能,其高溶液黏度和独特的黏弹性使其成为骨关节炎软骨修复的热点[36-38]。研究表明,将外源性透明质酸注入关节腔,既可直接弥补关节腔内的透明质酸,同时也可刺激滑膜细胞增加透明质酸的合成,增加关节润滑功能[39]。壳聚糖及其衍生物作为一种优良的递送系统被广泛用于诊断和治疗研究,壳聚糖不仅可以形成良好的支架结构用于组织再生研究,也可通过乳化交联、蒸发溶剂、喷雾干燥等方法制备成壳聚糖微球[40],随后在微球表面接入功能基团,以吸附或包裹的方式灵活负载不同药物。槲皮素是一种黄酮类化合物,具有良好的抗氧化和抗炎特性[41],可以通过抑制软骨细胞的炎症和凋亡等发挥抗骨关节炎作用[42],但存在着稳定性差和易降解、水溶性不足等缺陷,将其制备成纳米颗粒可有效提高其利用度。巨噬细胞在骨关节炎中发挥着重要作用,促炎M1型巨噬细胞和抗炎M2型巨噬细胞之间的失衡始终伴随着骨关节炎的炎症进程[43-45]。而随着关节软骨被破坏,新的软骨将由间充质干细胞分化而来[10],转化生长因子β1可促进其向软骨细胞分化、加速软骨的修复[46]。 EL-REFAIE等[47]以1%透明质酸为原料制备了一种新型的自组装透明质酸凝胶核心弹性纳米囊泡复合水凝胶,用于无创透皮传递透明质酸,体内研究显示,与普通透明质酸凝胶相比,制作的弹性纳米囊泡复合水凝胶对膝关节的透皮渗透增加了6倍。YAO等[48]以巯基多面体低聚倍半硅氧烷为纳米构建平台,将聚乙二醇、2-([1,1-联苯]-4-基氨基甲酰)苯甲酸、氢化大豆磷脂酰胆碱和荧光素连接,构建具有荧光可视化的软骨修复纳米材料,再利用微流控技术制备载此种纳米材料的微流控透明质酸甲基丙烯酸酯微球,将最终得到的纳米复合水凝胶用于关节腔的原位注射,这种材料在关节空间形成缓冲润滑层,减少了关节软骨之间的摩擦,同时通过电磁力将包裹着带正电荷的巯基多面体低聚倍半硅氧烷/聚乙二醇/苯甲酸/氢化大豆磷脂酰胆碱纳米颗粒释放到深层软骨中,促进骨髓间充质干细胞向软骨细胞分化,并通过荧光可视化来确定药物的位置,了解软骨的修复进度。LI等[49]制备出一种负载抗炎肽的乙二醇壳聚糖/岩藻糖丹纳米复合水凝胶,评估了该水凝胶对白细胞介素1β刺激大鼠软骨细胞的抗炎作用和软骨保护作用,结果表明该水凝胶不仅能抑制炎症因子白细胞介素6和肿瘤坏死因子α的表达,还能增强软骨形成标志物Ⅱ型胶原、蛋白聚糖和Sox9基因的表达;进一步在大鼠骨关节炎模型中发现,关节内注射该水凝胶可以减少糖胺聚糖的损失、降低炎症细胞因子的释放水平,micro-CT定量检测也验证了该水凝胶可以减少骨赘形成、增加骨小梁数量、提高胫骨软骨下骨密度、增加骨体积/总体积的比例。CHEN等[50]在研究壳聚糖微球和光交联水凝胶包裹的克唑替尼降低软骨血管生成协同治疗效果时发现,克唑替尼可以通过促进抗血管生成和阻碍细胞外信号调节激酶1/2信号通路来软骨基质降解。CHEN等[51]研究壳聚糖微球和光交联甲基丙烯酸明胶水凝胶包裹的青藤碱对促进软骨细胞自噬的协同治疗作用,结果表明,此种水凝胶可以通过激活软骨自噬来改善白细胞介素1β引起的病理改变,部分改善软骨基质的退化。ZHOU等[52]合成了具有乙烯基的天然聚合物,即马来酸盐壳聚糖和甲基丙烯酸化丝素蛋白微/纳米颗粒,将马来酸盐壳聚糖和甲基丙烯酸化丝素蛋白微/纳米颗粒在水溶液中光交联制备马来酸盐壳聚糖/甲基丙烯酸化丝素蛋白微纳米复合水凝胶,且当马来酸盐壳聚糖含量为0.1%时,水凝胶的压缩模量为(0.32±0.07) MPa,这正相当于关节软骨的压缩模量。随后将负载了转化生长因子β1微纳米复合水凝胶与小鼠关节软骨细胞联合培养,结果显示这种微纳米复合水凝胶有助于软骨细胞的生长。Permatasari等[53]分析了槲皮素纳米颗粒外用凝胶的制备及其对骨关节炎的治疗作用,结果表明,槲皮素纳米凝胶在组织学上具有减轻炎症、预防软骨损伤的作用,相较于关节内注射给药系统具有创伤更小的优势。MA等[54]将巨噬细胞膜作为“壳”、炎症反应纳米凝胶作为“蛋黄”,应用明胶和硫酸软骨素通过离子键和氢键的物理相互作用制备了具有蛋黄壳结构的人工M2巨噬细胞纳米水凝胶,使其在炎症急性发作时实现爆发释放下调炎症反应,在低炎症活动时实现持续缓慢释放以修复软骨。ZHU等[55]通过核壳电喷雾技术将转化生长因子β1嵌入可打印树脂纳米球中,再利用桌面立体平版3D生物打印机来制造一种新型的细胞负载软骨组织结构纳米复合水凝胶,结果显示转化生长因子β1包埋在纳米球中可持续释放21 d,并促进包埋间充质干细胞的软骨分化。有机纳米颗粒复合水凝胶的应用见表2。"

(3)纳米纤维复合水凝胶:纳米纤维的应用可以增强水凝胶的力学性能,用于水凝胶支架的制备,其常用的材料有海藻酸盐、聚乙二醇、羟基磷灰石、聚己内酯、明胶等。海藻酸盐是制作水凝胶的常用材料,修饰后的海藻酸盐复合支架具有优异的软骨修复能力[56]。羟基磷灰石是一种典型的生物活性材料,骨质的主要无机质成分是羟基磷灰石,将羟基磷灰石植入体内有助于新的成骨生成[57]。 FORMICA等[58]用一种聚阴离子功能化海藻酸盐来模拟天然细胞外基质的糖胺聚糖成分,创建出稳定、超多孔和亲水的纳米纤维网络,然后将软骨细胞/海藻酸盐溶液渗透到纤维网中,利用静电纺丝组件负载地塞米松增强该系统,最终得到既具有诱导软骨分化又兼抗炎作用的纳米复合水凝胶支架。聚乙二醇是广泛应用在医药和生物材料领域的聚合物,其表面的氢键能更好地结合水分子,通过不同制备方法可得到不同的聚乙二醇衍生物。GUNES等[59]采用聚乙二醇二缩水甘油醚制备了聚(3-羟基丁酸-co-3-羟基戊酸 )纳米纤维增强羧甲基壳聚糖/丝素水凝胶复合支架材料,当聚乙二醇二缩水甘油醚与纳米纤维聚合物含量相同时,这种纳米复合水凝胶的含水量(91.4±0.7)%和抗压强度(457±85) kPa与正常软骨相当,并且能够支持骨髓间充质干细胞向软骨细胞分化。MOEINZADEH等[60]将定向羟基磷灰石纳米纤维平行于凝胶表面方向加入,结果显示加入了定向纳米纤维的水凝胶可以促进人间充质干细胞浅表区软骨分化中Ⅱ型胶原蛋白的表达。除了间充质干细胞,脂肪来源干细胞也被常用作软骨细胞再生的来源细胞。NAJAFI等[61]将脂肪来源干细胞封装在硫酸盐海藻酸盐水凝胶中,然后将它们添加到聚己内酯/明胶静电纺丝纳米纤维和细胞外基质粉末中以模拟软骨结构和特征,得到细胞外基质含量为4%的3层复合软骨支架材料,用于促进软骨的再生。纳米纤维复合水凝胶的应用见表3。"

| [1] 杨连甲,唐农轩.骨科临床病理学[M].西安:世界图书出版西安有限公司, 2020:150. [2] 汪国翔,章晓云.骨关节炎病变过程中炎症细胞因子及相关信号通路的作用机制[J].中国组织工程研究,2021,25(14):2266-2273. [3] 易南星,梁倩倩,张伟强,等.骨性关节炎相关滑膜炎症研究进展[J].昆明医科大学学报,2019,40(3):136-139. [4] 李元城,张卫国.关节软骨损伤治疗进展[J].解放军医学杂志,2013,38(5): 423-427. [5] CONAGHAN PG, ARDEN N, AVOUAC B, et al. Safety of Paracetamol in Osteoarthritis: What Does the Literature Say? Drugs Aging. 2019;36(Suppl 1):7-14. [6] MACHADO GC, MAHER CG, FERREIRA PH, et al. Efficacy and safety of paracetamol for spinal pain and osteoarthritis: systematic review and meta-analysis of randomised placebo controlled trials. BMJ. 2015;350:h1225. [7] MELLATI A, HASANZADEH E, GHOLIPOURMALEKABADI M, et al. Injectable nanocomposite hydrogels as an emerging platform for biomedical applications: A review. Mater Sci Eng C Mater Biol Appl. 2021;131:112489. [8] ZHAO H, LIU M, ZHANG Y, et al. Nanocomposite hydrogels for tissue engineering applications. Nanoscale. 2020;12(28):14976-14995. [9] 韩明睿,刘倩倩,孙洋.骨关节炎发病机制及药物调控新进展[J].中国药理学通报,2022,38(6):807-812. [10] LI M, YIN H, YAN Z, et al. The immune microenvironment in cartilage injury and repair. Acta Biomater. 2021;140:23-42. [11] KOEN R. Advanced nanogel engineering for drug delivery. Soft Matter. 2008;5(4): 707-715. [12] 杨洁.基于基因工程多肽杂化纳米水凝胶的制备及在生物医学中的应用[D].武汉:华中科技大学,2016. [13] WANG Y, YUAN K, SHANG Z, et al. Construction of nanohydrogels for enhanced delivery of hydrophilic and hydrophobic drugs and improving chemotherapy efficacy. Eur Polym J. 2023;186:111838-111846. [14] GHIMIRE A, ZORE OZ, THILAKARATHNE VK, et al. “Stable-on-the-Table” Biosensors: Hemoglobin-Poly (Acrylic Acid) Nanogel BioElectrodes with High Thermal Stability and Enhanced Electroactivity. Sensors (Basel). 2015;15(9):23868-23885. [15] 苏日辉,夏凌,李攻科,等.智能水凝胶在分离分析中的应用研究进展[J].分析科学学报,2019,35(3):377-384. [16] ZHANG Y, ZHANG P, GAO X, et al. Preparation of exosomes encapsulated nanohydrogel for accelerating wound healing of diabetic rats by promoting angiogenesis. Mater Sci Eng C Mater Biol Appl. 2021;120:111271. [17] QUAZI MZ, PARK N. DNA Hydrogel-Based Nanocomplexes with Cancer-Targeted Delivery and Light-Triggered Peptide Drug Release for Cancer-Specific Therapeutics. Biomacromolecules. 2023;24(5):2127-2137. [18] THOMAS J, GUPTA N, JOSEPH JP, et al. Mechanical Integrity in a Dynamic Interpenetrating Hydrogel Network of Supramolecular Peptide-Polysaccharide Supports Enhanced Chondrogenesis. ACS Biomater Sci Eng. 2021;7(12):5798-5809. [19] HSU MN, LUO R, KWEK KZ, et al. Sustained release of hydrophobic drugs by the microfluidic assembly of multistage microgel/poly (lactic-co-glycolic acid) nanoparticle composites. Biomicrofluidics. 2015;9(5):052601. [20] SUN P, JIAO J, WANG X, et al. Nanomedicine hybrid and catechol functionalized chitosan as pH-responsive multi-function hydrogel to efficiently promote infection wound healing. Int J Biol Macromol. 2023;238:124106. [21] CORREA S, GROSSKOPF AK, KLICH JH, et al. Injectable liposome-based supramolecular hydrogels for the programmable release of multiple protein drugs. Matter. 2022;5(6):1816-1838. [22] MA L, YE H, LIU L, et al. Polypropylene membranes with high adsorption capacity and anti-adhesion properties achieved by hydrophobic interactions and hydrogen bonded self-assembly for uranium extraction from seawater. Chem Eng J. 2023; 451(P2). doi.org/10.1016/j.cej.2022.138696 [23] OREFFO RO, DRIESSENS FC, PLANELL JA, et al. Growth and differentiation of human bone marrow osteoprogenitors on novel calcium phosphate cements. Biomaterials. 1998;19(20):1845-1854. [24] BOSE S, TARAFDER S. Calcium phosphate ceramic systems in growth factor and drug delivery for bone tissue engineering: A review. Acta Biomater. 2012;8(4): 1401-1421. [25] LIANG H, JIN C, MA L, et al. Accelerated Bone Regeneration by Gold-Nanoparticle-Loaded Mesoporous Silica through Stimulating Immunomodulation. Acs Appl Mater Inter. 2019;11(44):41758-41769. [26] YANG Q, YIN H, XU T, et al. Engineering 2D Mesoporous Silica@MXene-Integrated 3D-Printing Scaffolds for Combinatory Osteosarcoma Therapy and NO-Augmented Bone Regeneration. Small. 2020;16(14):e1906814. [27] ZHOU P, XIA Y, CHENG X, et al. Enhanced bone tissue regeneration by antibacterial and osteoinductive silica-HACC-zein composite scaffolds loaded with rhBMP-2. Biomaterials. 2014;35(38):10033-10045. [28] KIM KJ, JOE YA, KIM MK, et al. Silica nanoparticles increase human adipose tissue-derived stem cell proliferation through ERK1/2 activatio. Int J Nanomed. 2015;10:2261-2272. [29] ANSARI MY, AHMAD N, HAQQI TM. Oxidative stress and inflammation in osteoarthritis pathogenesis: Role of polyphenols. Biomed Pharmacother. 2020; 129:110452. [30] ARRA M, SWARNKAR G, KE K, et al. LDHA-mediated ROS generation in chondrocytes is a potential therapeutic target for osteoarthritis. Nat Commun. 2020;11(1):3427. [31] BOLDUC JA, COLLINS JA, LOESER RF. Reactive Oxygen Species, Aging and Articular Cartilage Homeostasis. Free Radical Bio Med. 2018;132:73-82. [32] MOTTA F, BARONE E, SICA A, et al. Inflammaging and Osteoarthritis. Clin Rev Allerg Immu. 2022;64(2):222-238. [33] YAO H, KANG J, LI W, et al. Novel β-TCP/PVA bilayered hydrogels with considerable physical and bio-functional properties for osteochondral repair. Biomed Mater. 2017;13(1):015012. [34] NEDUNCHEZIAN S, WU C, WU S, et al. Characteristic and Chondrogenic Differentiation Analysis of Hybrid Hydrogels Comprised of Hyaluronic Acid Methacryloyl (HAMA), Gelatin Methacryloyl (GelMA), and the Acrylate-Functionalized Nano-Silica Crosslinker. Polymers-Basel. 2022;14(10):2003. [35] YANG G, GAN M, ZHU J, et al. A multifunctional anti-inflammatory drug that can specifically target activated macrophages, massively deplete intracellular H2O2, and produce large amounts CO for a highly efficient treatment of osteoarthritis. Biomaterials. 2020;255:120155. [36] 凌沛学,梁虹,贺艳丽,等.透明质酸钠在关节疾病中的应用[J].中国修复重建外科杂志,2002,16(1):1-4. [37] 许鹏,王效东,郭雄.透明质酸与骨关节炎[J].中华风湿病学杂志,2002,6(5): 360-363. [38] WANG M, DENG Z, GUO Y, et al. Designing functional hyaluronic acid-based hydrogels for cartilage tissue engineering. Mater Today Bio. 2022;17:100495. [39] 李棋,唐新,裴福兴,等.透明质酸在骨关节疾病中的应用[J].中国组织工程研究与临床康复,2010,14(47):8835-8839. [40] 张越,师宪宪,于奕峰.壳聚糖微球的制备方法研究进展[J].河北科技大学学报,2013,34(5):434-439. [41] 祝珊珊,谭博文,秦飞,等.槲皮素通过PTEN/PI3K/JNK信号通路减轻小鼠RAW264.7巨噬细胞炎症[J]. 中国病理生理杂志,2023,39(3):510-519. [42] HU Y, GUI Z, ZHOU Y, et al. Quercetin alleviates rat osteoarthritis by inhibiting inflammation and apoptosis of chondrocytes, modulating synovial macrophages polarization to M2 macrophages. Free Radical Bio Med. 2019;145(C):146-160. [43] FERNANDES TL, GOMOLL AH, LATTERMANN C, et al. Macrophage: A Potential Target on Cartilage Regeneration. Front Immunol. 2020;11:111. [44] WU CL, HARASYMOWICZ NS, KLIMAK MA, et al. The role of macrophages in osteoarthritis and cartilage repair. Osteoarthr Cartilage. 2020;28(5):544-554. [45] ZHANG L, XING R, HUANG Z, et al. Inhibition of Synovial Macrophage Pyroptosis Alleviates Synovitis and Fibrosis in Knee Osteoarthritis. Mediat Inflamm. 2019; 2019:2165918. [46] YING J, WANG P, ZHANG S, et al. Transforming growth factor-beta1 promotes articular cartilage repair through canonical Smad and Hippo pathways in bone mesenchymal stem cells. Life Sci. 2018;192:84-90. [47] EL-REFAIE WM, ELNAGGAR YSR, EL-MASSIK MA, et al. Novel Self-assembled, Gel-core Hyaluosomes for Non-invasive Management of Osteoarthritis: In-vitro Optimization, Ex-vivo and In-vivo Permeation. Pharm Res. 2015;32(9):2901-2911. [48] YAO Y, WEI G, DENG L, et al. Visualizable and Lubricating Hydrogel Microspheres Via NanoPOSS for Cartilage Regeneration. Adv Sci. 2023;10(15):e2207438. [49] LI T, YANG J, WENG C, et al. Intra-articular injection of anti-inflammatory peptide-loaded glycol chitosan/fucoidan nanogels to inhibit inflammation and attenuate osteoarthritis progression. Int J Biol Macromol. 2021;170:469-478. [50] CHEN P, MEI S, XIA C, et al. The amelioration of cartilage degeneration by photo-crosslinked GelHA hydrogel and crizotinib encapsulated chitosan microspheres. Oncotarget. 2017;8(18):30235-30251. [51] CHEN R, XIA C, MEI S, et al. Intra-articular delivery of sinomenium encapsulated by chitosan microspheres and photo-crosslinked GelMA hydrogel ameliorates osteoarthritis by effectively regulating autophagy. Biomaterials. 2016;81:1-13. [52] ZHOU Y, LIANG K, ZHAO S, et al. Photopolymerized maleilated chitosan/methacrylated silk fibroin micro/nanocomposite hydrogels as potential scaffolds for cartilage tissue engineering. Int J Biol Macromol. 2018;108:383-390. [53] Permatasari DA, Karliana D, Iskandarsyah I, et al. Quercetin prevent proteoglycan destruction by inhibits matrix metalloproteinase-9, matrix metalloproteinase-13, a disintegrin and metalloproteinase with thrombospondin motifs-5 expressions on osteoarthritis model rats. J Adv Pharm Technol. 2019; 10(1):2-8. [54] MA Y, YANG H, ZONG X, et al. Artificial M2 Macrophages for Disease-Modifying Osteoarthritis Therapeutics. Biomaterials. 2021;274:120865. [55] ZHU W, CUI H, OUALAMB B, et al. 3D bioprinting mesenchymal stem cell-laden construct with core–shell nanospheres for cartilage tissue engineering. Nanotechnology. 2018; 29(18):185101. [56] 李诚,郑国爽,蒯贤东,等.海藻酸盐支架修复关节软骨[J].中国组织工程研究,2023,27(7):1080-1088. [57] 王迎军,刘康时.生物医学材料的研究与发展[J].中国陶瓷,1998(5):26-29,37. [58] FORMICA FA, ÖZTÜRK E, HESS SC, et al. A Bioinspired Ultraporous Nanofiber-Hydrogel Mimic of the Cartilage Extracellular Matrix. Adv Healthc Mater. 2016; 5(24):3129-3138. [59] GUNES OC, ALBAYRAK AZ, TASDEMIR S, et al. Wet-electrospun PHBV nanofiber reinforced carboxymethyl chitosan-silk hydrogel composite scaffolds for articular cartilage repair. J Biomater Appl. 2020;35(4-5):515-531. [60] MOEINZADEH S, PAJOUM SHARIATI SR, JABBARI E. Comparative effect of physicomechanical and biomolecular cues on zone-specific chondrogenic differentiation of mesenchymal stem cells. Biomaterials. 2016;92:57-70. [61] NAJAFI R, CHAHSETAREH H, PEZESHKI-MODARESS M, et al. Alginate sulfate/ECM composite hydrogel containing electrospun nanofiber with encapsulated human adipose-derived stem cells for cartilage tissue engineering. Int J Biol Macromol. 2023;238:124098. [62] KLEIN J. Chemistry. Repair or replacement--a joint perspective. Science. 2009; 323(5910):47-48. [63] MOSTAKHDEMIN M, NAND A, RAMEZANI M. A novel assessment of microstructural and mechanical behaviour of bilayer silica-reinforced nanocomposite hydrogels as a candidate for artificial cartilage. J Mech Behav Biomed. 2021;116:104333. [64] GRIMAUDO MA, CONCHEIRO A, ALVAREZ-LORENZO C. Nanogels for regenerative medicine. J Control Release. 2019;313:148-160. [65] CAUSA F, NETTI PA, AMBROSIO L. A multi-functional scaffold for tissue regeneration: The need to engineer a tissue analogue. Biomaterials. 2007;28(34):5093-5099. [66] GOLDBERG M, LANGER R, JIA X. Nanostructured materials for applications in drug delivery and tissue engineering. J Biomater Sci Polym Ed. 2007;18(3):241-268. [67] VAN RIJT S, HABIBOVIC P. Enhancing regenerative approaches with nanoparticles. J R Soc Interface. 2017;14(129):20170093. [68] FARR J, GRACITELLI GC, SHAH N, et al. High Failure Rate of a Decellularized Osteochondral Allograft for the Treatment of Cartilage Lesions. Am J Sports Med. 2016;44(8):2015-2022. [69] GUO T, TIAN X, LI B, et al. Repair of articular cartilage and subchondral defects in rabbit knee joints with a polyvinyl alcohol/nano-hydroxyapatite/polyamide 66 biological composite material. J Orthop Surg Res. 2017;12(1):176. [70] YANG J, ZHANG YS, YUE K, et al. Cell-laden hydrogels for osteochondral and cartilage tissue engineering. Acta Biomater. 2017;57:1-25. [71] YANG Z, YI P, LIU Z, et al. Stem Cell-Laden Hydrogel-Based 3D Bioprinting for Bone and Cartilage Tissue Engineering. Front Bioeng Biotech. 2022;10:865770. |
| [1] | Li Yongjie, Fu Shenyu, Xia Yuan, Zhang Dakuan, Liu Hongju. Correlation of knee extensor muscle strength and spatiotemporal gait parameters with peak knee flexion/adduction moment in female patients with knee osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(9): 1354-1358. |
| [2] | Qi Haodong, Lu Chao, Xu Hanbo, Wang Mengfei, Hao Yangquan. Effect of diabetes mellitus on perioperative blood loss and pain after primary total knee arthroplasty [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(9): 1383-1387. |
| [3] | Du Changling, Shi Hui, Zhang Shoutao, Meng Tao, Liu Dong, Li Jian, Cao Heng, Xu Chuang. Efficacy and safety of different applications of tranexamic acid in high tibial osteotomy [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(9): 1409-1413. |
| [4] | Yu Weijie, Liu Aifeng, Chen Jixin, Guo Tianci, Jia Yizhen, Feng Huichuan, Yang Jialin. Advantages and application strategies of machine learning in diagnosis and treatment of lumbar disc herniation [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(9): 1426-1435. |
| [5] | Chen Kaijia, Liu Jingyun, Cao Ning, Sun Jianbo, Zhou Yan, Mei Jianguo, Ren Qiang. Application and prospect of tissue engineering in treatment of osteonecrosis of the femoral head [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(9): 1450-1456. |
| [6] | Bai Chen, Yang Wenqian, Meng Zhichao, Wang Yuze. Strategies for repairing injured anterior cruciate ligament and promoting graft healing [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(9): 1457-1463. |
| [7] | Huang Xiarong, Hu Lizhi, Sun Guanghua, Peng Xinke, Liao Ying, Liao Yuan, Liu Jing, Yin Linwei, Zhong Peirui, Peng Ting, Zhou Jun, Qu Mengjian. Effect of electroacupuncture on the expression of P53 and P21 in articular cartilage and subchondral bone of aged rats with knee osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(8): 1174-1179. |
| [8] | Zhao Garida, Ren Yizhong, Han Changxu, Kong Lingyue, Jia Yanbo. Mechanism of Mongolian Medicine Erden-uril on osteoarthritis in rats [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(8): 1193-1199. |
| [9] | Li Rui, Zhang Guihong, Wang Tao, Fan Ping. Effect of ginseng polysaccharide on the expression of prostaglandin E2/6-keto-prostaglandin 1alpha in traumatic osteoarthritis model rats [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(8): 1235-1240. |
| [10] | Lin Zeyu, Xu Lin. Research progress in gout-induced bone destruction mechanism [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(8): 1295-1300. |
| [11] | Ma Shuwei, He Sheng, Han Bing, Zhang Liaoyun. Exosomes derived from mesenchymal stem cells in treatment of animals with acute liver failure: a meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(7): 1137-1142. |
| [12] | Zhang Kefan, Shi Hui. Research status and application prospect of cytokine therapy for osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(6): 961-967. |
| [13] | Zhang Zeyi, Yang Yimin, Li Wenyan, Zhang Meizhen. Effect of foot progression angle on lower extremity kinetics of knee osteoarthritis patients of different ages: a systematic review and meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(6): 968-975. |
| [14] | Li Jiaqi, Huang Yuanli, Li Yan, Wang Chunren, Han Qianqian. Mechanism and influencing factors in molecular weight degradation of non-cross-linked hyaluronic acid [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(5): 747-752. |
| [15] | Xu Rong, Wang Haojie, Geng Mengxiang, Meng Kai, Wang Hui, Zhang Keqin, Zhao Huijing. Research advance in preparation and functional modification of porous polytetrafluoroethylene artificial blood vessels [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(5): 759-765. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||