Chinese Journal of Tissue Engineering Research ›› 2025, Vol. 29 ›› Issue (12): 2429-2437.doi: 10.12307/2025.383
Previous Articles Next Articles
Liquiritin inhibits osteoclast differentiation and alleviates bone loss
Zhang Wensheng1, 2, 3, 4, Guo Haiwei1, 2, 3, 4, Weng Rui1, 2, 3, 4, Mo Ling1, 2, 3, 4, Song Zhenjie1, 2, 3, 4, Tian Han2, 3, 4, Zhong Yelin2, 3, 4, Wang Yuancheng2, 3, 4, Tang Hanwu1, 2, 3, 4, Liu Caijun1, 2, 3, 4, Yuan Chao1, 2, 3, 4, Li Ying1, 2, 3, 4
- 1Guangdong Provincial Traditional Chinese Medicine Orthopedic Research Institute, Guangzhou 510240, Guangdong Province, China; 2Third Affiliated Hospital of Guangzhou University of Chinese Medicine, Guangzhou 510240, Guangdong Province, China; 3Guangzhou University of Chinese Medicine, Guangzhou 510006, Guangdong Province, China; 4Lingnan Medical Research Center, Guangzhou 510006, Guangdong Province, China
-
Received:2024-04-07Accepted:2024-06-01Online:2025-04-28Published:2024-09-09 -
Contact:Li Ying, MD, Chief physician, Doctoral supervisor, Guangdong Provincial Traditional Chinese Medicine Orthopedic Research Institute, Guangzhou 510240, Guangdong Province, China; Third Affiliated Hospital of Guangzhou University of Chinese Medicine, Guangzhou 510240, Guangdong Province, China; Guangzhou University of Chinese Medicine, Guangzhou 510006, Guangdong Province, China; Lingnan Medical Research Center, Guangzhou 510006, Guangdong Province, China -
About author:Zhang Wensheng, Master, Attending physician, Guangdong Provincial Traditional Chinese Medicine Orthopedic Research Institute, Guangzhou 510240, Guangdong Province, China; Third Affiliated Hospital of Guangzhou University of Chinese Medicine, Guangzhou 510240, Guangdong Province, China; Guangzhou University of Chinese Medicine, Guangzhou 510006, Guangdong Province, China; Lingnan Medical Research Center, Guangzhou 510006, Guangdong Province, China -
Supported by:the Natural Science Foundation of Guangdong Province (General Program), No. 2022A1515011404 (to LY); Open Fund of Guangdong Provincial Traditional Chinese Medicine Orthopedic Research Institute, Nos. GYH202102-04 (to YC) and GYH202101-03 (to LY)
CLC Number:
Cite this article
Zhang Wensheng, Guo Haiwei, Weng Rui, Mo Ling, Song Zhenjie, Tian Han, Zhong Yelin, Wang Yuancheng, Tang Hanwu, Liu Caijun, Yuan Chao, Li Ying. Liquiritin inhibits osteoclast differentiation and alleviates bone loss [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(12): 2429-2437.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
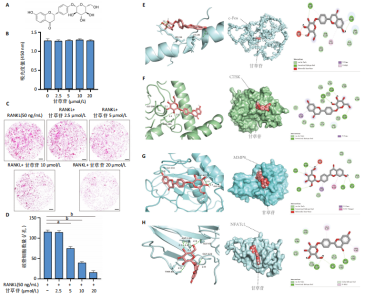
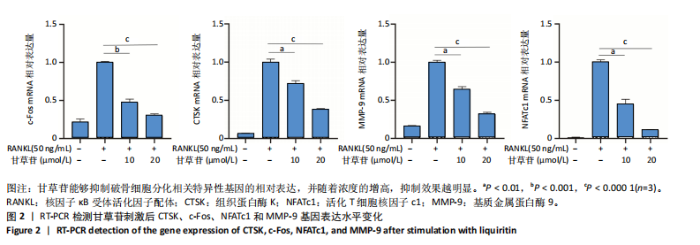
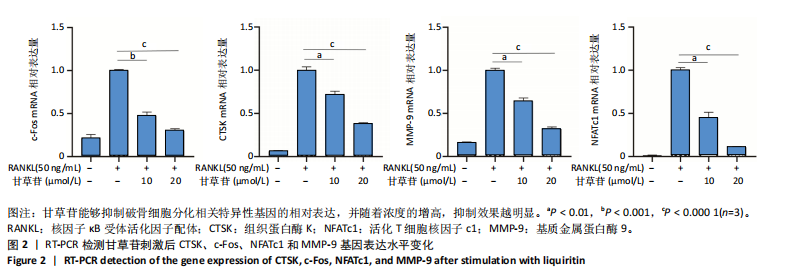
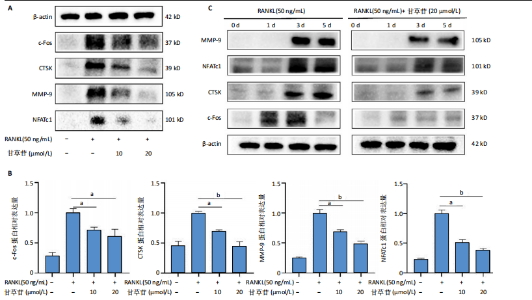
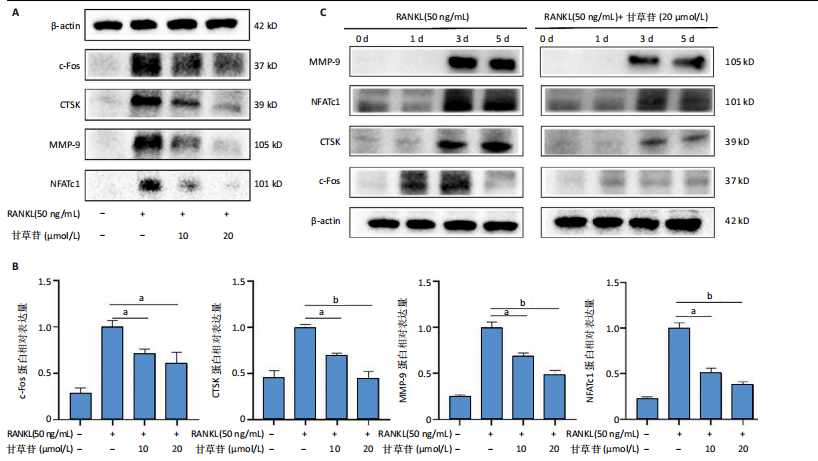
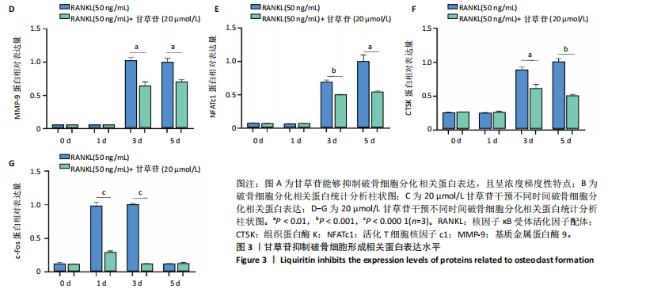
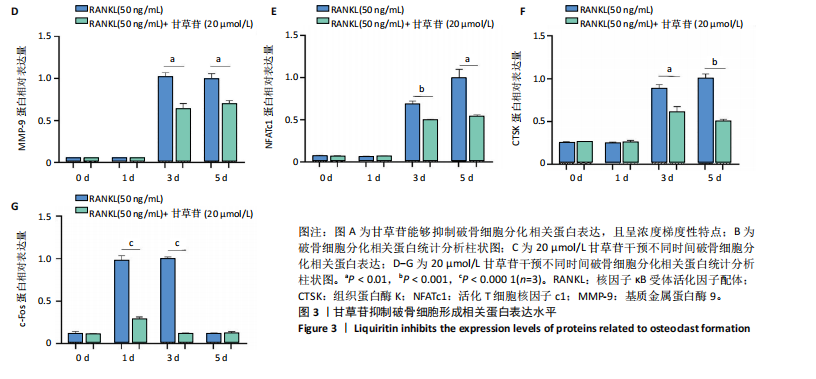
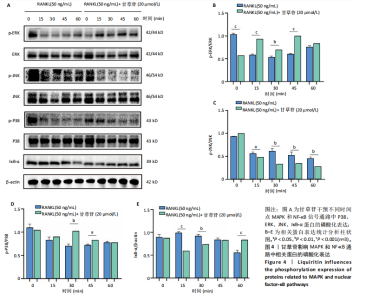
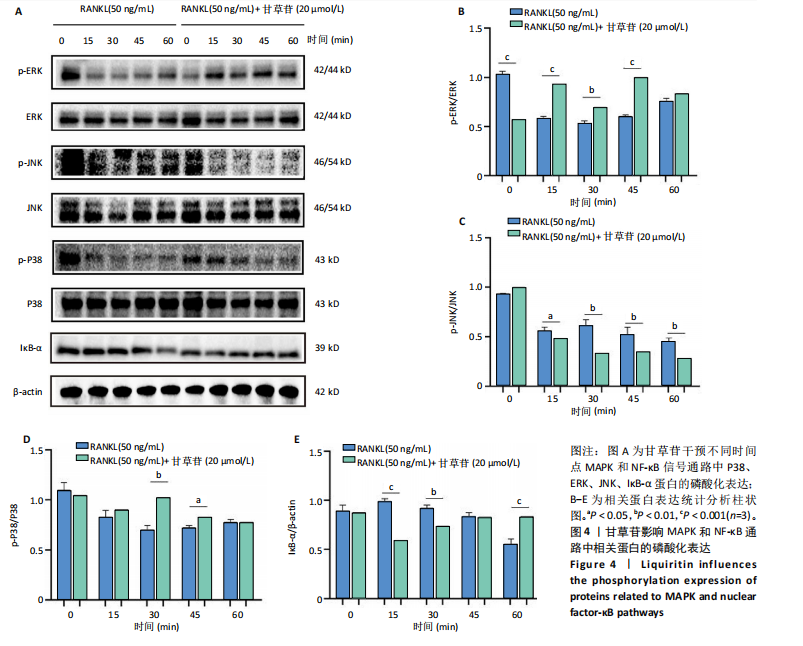
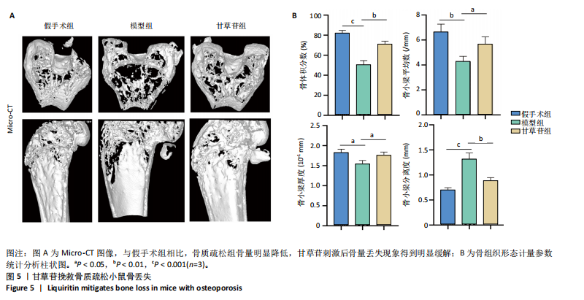
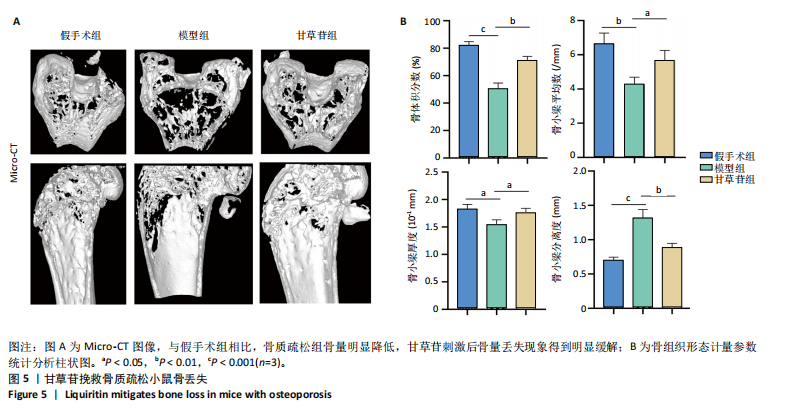
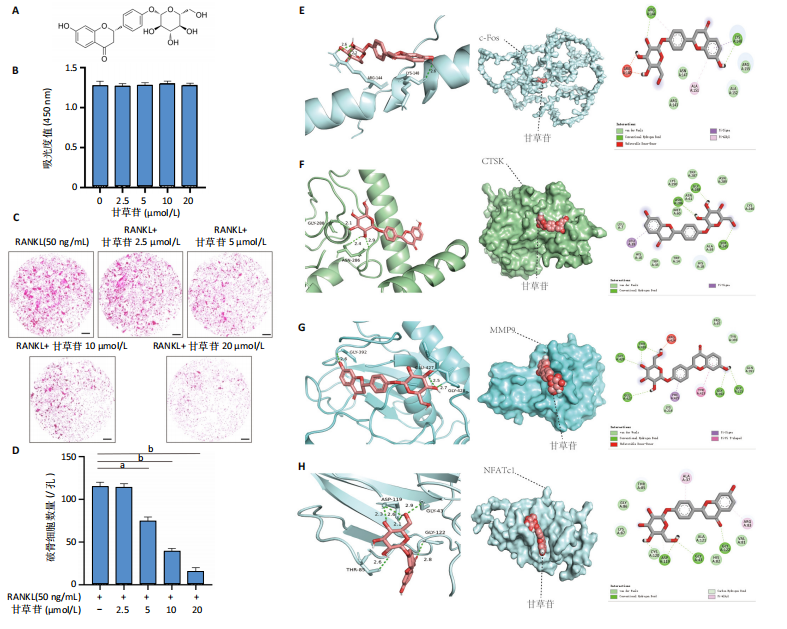
2.1 甘草苷无细胞毒性/增殖作用 甘草苷的化学结构见图1A;CCK-8结果表明,在20 μmol/L及以下浓度甘草苷对骨髓巨噬细胞不具有显著毒性或增殖效应,见图1B。 2.2 甘草苷抑制破骨细胞形成以及与破骨细胞特异性蛋白有高结合力 为了研究甘草苷是否能抑制成熟破骨细胞形成,首先以含有核因子κB受体活化因子配体(50 ng/mL)、巨噬细胞集落刺激因子(50 ng/mL)和不同浓度梯度甘草苷(0,2.5,5,10,20 μmol/L)刺激骨髓巨噬细胞诱导破骨细胞分化。结果显示,甘草苷显著抑制成熟破骨细胞分化,并且随着浓度增加,抑制效果更强,见图1C,D。分子对接技术结果表明甘草苷与活化T细胞核因子c1、组织蛋白酶K、c-Fos、基质金属蛋白酶9均具有较高的亲和力,亲和力分别为-32.76,-32.34,-23.94, -30.66 kJ/mol,同时通过AutoDock软件对结果进行可视化分析,图1E-H分别展示了甘草苷与活化T细胞核因子c1、组织蛋白酶K、c-Fos、基质金属蛋白酶9蛋白的3D对接结构与可视化对接。 2.3 甘草苷能抑制破骨细胞相关基因表达 为了探讨甘草苷在抑制破骨细胞分化中对特异性基因表达的影响,采用RT-PCR检测甘草苷刺激后组织蛋白酶K、c-Fos、活化T细胞核因子c1和基质金属蛋白酶9等基因表达水平的变化,结果显示在甘草苷的作用下,各基因的相对表达水平均呈下降的趋势,浓度越高,下降趋势越明显,见图2。 2.4 甘草苷能抑制破骨细胞相关蛋白表达 如图3A,B所示,在核因子κB受体活化因子配体的刺激下,活化T细胞核因子c1、c-Fos、组织蛋白酶K与基质金属蛋白酶9蛋白表达明显上升,不同浓度甘草苷(10,20 μmol/L)刺激后,破骨细胞分化特异性相关蛋白表达呈浓度梯度性的下降趋势。如图3C-F所示,在核因子κB受体活化因子配体的刺激下,第3,5天时活化T细胞核因子c1、组织蛋白酶K与基质金属蛋白酶9的蛋白表达明显上升,20 μmol/L甘草苷能够降低活化T细胞核因子c1、组织蛋白酶K与基质金属蛋白酶9的蛋白表达。如图3G所示,在核因子κB受体活化因子配体的刺激下,在第1,3天时c-Fos的蛋白表达明显上升,20 μmol/L甘草苷能够降低c-Fos的蛋白表达。 2.5 甘草苷通过MAPK/NF-κB抑制破骨细胞生成 如图4所示,甘草苷能够在第15,30,45,60分钟有效降低JNK的磷酸化表达水平。但对于ERK和P38的磷酸化表达,甘草苷未表现出明显的抑制趋势。在NF-κB通路中,甘草苷在第15,30分钟时无法缓解IκB-α的降解现象,但在第60分钟时,IκB-α的表达呈上升趋势,说明甘草苷在第60分钟时能够挽救该蛋白的降解。 2.6 甘草苷能减少骨丢失 24只小鼠均进入结果分析。Micro-CT结果显示:甘草苷有效缓解了由于破骨细胞过度活跃引起的骨质疏松。与假手术组相比,骨质疏松组显示出骨小梁数量减少和骨密度显著降低,见图5A,甘草苷组这种现象得到了明显缓解,骨密度和骨小梁厚度均增加,骨小梁分离度显著下降,见图5B。"
| [1] BROWN JP. Long-Term Treatment of Postmenopausal Osteoporosis. Endocrinol Metab (Seoul). 2021;36(3):544-552. [2] LANGDAHL BL. Overview of treatment approaches to osteoporosis. Br J Pharmacol. 2021;178(9):1891-1906. [3] CHAN WCW, TAN Z, TO MKT, et al. Regulation and Role of Transcription Factors in Osteogenesis. Int J Mol Sci. 2021;22(11):5445. [4] HONG G, CHEN Z, HAN X, et al. A novel RANKL-targeted flavonoid glycoside prevents osteoporosis through inhibiting NFATc1 and reactive oxygen species. Clin Transl Med. 2021;11(5):e392. [5] CLYNES MA, HARVEY NC, CURTIS EM, et al. The epidemiology of osteoporosis. Br Med Bull. 2020;133(1):105-117. [6] QIN J, CHEN J, PENG F, et al. Pharmacological activities and pharmacokinetics of liquiritin: A review. J Ethnopharmacol. 2022;293: 115257. [7] YANG X, DANG X, ZHANG X, et al. Liquiritin reduces lipopolysaccharide-aroused HaCaT cell inflammation damage via regulation of microRNA-31/MyD88. Int Immunopharmacol. 2021;101(Pt B):108283. [8] 张建新,杨孝来.甘草苷抑制吗啡引起的神经损伤作用及机制[J]中国临床药理学与治疗学,2024,29(5):554-560. [9] QIU M, CHENG L, XU J, et al. Liquiritin reduces chondrocyte apoptosis through P53/PUMA signaling pathway to alleviate osteoarthritis. Life Sci. 2024;343:122536. [10] GUO D, WANG Q, LI A, et al. Liquiritin targeting Th17 cells differentiation and abnormal proliferation of keratinocytes alleviates psoriasis via NF-κB and AP-1 pathway. Phytother Res. 2024;38(1): 174-186. [11] ZHOU H, YANG T, LU Z, et al. Liquiritin exhibits anti-acute lung injury activities through suppressing the JNK/Nur77/c-Jun pathway. Chin Med. 2023;18(1):35. [12] WANG R, CHEN Y, WANG Z, et al. Antidepressant effect of licorice total flavonoids and liquiritin: A review. Heliyon. 2023;9(11):e22251. [13] XIA X, ZHANG Y, ZHU L, et al. Liquiritin apioside alleviates colonic inflammation and accompanying depression-like symptoms in colitis by gut metabolites and the balance of Th17/Treg. Phytomedicine. 2023;120:155039. [14] IANTOMASI T, ROMAGNOLI C, PALMINI G, et al. Oxidative Stress and Inflammation in Osteoporosis: Molecular Mechanisms Involved and the Relationship with microRNAs. Int J Mol Sci. 2023;24(4):3772. [15] WU D, CLINE-SMITH A, SHASHKOVA E, et al. T-Cell Mediated Inflammation in Postmenopausal Osteoporosis. Front Immunol. 2021; 12:687551. [16] ROTTA D, FASSIO A, ROSSINI M, et al. Osteoporosis in Inflammatory Arthritides: New Perspective on Pathogenesis and Treatment. Front Med (Lausanne). 2020;7:613720. [17] DENG W, HUANG Y, LI H, et al. Dehydromiltirone inhibits osteoclast differentiation in RAW264.7 and bone marrow macrophages by modulating MAPK and NF-κB activity. Front Pharmacol. 2022;13: 1015693. [18] CHEN K, LIAO S, LI Y, et al. Osteoblast-derived EGFL6 couples angiogenesis to osteogenesis during bone repair. Theranostics. 2021; 11(20):9738-9751. [19] YAN C, ZHANG J, AN F, et al. Research Progress of Ferroptosis Regulatory Network and Bone Remodeling in Osteoporosis. Front Public Health. 2022;10:910675.
[20] FISCHER V, HAFFNER-LUNTZER M. Interaction between bone and immune cells: Implications for postmenopausal osteoporosis. Semin Cell Dev Biol. 2022;123:14-21. [21] 吴凤爱,逯青霞,侯贵明,等.甘草苷对血管紧张素Ⅱ诱导的心肌细胞肥大的影响[J].中国临床药理学杂志,2023,39(19):2780-2784. [22] 曲欣妮,李文标,王利,等.基于网络药理学及实验验证探讨甘草苷治疗帕金森病的作用机制[J].世界科学技术-中医药现代化, 2023,25(5):1689-1701. [23] LIU C, YUAN D, ZHANG C, et al. Liquiritin Alleviates Depression-Like Behavior in CUMS Mice by Inhibiting Oxidative Stress and NLRP3 Inflammasome in Hippocampus. Evid Based Complement Alternat Med. 2022;2022:7558825. [24] THU VT, YEN NTH, LY NTH. Liquiritin from Radix Glycyrrhizae Protects Cardiac Mitochondria from Hypoxia/Reoxygenation Damage. J Anal Methods Chem. 2021;2021:1857464. [25] IBÁÑEZ L, NÁCHER-JUAN J, TERENCIO MC, et al. Osteostatin Inhibits M-CSF+RANKL-Induced Human Osteoclast Differentiation by Modulating NFATc1. Int J Mol Sci. 2022;23(15):8551. [26] UDAGAWA N, KOIDE M, NAKAMURA M, et al. Osteoclast differentiation by RANKL and OPG signaling pathways. J Bone Miner Metab. 2021; 39(1):19-26. [27] YANG Y, LIU Z, WU J, et al. Nrf2 Mitigates RANKL and M-CSF Induced Osteoclast Differentiation via ROS-Dependent Mechanisms. Antioxidants (Basel). 2023;12(12):2094. [28] FAN J, XU C, SHI H, et al. Hesperetin affects osteoclast differentiation via MAPK signaling pathway. Adv Clin Exp Med. 2023 Dec 12. doi: 10.17219/acem/174393. [29] KOGA Y, TSURUMAKI H, AOKI-SAITO H, et al. Roles of Cyclic AMP Response Element Binding Activation in the ERK1/2 and p38 MAPK Signalling Pathway in Central Nervous System, Cardiovascular System, Osteoclast Differentiation and Mucin and Cytokine Production. Int J Mol Sci. 2019;20(6):1346. [30] LI Y, ZHUANG Q, TAO L, et al. Urolithin B suppressed osteoclast activation and reduced bone loss of osteoporosis via inhibiting ERK/NF-κB pathway. Cell Prolif. 2022;55(10):e13291. [31] CHOI EB, AGIDIGBI TS, KANG IS, et al. ERK Inhibition Increases RANKL-Induced Osteoclast Differentiation in RAW 264.7 Cells by Stimulating AMPK Activation and RANK Expression and Inhibiting Anti-Osteoclastogenic Factor Expression. Int J Mol Sci. 2022;23(21):13512. [32] FUJII S, ISHIBASHI T, KOKURA M, et al. RAF1-MEK/ERK pathway-dependent ARL4C expression promotes ameloblastoma cell proliferation and osteoclast formation. J Pathol. 2022;256(1):119-133. [33] DONG Y, SONG K, WANG P, et al. Blocking the cytohesin-2/ARF1 axis by SecinH3 ameliorates osteoclast-induced bone loss via attenuating JNK-mediated IRE1 endoribonuclease activity. Pharmacol Res. 2022; 185:106513. [34] MA Z, YU R, ZHAO J, et al. Constant hypoxia inhibits osteoclast differentiation and bone resorption by regulating phosphorylation of JNK and IκBα. Inflamm Res. 2019;68(2):157-166. [35] YANG J, ZHANG L, DING Q, et al. Flavonoid-Loaded Biomaterials in Bone Defect Repair. Molecules. 2023;28(19):6888. [36] CHENG LY, JIN XL, CHEN XW, et al. Icariin inhibits thioacetamide-induced osteoclast differentiation through RANKL-p38/ERK-NFAT pathway. Zhongguo Zhong Yao Za Zhi. 2022;47(21):5882-5889. [37] SHEN Y, WANG Z, TAN J, et al. TRAF6/ERK/p38 pathway is involved in interleukin-17-mediated autophagy to promote osteoclast precursor cell differentiation. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2021;50(2): 162-170. [38] GAO Y, CHEN N, FU Z, et al. Progress of Wnt Signaling Pathway in Osteoporosis. Biomolecules. 2023;13(3):483. [39] XUE C, LUO H, WANG L, et al. Aconine attenuates osteoclast-mediated bone resorption and ferroptosis to improve osteoporosis via inhibiting NF-κB signaling. Front Endocrinol (Lausanne). 2023;14:1234563. [40] JI H, PAN Q, CAO R, et al. Garcinone C attenuates RANKL-induced osteoclast differentiation and oxidative stress by activating Nrf2/HO-1 and inhibiting the NF-kB signaling pathway. Heliyon. 2024;10(3): e25601. [41] CHANG J, WANG Z, TANG E, et al. Inhibition of osteoblastic bone formation by nuclear factor-kappaB. Nat Med. 2009;15(6):682-689. [42] YASUDA H. Discovery of the RANKL/RANK/OPG system. J Bone Miner Metab. 2021;39(1):2-11. [43] HONG G, ZHOU L, ZHENG G, et al. A novel Glycyrrhiza glabra extract liquiritin targeting NFATc1 activity and ROS levels to counteract ovariectomy-induced osteoporosis and bone loss in murine model. Front Pharmacol. 2023;14:1287827. |
| [1] | Chen Yueping, Chen Feng, Peng Qinglin, Chen Huiyi, Dong Panfeng . Based on UHPLC-QE-MS, network pharmacology, and molecular dynamics simulation to explore the mechanism of Panax notoginseng in treating osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1751-1760. |
| [2] | Chen Shuai, Jin Jie, Han Huawei, Tian Ningsheng, Li Zhiwei . Causal relationship between circulating inflammatory cytokines and bone mineral density based on two-sample Mendelian randomization [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1556-1564. |
| [3] | Yang Lei, Liu Sanmao, Sun Huanwei, Che Chao, Tang Lin. Comparison of six machine learning models suitable for use in medicine: support for osteoporosis screening and initial diagnosis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(35): 7499-7510. |
| [4] | Fang Yuan, Qian Zhiyong, He Yuanhada, Wang Haiyan, Sha Lirong, Li Xiaohe, Liu Jing, He Yachao, Zhang Kai, Temribagen. Mechanism of Mongolian medicine Echinops sphaerocephalus L. in proliferation and angiogenesis of vascular endothelial cells [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(35): 7519-7528. |
| [5] | Jiang Qiyu, Zeng Huiyan. A novel analysis and prediction method for potential mechanisms of traditional Chinese medicine based on artificial intelligence and omics data-driven approach [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(35): 7552-7561. |
| [6] |
Li Zhipeng, Xing Rongxin, Hu Lianghong.
Roles of SOX5 in bone metabolism and prevention of bone diseases and the relationship with exercise#br#
#br#
[J]. Chinese Journal of Tissue Engineering Research, 2025, 29(35): 7589-7600.
|
| [7] | Wang Tong, Zheng Yu, Jia Chengming, Yang Hu, Zhang Guangfei, Ji Yaoyao. Action mechanism of Gegenmaqi prescription in treatment of periarthritis of shoulder combined with type 2 diabetes based on TCMSP database [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(35): 7669-7678. |
| [8] | Zhang Xin, Guo Baojuan, Xu Huixin, Shen Yuzhen, Yang Xiaofan, Yang Xufang, Chen Pei. Protective effects and mechanisms of 3-N-butylphthalide in Parkinson’s disease cell models [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(30): 6466-6473. |
| [9] | Wu Wangxiang, Ran Dongcheng, Xu Jiamu, Xu Jiafu, Chen Jingjing, Wang Chunqing. Long noncoding RNAs related to osteoporosis: current research status and developmental trends [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(29): 6360-6368. |
| [10] | Li Su, Wang Qinghua, Da Mengting, Yang Rui, Chen Daozhen. Action mechanism by which gambogic acid down-regulates expression of protein C receptor to kill triple negative breast cancer stem cells [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(23): 4888-4898. |
| [11] | Ji Mingyi, Ji Xinyi, Xu Junfeng. Mechanism by which melatonin promotes bone regeneration and its application in oral implantation [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(18): 3868-3876. |
| [12] | Lyu Hao, Zhang Ge, Hu Zhimu, Wang Yan, Chu Qingsong, Zhou Yao, Jiang Ting, Wang Jiuxiang. Inflammation, metabolites and osteoporosis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(17): 3697-3704. |
| [13] | Lai Yue, Lin Xuan, Xu Miao, Liu Huan, Shen Jianlin, Huang Wenhua. Medication pattern and mechanism of marine traditional Chinese medicine in the treatment of osteoporosis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(17): 3713-3723. |
| [14] | Gu Yufeng, Deng Bingying, Li Niren, Zeng Yixuan, Lu Sifan, Zhu Chen, Chen Lei, Liu Yi. Mechanisms of total flavonoids from Sophora flavescens for the treatment of non-alcoholic fatty liver disease and experimental validation in zebrafish [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(14): 2969-2978. |
| [15] | Chen Xinfei, Dai Yahui, Xie Bingying, Huang Xiaobin, Huang Huimin, Huang Jingwen, Li Shengqiang, Ge Jirong. Metabolomics analysis of the lumbar spine after alendronate sodium intervention in ovariectomized rats with osteoporosis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(11): 2277-2284. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||