Chinese Journal of Tissue Engineering Research ›› 2025, Vol. 29 ›› Issue (18): 3868-3876.doi: 10.12307/2025.669
Previous Articles Next Articles
Mechanism by which melatonin promotes bone regeneration and its application in oral implantation
Ji Mingyi1, Ji Xinyi2, Xu Junfeng3
- 1Zhejiang Chinese Medical University School of Stomatology, Hangzhou 310053, Zhejiang Province, China; 2Department of Stomatology, Kunming Medical University Haiyuan College, Kunming 650106, Yunnan Province, China; 3Department of Stomatology, Tongde Hospital, Hangzhou 310012, Zhejiang Province, China
-
Received:2024-07-11Accepted:2024-08-21Online:2025-06-28Published:2024-11-29 -
Contact:Xu Junfeng, MS, Master’s supervisor, Chief physician, Department of Stomatology, Tongde Hospital, Hangzhou 310012, Zhejiang Province, China -
About author:Ji Mingyi, Master candidate, Physician, Zhejiang Chinese Medical University School of Stomatology, Hangzhou 310053, Zhejiang Province, China
CLC Number:
Cite this article
Ji Mingyi, Ji Xinyi, Xu Junfeng. Mechanism by which melatonin promotes bone regeneration and its application in oral implantation[J]. Chinese Journal of Tissue Engineering Research, 2025, 29(18): 3868-3876.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
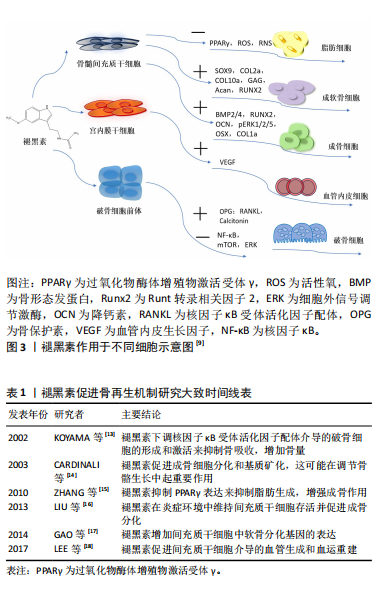
2.1 褪黑素促进骨再生 2.1.1 褪黑素对成骨细胞的作用机制 褪黑素在骨髓间充质干细胞向成骨细胞谱系的分化中起着至关重要的作用。通过激活磷脂酰肌醇3-激酶/蛋白激酶B(phosphatidylinositide 3-kinases/protein kinase B,PI3K/AKT)、骨形态发生蛋白/Smad和Wnt/β-连环蛋白(β-catenin)信号通路,褪黑素诱导成骨细胞分化,增加成骨转录因子Runt转录相关因子2(Runt-related transcription factor 2,Runx2)、Dlx5和osterix的表达水平,并减少如过氧化物酶体增殖物激活受体γ(peroxisome proliferator-activated receptor γ,PPARγ)这类成脂标记物产生,而这可以改善骨骼的数量和质量[19-20]。血小板衍生生长因子/AKT信号通路也参与成骨细胞的分化[21]。在骨折部位,褪黑素上调神经肽Y和神经肽受体Y1的表达,促进骨髓间充质干细胞向成骨细胞分化[22]。褪黑素也分别与骨形态发生蛋白4、骨形态发生蛋白9联合使用,刺激碱性磷酸酶活性和基质矿化[23-24]。软骨内成骨是骨形成的一种重要形式,褪黑素具有增加骨髓间充质干细胞中软骨分化基因表达的作用[17]。 褪黑素对成骨细胞的双重作用具有浓度依赖性。低浓度褪黑素(1 μmol/L)促进成骨细胞的增殖分化,100 μmol/L地塞米松降低小鼠胚胎成骨细胞前体细胞(mouse embryo osteoblast precursor cells,MC3T3-E1)中Runx2的表达,减少成骨细胞的分化和矿化,但在1 μmol/L的褪黑素处理后发生逆转[25]。高浓度的褪黑素(1 mmol/L)抑制细胞外信号调节激酶(extracellular regulated protein kinases,ERK)磷酸化和活化,下调Cyclin D1、CDK4、Cyclin B1和CDK1的基因表达,总体上影响了成骨细胞的增殖[26]。最近发现,高剂量的褪黑素可能是有毒的,并加剧骨质流失[27]。有研究人员认为,高水平的褪黑素通过上调基质相互作用分子1(STIM1)和下调ERK通路来刺激成骨细胞的凋亡[28]。Septin 4对内质网应激诱导和成骨细胞凋亡具有直接影响,较高浓度的褪黑素刺激Septin 4表达,也导致内质网应激积累和成骨细胞减少[29]。Septin 7表达水平与褪黑素浓度呈正相关。实验分析表明,Septin 7通过抑制CHOP和caspase-3凋亡以及抑制内质网应激积累来保护成骨细胞系免受褪黑素诱导的凋亡[30]。 褪黑素在多种病理条件下促进成骨。在缺氧条件下,小鼠胚胎成骨细胞前体细胞(MC3T3-E1)的成骨分化和矿化受到抑制,褪黑素激活 p38丝裂原活化蛋白激酶(p38 mitogen-activated protein kinase,p38 MAPK)和蛋白激酶 D1(Prkd1)信号通路来增强成骨细胞的分化,从而缓解这种情况[31]。铁超载会显著降低骨髓间充质干细胞的成骨分化,但褪黑素可以阻断p53/ERK/p38信号通路的磷酸化和激活来逆转铁失衡效应,从而增强骨髓间充质干细胞分化以及抑制骨丢失,在铁超载诱导的成骨分化功能障碍和衰老中发挥保护作用[32-33]。褪黑素对镉诱导的人骨髓间充质干细胞骨代谢紊乱和骨髓脂肪细胞蓄积也有保护作用[34]。 2.1.2 褪黑素对破骨细胞的作用机制 骨再生的控制不仅需要成骨细胞的调节,还需要破骨细胞的参与。破骨细胞源于造血干细胞,核因子κB受体活化因子配体/核因子κB受体活化因子(receptor activator of nuclear factor-κB ligand/receptor activator of nuclear factor-κB,RANKL/RANK)通路的活化诱导破骨细胞的形成。骨保护素是基质细胞产生的诱饵受体,与RANKL结合并阻止其与 RANK 的相互作用。同时,巨噬细胞集落刺激因子促进破骨细胞前体的增殖和破骨细胞的存活[35]。 虽然证据有限,但褪黑素调节破骨细胞活动的作用正在显现出来。例如,褪黑素减少与骨吸收增加有关,提示褪黑素可能是一种内源性破骨细胞抑制剂[36]。血清中的低褪黑素水平与骨质疏松症患者的低骨密度也表现出了相关性[37]。通过抑制MT2受体依赖的核因子κB信号通路促进间充质干细胞的成骨分化,并由RANKL旁分泌下调破骨细胞的生成,表明褪黑素可能通过骨保护素/RANKL/RANK轴影响成骨细胞系细胞和破骨细胞系细胞之间的相互作用[38]。另有说法是褪黑素可能下调核因子κB信号通路和活化T细胞核因子c-1 (NFATc1)转录因子直接抑制破骨细胞的分化,而不涉及褪黑素受体[39]。褪黑素还上调骨细胞分泌降钙素发挥对破骨细胞的抑制作用[40]。在细胞水平上,褪黑素抑制 RAW 264.7 细胞在低浓度RANKL和巨噬细胞集落刺激因子诱导下分化为破骨细胞[35]。褪黑素的抑制破骨细胞分化的能力也让它成为太空飞行中预防骨丢失的潜在药物[41]。 2.1.3 褪黑素具有促进血管生成的作用 骨修复需要血液供应和血运重建来恢复受损区域,血管化是骨再生的前提和关键环节。褪黑素促进血管生成的机制是通过上调血管内皮生长因子水平促进骨髓间充质干细胞介导的血管生成,即促进骨生成-血管生成偶联[42]。将骨髓间充质干细胞经褪黑素处理,在得到具有更高表达水平的成骨相关标志物的同时,也得到更高表达水平的血管生成相关标志物,如血管内皮生长因子、血管生成素2和血管生成素4 [43]。褪黑素纳米复合支架的成功制备,实现了褪黑素的长效缓释和成骨能力的增强。将褪黑素包埋丝素电纺丝(melatonin-encapsuled silk Fibronin electrospun,SF@MT)纳米纤维植入大鼠临界尺寸的颅骨缺损中,通过激活PI3K/Akt信号通路,可显著促进骨基质沉积和新血管形成[44]。负载褪黑素的纳米纤维海绵支架(Scaffold@MT)的植入也显著改善了大鼠股骨远端缺损的血管化骨再生[45]。通过核因子红系2相关因子2(nuclear factor-erythroid 2-Related Factor 2,Nrf2)/血红素加氧酶1(heme oxygenase,HO-1)信号通路,褪黑素纳米复合支架可减少血管内皮细胞的氧化应激损伤以及直接刺激血管内皮生长因子的产生,从而逆转糖尿病条件下骨生成-血管生成的解偶联并促进成骨[46]。相关的骨组织工程的进展进一步证实褪黑素具有改善缺血组织血管生成的潜力,进而加速骨修复。 然而,也有研究指出,在肿瘤、年龄相关性眼病和低氧环境中,褪黑素抑制组织内新血管生成,这与骨愈合过程中的观察结果相矛盾。褪黑素在不同病理生理条件下调控血管生成有一些看似矛盾的机制,需要进一步研究来解释[47]。 2.1.4 褪黑素具有抗炎和抗氧化功能 自由基的产生是骨降解的关键事件,有助于骨降解过程。褪黑素是直接的自由基清除剂和间接的抗氧化剂,通过激活抗氧化防御系统,维持骨髓间充质干细胞在长期传代后的自我更新和分化[48]。当褪黑素在一定浓度下,其促成骨、抗炎和抗氧化作用可发挥到极致[49]。高葡萄糖环境会降低细胞活力并诱导细胞凋亡,而褪黑素可以影响PERK-eIF 2 α-ATF 4-CHOP信号通路减少内质网应激诱导的细胞凋亡,从而保护成骨细胞免受高糖诱导的变化[50]。褪黑素也能缓解糖皮质激素所致的成骨分化障碍,这是通过骨形态发生蛋白/Smad信号通路,并伴随由Smurf2介导的泛素化途径参与实现的[51]。褪黑素在Akt/Nrf 2/HO-1信号通路的激活也能缓解糖皮质激素诱导的成骨细胞凋亡,预防和改善糖皮质激素引起的骨质丢失[52]。此外,褪黑素上调SIRT1/Nrf2/转化生长因子β/骨形态发生蛋白的表达,同时抑制核因子κB通路和软骨基质降解的能力,表明褪黑素在减缓软骨退化和调节软骨再生方面的潜力[53]。褪黑素的应用不仅能减轻实验诱导的大鼠根尖周炎的炎症并减少骨吸收[54],还有助于控制高脂饮食大鼠的根尖周炎病变[55]。在小鼠的牙周炎模型中,褪黑素衍生碳点(melatonin-derived carbon dots,MT-CDs)可能通过调节Nrf2/HO-1信号通路来清除活性氧,防止细胞损伤和炎症因子的产生,缓解牙槽骨的退缩[56]。褪黑素也在拔牙后发挥抗炎作用,表现在减轻疼痛程度和肿胀方面[57]。 2.2 褪黑素在口腔种植中的应用进展 2.2.1 褪黑素促进种植体骨结合 口腔种植成功的前提是骨结合。“骨结合”这一概念原本用来描述在光学显微镜下骨组织与种植体之间的接触,但目前的含义已经包括种植体植入后,种植体周围骨愈合和再生期间发生的所有事件[58]。骨折愈合和骨结合在很多方面相类似,但它们又不尽相同[59]。对于骨结合的确切分子机制尚不清楚,但已确定成功的骨结合大致可以分为:①止血期,血小板通过纤维蛋白原聚合附着在伤口上,持续几分钟到几个小时;②急性炎症期,以炎症细胞迁移和炎症递质释放为特征,通常在手术后持续10 min至几天;③增生期,在此期间沉积新的细胞外基质和血管,同时形成新的骨骼,持续几天到几周;④重塑期,在破骨细胞和成骨细胞的相互作用下,根据机体功能的需要对骨骼进行重塑,这一过程可能持续数年。骨结合的这4个阶段并不明显,也受到个体差异影响[60]。依据现有的研究可以大致推断出应用褪黑素在骨结合的不同阶段可能起到的相应作用。在种植术后的急性炎症期,出现血栓形成、缺血和再灌注损伤以及炎症细胞的浸润,中性粒细胞产生自由基,通过脂质过氧化引发一系列连锁反应和细胞膜损伤。褪黑素作为自由基清除剂和抗氧化剂,给药后可能起到抑制自由基和调节抗氧化酶活性的作用。增生期,发生血管生成分化、成骨细胞和成纤维细胞浸润、胶原沉积和肉芽形成。推测这一阶段的褪黑素可能在积极促进血管和骨的生成。重塑期,褪黑素可能通过减少炎症细胞因子、氧化应激参数和牙周损伤来预防种植体周围炎。增生期和重塑期是骨结合最关键的时期,直接决定了骨结合的成功与否。大多数研究报告称,褪黑素对骨结合的促进作用主要体现在植入后的2-8周[61]。 诸多动物模型证实,在种植中使用外源性褪黑素可以促进成骨。CUTANDO等[62]在比格犬的下颌骨植入种植体前将褪黑素粉末置于种植窝内,在2周后以组织形态计量学为指标,通过对种植体-骨结合率、螺纹间骨密度、骨新生区、种植体周围骨密度对比发现,各项参数及种植体周围总骨量均有显著增加,提示褪黑素局部应用可能在种植体的植入中起到仿生剂的作用。GUARDIA等[63](5周和8周)、TRESGUERRES等[64](4周)、DUNDAR等[65](4周)也证实了这一结论。CALVO-GUIRADO等[66]对比了褪黑素在钛和微槽氧化锆种植体中的效果,发现局部应用褪黑素可在1周时增加钛和微槽氧化锆种植体的种植体-骨结合率值,4周后,加入褪黑素的微槽氧化锆种植体组的种植体-骨结合率为(47.5±2.2)%,高于其他所有组(P < 0.05)。 褪黑素与其他分子联合使用促进骨形成。褪黑素和成纤维细胞生长因子2(FGF-2)促进成骨的能力近乎相同,成纤维细胞生长因子控制骨祖细胞增殖,而褪黑素在成骨细胞分化中更重要,但它们的协同作用极大增强了钛种植体表面的新骨形成[67]。一项在比格犬上使用褪黑素和生长激素协同作用的研究中,研究人员在2,5周时观察到骨结合参数显著改善;然而,在第8周仅在新骨形成上表现出显著的统计学差异,这支持了褪黑素在骨愈合早期更有效的观点[68]。SALOMO-COLL等[69]在对猎狐犬的即刻种植植入前将种植体浸泡在5%的褪黑素溶液(MI组)中,对比浸泡在10%的维生素D(CI组)和未浸泡组,12周以后,MI组的总种植体-骨结合率值为(48.36±7.45)%,CI组为(44.82±10.98)%,组间差异具有统计学意义(P=0.035),而且得出褪黑素可以改善种植体的骨结合、减少舌侧骨吸收的结论;并进一步推导在骨结合的早期阶段,无论植入物表面特性如何,局部应用褪黑素都会增加种植体-骨结合率。松果体的缺失会阻碍骨结合过程中的骨修复进程,但可以补充褪黑素恢复[70]。褪黑素的全身应用也能通过促进Runx2、降钙素和骨保护素基因表达和抑制RANKL基因表达,改善老年雌性骨质疏松大鼠羟基磷灰石涂层-钛种植体的骨结合[71]。最近的研究表明,同样是骨质疏松条件下,上午9点全身给予褪黑素可能比晚上9点更有效地改善去卵巢大鼠的股骨干骺端骨髓腔内钛棒的初始骨结合[72]。 褪黑素-骨结合的动物实验研究,详见表2。 在种植临床上,褪黑素得到小范围应用。 EL-GAMMAL等[73]在上颌后牙区种植时局部应用褪黑素凝胶,使用一体式种植体并在2周以后负重,发现在1个月时褪黑素组的植入物稳定性明显更高(P=0.01);1年的随访期发现褪黑素的使用减少骨吸收和促进种植体的稳定性,提示在低骨密度区如上颌后牙区局部应用褪黑素使种植体即刻负重成为可能。HAZZAA等[74]在美学区联合应用褪黑素和自体骨移植行即刻种植,在第6个月和第9个月的随访期,发现褪黑素组的骨组织参数(骨密度、边缘骨丢失)与对照组存在显著差异,软组织评估(牙龈指数、探诊深度)也有较好的表现,提示与单独使用自体骨移植"
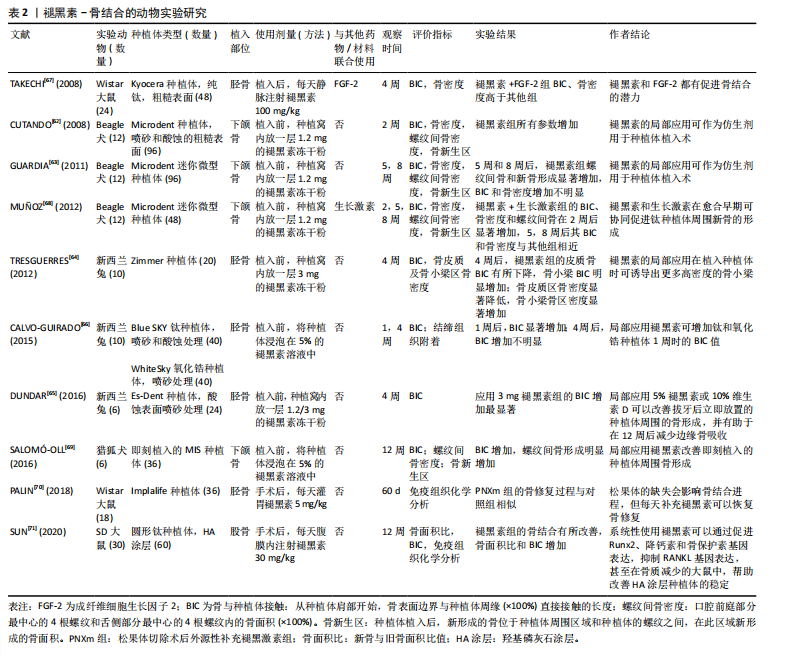

相比,褪黑素和自体骨移植在即刻种植体周围的结合应用可改善自体骨移植愈合过程中骨吸收量大的局限性,是一种有价值的选择。在另一项相关的研究中,HAZZAA等[75]对自体骨移植联合应用褪黑素凝胶组和自体骨移植对照组行植入2个月后早期负荷,种植体周探诊深度、软组织厚度及影像学分析结果证实自体骨移植联合应用褪黑素方法的可行性。RAVI KIRAN等[76]的研究也为褪黑素在即刻种植中使用可以改善骨结合提供了证据。 动物实验和临床研究表明,无论是局部应用于种植窝内、作为涂层覆盖种植体,还是在植入时注射在种植体周围,褪黑素都可以促进种植体-骨接触,增加种植体周围骨量和骨密度,特别是在愈合的早期阶段,更有利于骨结合[77]。 2.2.2 褪黑素在种植体周围炎的潜在作用 种植体周围炎是发生在口腔种植体周围组织中的病理状态,其特征在于种植体周围结缔组织中的炎症和支持骨的进行性丧失[78]。种植体周围炎与牙周炎在组织病理学方面的表现具有相似性[79]。唾液和龈沟液中的褪黑素水平与牙周炎的严重程度呈反比,提示褪黑素可以作为诊断牙周病的一个重要的生物标志物[80]。褪黑素对牙周炎的治疗效果主要表现在有助于减轻氧化应激、改善牙周参数,降低RANKL、碱性和酸性磷酸酶水平[81]。重度牙周炎患者在接受非手术牙周治疗后口服褪黑素,探诊深度明显减少[82]。在非手术牙周治疗的基础上,补充褪黑素可能会改善患有牙周炎的2型糖尿病患者的炎症和牙周状况[83]。这些证据表明褪黑素辅助治疗能有效控制牙周炎症。但牙周组织和种植体周围组织间的差异,使种植体周软组织对病变的防御能力弱于天然牙,从而导致种植体周围炎的进展较牙周炎更迅速[84]。由于治疗效果不明确,最佳临床治疗方案尚存在争议,预防是处理种植体周围炎的首选方法[85]。然而,到目前为止预防种植周炎的方法主要局限于控制病原菌和牙菌斑[86]。根据种植体周围炎的发病机制,减少炎性细胞因子的产生,抑制牙槽骨吸收,促进新骨形成,可能是预防种植体周围炎的有效策略。 尽管褪黑素预防种植体周围炎的具体机制和作用程度仍不清楚,但已有研究承认其效力。氯己定在种植临床使用广泛,但被证明是对成骨细胞有害,而褪黑素的使用可以保护成骨细胞免受氯己定的损害。PROKSCH等[87]观察不同浓度氯己定对MC3T3成骨细胞的反应,发现氯己定导致活性氧和超氧化物水平升高,成骨细胞形态不良,并以浓度依赖的方式严重减少了成骨细胞活力和代谢活性;但是,同时供应褪黑素可减少活性氧和超氧化物的产生,支持细胞形态发生和生长,将氯己定损伤成骨细胞的程度从坏死转变为早期凋亡,表明褪黑素在氯己定的背景下保护成骨细胞,为褪黑素治疗牙周炎和种植体周围炎提供依据。钛离子使线粒体的活性氧大量生成,导致种植体周围氧化应激和自噬损伤,成骨细胞受损,而褪黑素通过SIRT3/SOD2/mROS信号通路减少活性氧的生成,降低细胞自噬水平,恢复成骨细胞活性,可作为钛离子损伤成骨细胞所致的种植体周围炎的潜在药物[88]。WU等[89]研究发现预防性使用褪黑素可降低大鼠种植体周围炎模型体内促炎细胞因子水平和破骨细胞数量,减少牙槽骨吸收,并且降低种植体周围炎的发生率。在机制上,褪黑素降低了Toll样受体4蛋白水平,并抑制了核因子κB的激活,从而下调了肿瘤坏死因子、白细胞介素1β和白细胞介素6的水平,表明褪黑素通过抑制Toll样受体4/核因子κB信号来预防种植体周围炎症。 褪黑素促进骨再生的生物作用使其具有应用于口腔种植相关的再生医学的潜力。CERQUEIRA团队[90]使用溶胶-凝胶涂层作为褪黑素在钛种植体上释放的载体;XIAO团队[91]开发出了一种新型的复合黏附型水凝胶缓释系统——明胶甲基丙烯酰-多巴胺@褪黑素(GelMA-DOPA@MT),实现了褪黑素在局部区域的持续释放,并将人骨髓间充质干细胞分化为成骨细胞。"

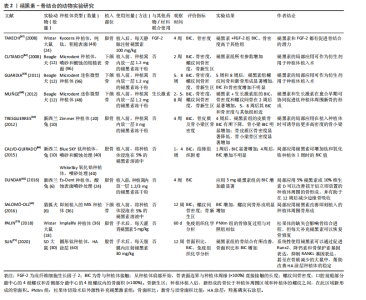
| [1] HATHAWAY‐SCHRADER JD, Novince CM. Maintaining homeostatic control of periodontal bone tissue. J Periodontology 2000. 2021;86(1): 157-187. [2] 魏洁雅,徐思群,周学东,等.牙槽骨修复重建分子调控机制的研究新进展[J].四川大学学报(医学版),2024,55(1):31-38. [3] RESNIK R. Misch’s Contemporary Implant Dentistry E-Book. M Elsevier Health Sciences. 2020. [4] LERNER AB, CASE JD, TAKAHASHI Y, et al. Isolation of melatonin, the pineal gland factor that lightens melanocytes1. J JACS Au. 1958;80(10): 2587-2587. [5] WANG B, WEN H, SMITH W, et al. Regulation effects of melatonin on bone marrow mesenchymal stem cell differentiation. J Cell Physiol. 2019;234(2):1008-1015. [6] CUTANDO A, ANEIROS-FERNÁNDEZ J, LÓPEZ-VALVERDE A, et al. A new perspective in oral health: potential importance and actions of melatonin receptors MT1, MT2, MT3, and RZR/ROR in the oral cavity. J Arch Oral Biol. 2011;56(10):944-950. [7] CONTI A, CONCONI S, HERTENS E, et al. Evidence for melatonin synthesis in mouse and human bone marrow cells. J Pineal Res. 2000; 28(4):193-202. [8] MUNMUN F, WITT‐ENDERBY PA. Melatonin effects on bone: implications for use as a therapy for managing bone loss. J Pineal Res. 2021;71(1):e12749. [9] HUANG J, LI Y, HE C. Melatonin having therapeutic bone regenerating capacity in biomaterials. Curr Pharm Biotechnol. 2022;23(5):707-718. [10] SACK RL, LEWY AJ, ERB DL, et al. Human melatonin production decreases with age. J Pineal Res. 1986;3(4):379-388. [11] KOTLARCZYK MP, LASSILA HC, O’NEIL CK, et al. Melatonin osteoporosis prevention study (MOPS): a randomized, double‐blind, placebo‐controlled study examining the effects of melatonin on bone health and quality of life in perimenopausal women. J Pineal Res. 2012;52(4): 414-426. [12] LU X, YU S, CHEN G, et al. Insight into the roles of melatonin in bone tissue and bone related diseases. Int J Mol Med. 2021;47(5):1-19. [13] KOYAMA H, NAKADE O, TAKADA Y, et al. Melatonin at pharmacologic doses increases bone mass by suppressing resorption through down‐regulation of the RANKL‐mediated osteoclast formation and activation. J Bone Miner. Res. 2002;17(7):1219-1229. [14] CARDINALI DP, LADIZESKY MG, BOGGIO V, et al. Melatonin effects on bone: experimental facts and clinical perspectives. J Pineal Res. 2003;34(2):81-87. [15] ZHANG L, SU P, XU C, et al. Melatonin inhibits adipogenesis and enhances osteogenesis of human mesenchymal stem cells by suppressing PPARγ expression and enhancing Runx2 expression. J Pineal Res. 2010;49(4):364-372. [16] LIU X, GONG Y, XIONG K, et al. Melatonin mediates protective effects on inflammatory response induced by interleukin‐1 beta in human mesenchymal stem cells. J Pineal Res. 2013;55(1):14-25. [17] GAO W, LIN M, LIANG A, et al. Melatonin enhances chondrogenic differentiation of human mesenchymal stem cells. J Pineal Res. 2014; 56(1):62-70. [18] LEE JH, HAN YS, LEE SH. Potentiation of biological effects of mesenchymal stem cells in ischemic conditions by melatonin via upregulation of cellular prion protein expression. J Pineal Res. 2017; 62(2):e12385. [19] MALAKOTI F, ZARE F, ZAREZADEH R, et al. The role of melatonin in bone regeneration: A review of involved signaling pathways. J Biochimie. 2022;202:56-70. [20] CARDINALI DP. Melatonin as a chronobiotic/cytoprotective agent in bone. Doses involved. J Pineal Res. 2024;76(1):e12931. [21] ZHU G, MA B, DONG P, et al. Melatonin promotes osteoblastic differentiation and regulates PDGF/AKT signaling pathway. J Cell Biol Int. 2020;44(2):402-411. [22] DONG P, GU X, ZHU G, et al. Melatonin induces osteoblastic differentiation of mesenchymal stem cells and promotes fracture healing in a rat model of femoral fracture via neuropeptide Y/neuropeptide Y receptor Y1 signaling. J Pharmacology. 2018;102(5-6): 272-280. [23] JIANG T, XIA C, CHEN X, et al. Melatonin promotes the BMP9-induced osteogenic differentiation of mesenchymal stem cells by activating the AMPK/β-catenin signalling pathway. Stem Cell Res Ther. 2019;10(1):408. [24] LEE SH, HWANG JW, HAN Y, et al. Synergistic stimulating effect of 2-hydroxymelatonin and BMP-4 on osteogenic differentiation in vitro. J Biochem Biophys Res Commun. 2020;527(4):941-946. [25] ZHAO R, TAO L, QIU S, et al. Melatonin rescues glucocorticoid-induced inhibition of osteoblast differentiation in MC3T3-E1 cells via the PI3K/AKT and BMP/Smad signalling pathways. J Life Sci. 2020;257:118044. [26] LIU L, ZHU Y, XU Y, et al. Prevention of ERK activation involves melatonin‐induced G1 and G2/M phase arrest in the human osteoblastic cell line hFOB 1.19. J Pineal Res. 2012;53(1):60-66. [27] KOBAYASHI-SUN J, SUZUKI N, HATTORI A, et al. Melatonin suppresses both osteoblast and osteoclast differentiation through repression of epidermal Erk signaling in the zebrafish scale. J Biochem Biophys Res Commun. 2020;530(4):644-650. [28] QIU S, TAO ZB, TAO L, et al. Melatonin induces mitochondrial apoptosis in osteoblasts by regulating the STIM1/cytosolic calcium elevation/ERK pathway. J Life Sci. 2020;248:117455. [29] TAO L, ZHAO S, TAO Z, et al. Septin4 regulates endoplasmic reticulum stress and apoptosis in melatonin induced osteoblasts. J Mol Med Rep. 2020;22(2):1179-1186. [30] MENG X, ZHU Y, TAO L, et al. Overexpression of septin-7 inhibits melatonin-induced cell apoptosis in human fetal osteoblastic cells via suppression of endoplasmic reticulum stress Corrigendum in/10.3892/mmr. 2021.12538. J Mol Med Rep. 2018;17(3):4817-4822. [31] SON JH, CHO YC, SUNG IY, et al. Melatonin promotes osteoblast differentiation and mineralization of MC 3T3‐E1 cells under hypoxic conditions through activation of PKD/p38 pathways. J Pineal Res. 2014;57(4):385-392. [32] YANG F, YANG L, LI Y, et al. Melatonin protects bone marrow mesenchymal stem cells against iron overload‐induced aberrant differentiation and senescence. J Pineal Res. 2017;63(3):e12422. [33] YAN J, YAO Y, YAN S, et al. Chiral protein supraparticles for tumor suppression and synergistic immunotherapy: an enabling strategy for bioactive supramolecular chirality construction. J Nano Lett. 2020; 20(8):5844-5852. [34] KNANI L, BARTOLINI D, KECHICHE S, et al. Melatonin prevents cadmium‐induced bone damage: first evidence on an improved osteogenic/adipogenic differentiation balance of mesenchymal stem cells as underlying mechanism. J Pineal Res. 2019;67(3):e12597. [35] JARRAR H, ALTINDAL DÇI, GÜMÜŞDERELIOĞLU M. The inhibitory effect of melatonin on osteoclastogenesis of RAW 264.7 cells in low concentrations of RANKL and MCSF. Turk J Biol. 2020;44(6):427-436. [36] OSTROWSKA Z, KOS-KUDLA B, SWIETOCHOWSKA E, et al. Assessment of the relationship between dynamic pattern of nighttime levels of melatonin and chosen biochemical markers of bone metabolism in a rat model of postmenopausal osteoporosis. J Neuroendocrinol Lett. 2001;22(2):129-136. [37] SADADIWALA MH, SADADIWALA A. Effects of melatonin on bone: a case control study. Int J Res Med Sci. 2022;10(8):1703. [38] ZHOU Y, WANG C, SI J, et al. Melatonin up‐regulates bone marrow mesenchymal stem cells osteogenic action but suppresses their mediated osteoclastogenesis via MT2‐inactivated NF‐κB pathway. Br J Pharmacol. 2020;177(9):2106-2122. [39] KIM HJ, KIM HJ, BAE MK, et al. Suppression of osteoclastogenesis by melatonin: A melatonin receptor-independent action. Int J Mol Sci. 2017;18(6):1142. [40] NAKANO M, IKEGAME M, IGARASHI-MIGITAKA J, et al. Suppressive effect of melatonin on osteoclast function via osteocyte calcitonin. J Endocrinol. 2019;242(2):13-23. [41] IKEGAME M, HATTORI A, TABATA MJ, et al. Melatonin is a potential drug for the prevention of bone loss during space flight. J Pineal Res. 2019;67(3):e12594. [42] KUSUMBE AP, RAMASAMY SK, ADAMS RH. Coupling of angiogenesis and osteogenesis by a specific vessel subtype in bone. J Nature. 2014; 507(7492):323-328. [43] ZHENG S, ZHOU C, YANG H, et al. Melatonin accelerates osteoporotic bone defect repair by promoting osteogenesis–angiogenesis coupling. J Front Endocrinol. 2022;13:826660. [44] DENG L, HOU M, LV N, et al. Melatonin-encapsuled silk fibroin electrospun nanofibers promote vascularized bone regeneration through regulation of osteogenesis-angiogenesis coupling. J Mater Today Bio. 2024;25:100985. [45] LV N, HOU M, DENG L, et al. A sponge-like nanofiber melatonin-loaded scaffold accelerates vascularized bone regeneration via improving mitochondrial energy metabolism. J Mater Today Bio. 2024;26:101078. [46] CHEN T, WU Z, HOU Q, et al. The dual angiogenesis effects via Nrf2/HO-1 signaling pathway of melatonin nanocomposite scaffold on promoting diabetic bone defect repair. Int J Nanomedicine. 2024:19: 2709-2732. [47] MA Q, REITER R J, CHEN Y. Role of melatonin in controlling angiogenesis under physiological and pathological conditions. J Angiogenesis. 2020;23(2):91-104. [48] ALLEGRA M, REITER RJ, TAN DX, et al. The chemistry of melatonin’s interaction with reactive species. J Pineal Res. 2003;34(1):1-10. [49] QIU X, WANG X, QIU J, et al. Melatonin rescued reactive oxygen species‐impaired osteogenesis of human bone marrow mesenchymal stem cells in the presence of tumor necrosis factor‐alpha. J Stem Cells Int. 2019;2019(1):6403967. [50] ZHOU R, MA Y, TAO Z, et al. Melatonin inhibits glucose-induced apoptosis in osteoblastic cell line through PERK-eIF2α-ATF4 pathway. J Front Pharmacol. 2020;11:602307. [51] 赵锐,朱悦,陶琳,等.褪黑素缓解糖皮质激素抑制MC3T3-E1细胞成骨分化与矿化的作用机制[J].医学研究生学报,2021,34(1):8-13. [52] 吕文正.褪黑素调控Akt/Nrf 2/HO-1通路减轻糖皮质激素诱导成骨细胞凋亡[D].沈阳:中国医科大学,2023. [53] ZHAO M, QIU D, MIAO X, et al. Melatonin Delays Arthritis Inflammation and Reduces Cartilage Matrix Degradation through the SIRT1-Mediated NF-κB/Nrf2/TGF-β/BMPs Pathway. Int J Mol Sci. 2024;25(11):6202. [54] KIRMIZI D, SEHIRLI AÖ, SAYINER S, et al. Effects of melatonin against experimentally induced apical periodontitis in rats. Aust Endod J. 2024;50(2):218-226. [55] DOS SANTOS RM, DA SILVA MACHADO NE, CANTIGA-SILVA C, et al. Modulatory influence of melatonin on apical periodontitis in Wistar rats fed a high-fat diet. J Arch Oral Biol. 2023;153:105749. [56] XIN X, LIU J, LIU X, et al. Melatonin-Derived Carbon Dots with Free Radical Scavenging Property for Effective Periodontitis Treatment via the Nrf2/HO-1 Pathway. J ACS nano. 2024;18(11):8307-8324. [57] REFAHEE SM, ABOULMAGD I, RAGAB R, et al. The effect of local melatonin application following the removal of an impacted mandibular third molar. J Oral Maxillofac Surg. 2023;81(5):622-631. [58] ALBREKTSSON T, JOHANSSON C. Osteoinduction, osteoconduction and osseointegration. Eur Spine J. 2001;10(2):S96-S101. [59] COLNOT C, ROMERO DM, HUANG S, et al. Molecular Analysis of Healing at a Bone-Implant Interface. J Dent Res. 2007;86:862-867. [60] TERHEYDEN H, LANG NP, BIERBAUM S, et al. Osseointegration--communication of cells. Clin Oral Implants Res. 2012;23(10):1127-1135. [61] YI M, YIN Y, SUN J, et al. Hormone and implant osseointegration: Elaboration of the relationship among function, preclinical, and clinical practice. Front Mol Biosci. 2022:9:965753. [62] CUTANDO A, GÓMEZ-MORENO G, ARANA C, et al. Melatonin stimulates osteointegration of dental implants. J Pineal Res. 2008;45(2):174-179.
[63] GUARDIA J, GÓMEZ-MORENO G, FERRERA MJ, et al. Evaluation of effects of topic melatonin on implant surface at 5 and 8 weeks in Beagle dogs. J Clin Implant Dent Relat Res. 2011;134:262-268. [64] TRESGUERRES IF, CLEMENTE C, BLANCO L, et al. Effects of local melatonin application on implant osseointegration. J Clin Implant Dent Relat Res. 2012;14(3):395-399. [65] DUNDAR S, YAMAN F, SAYBAK A, et al. Evaluation of Effects of Topical Melatonin Application on Osseointegration of Dental Implant: An Experimental Study. J Oral Implantol. 2016;42(5):386-389. [66] CALVO-GUIRADO JL, AGUILAR SALVATIERRA A, GARGALLO ALBIOL J, et al. Zirconia with laser-modified microgrooved surface vs. titanium implants covered with melatonin stimulates bone formation. Experimental study in tibia rabbits. J Clin Oral Implants Res. 2015; 26(12):1421-1429. [67] TAKECHI M, TATEHARA S, SATOMURA K, et al. Effect of FGF-2 and melatonin on implant bone healing: a histomorphometric study. J Mater Sci Mater Med. 2008;19:2949-2952. [68] MUÑOZ F, LÓPEZ-PEÑA M, MIÑO N, et al. Topical application of melatonin and growth hormone accelerates bone healing around dental implants in dogs. J Clin Implant Dent Relat Res. 2012;14(2):226-235. [69] SALOMÓ-COLL O, MATÉ-SÁNCHEZ DE VAL JE, RAMÍREZ-FERNÁNDEZ MP, et al. Osseoinductive elements for promoting osseointegration around immediate implants: a pilot study in the foxhound dog. J Clin Oral Implants Res. 2016;27(12):e167-e175. [70] PALIN LP, POLO TOB, BATISTA FRS, et al. Daily melatonin administration improves osseointegration in pinealectomized rats. J Appl Oral Sci. 2018;26:e20170470. [71] SUN T, LI J, XING H, et al. Melatonin improves the osseointegration of hydroxyapatite-coated titanium implants in senile female rats. J Z Gerontol Geriatr. 2020;53(8):770-777. [72] ZHOU M, TAO Z. Systemic administration with melatonin in the daytime has a better effect on promoting osseointegration of titanium rods in ovariectomized rats. J Bone Joint Res. 2022;11:751-762. [73] EL-GAMMAL MY, SALEM AS, ANEES MM, et al. Clinical and Radiographic Evaluation of Immediate Loaded Dental Implants With Local Application of Melatonin: A Preliminary Randomized Controlled Clinical Trial. J Oral Implantol. 2016;42(2):119-125. [74] HAZZAA HH, ELKILANI N, ELSAYED SA, et al. Evaluation of Immediate Implants Augmented with Autogenous Bone/Melatonin Composite Graft in the Esthetic Zone: A Randomized Controlled Trial. J Prosthodont. 2019;28:e637-e642. [75] HAZZAA HH, SHAWKI NA, ABDELAZIZ LM, et al. Early Loading of Dental Implant Grafted with Autogenous Bone Alone or Combined with Melatonin Gel: A Randomized Clinical Trial. Austin J Dent. 2020; 7(2):1137. [76] RAVI KIRAN S, BAMMIDI N, KUMAR AK, et al. Evaluation of the Effect of Topical Melatonin Application on Immediately Placed Dental Implants Using Cone Beam Computed Tomography (CBCT). Cureus. 2022;14(5):e25233. [77] LÓPEZ-VALVERDE N, PARDAL-PELÁEZ B, LÓPEZ-VALVERDE A, et al. Role of Melatonin in Bone Remodeling around Titanium Dental Implants: Meta-Analysis. Coatings. 2021. doi: 10.3390/coatings11030271 [78] SCHWARZ F, DERKS J, MONJE A, et al. Peri‐implantitis. J Clin Periodontol. 2018;45:S246-S266. [79] CATON JG, ARMITAGE G, BERGLUNDH T, et al. A new classification scheme for periodontal and peri‐implant diseases and conditions–Introduction and key changes from the 1999 classification. J Periodontol. 2018;89:S1-S8. [80] LU X, YU S, CHEN G, et al. Insight into the roles of melatonin in bone tissue and bone related diseases. Int J Mol Med. 2021;47(5):82. [81] HOSSEINZADEH A, JAMSHIDI NAEINI A, SHEIBANI M, et al. Melatonin and oral diseases: possible therapeutic roles based on cellular mechanisms. Pharmacol Rep. 2024;76(3):487-503. [82] TINTO M, SARTORI M, PIZZI I, et al. Melatonin as host modulating agent supporting nonsurgical periodontal therapy in patients affected by untreated severe periodontitis: a preliminary randomized, triple‐blind, placebo‐controlled study. J Periodontal Res. 2020;55(1):61-67. [83] BAZYAR H, GHOLINEZHAD H, MORADI L, et al. The effects of melatonin supplementation in adjunct with non-surgical periodontal therapy on periodontal status, serum melatonin and inflammatory markers in type 2 diabetes mellitus patients with chronic periodontitis: a double-blind, placebo-controlled trial. J Inflammopharmacology. 2019;27:67-76. [84] 赖红昌.种植体周围炎与牙周炎的类比探究[J].口腔医学,2018, 38(12):1057-1061. [85] 周婷慧,欧国敏.种植体周炎临床治疗研究进展[J].中国实用口腔科杂志,2024,17(3):348-353. [86] ROCCUZZO M, LAYTON D M, ROCCUZZO A, et al. Clinical outcomes of peri‐implantitis treatment and supportive care: A systematic review. J Clin Oral Implants Res. 2018;29:331-350. [87] PROKSCH S, STROBEL SL, VACH K, et al. Melatonin as a candidate therapeutic drug for protecting bone cells from chlorhexidine‐induced damage. J Periodontol. 2014;85(12):e379-e389. [88] 王思钱.钛离子通过SIRT3/SOD2/mROS信号通路导致成骨细胞自噬及褪黑素逆转作用机制的研究[D].济南:山东大学,2020. [89] WU X, QIAO S, WANG W, et al. Melatonin prevents peri implantitis via suppression of TLR4/NF-κB. J Acta Biomater. 2021;134:325-336. [90] CERQUEIRA A, ROMERO-GAVILÁN F, ARAÚJO-GOMES N, et al. A possible use of melatonin in the dental field: Protein adsorption and in vitro cell response on coated titanium. J Mater Sci Eng C Mater Biol Appl. 2020;116:111262. [91] XIAO L, LIN J, CHEN R, et al. Sustained Release of Melatonin from GelMA Liposomes Reduced Osteoblast Apoptosis and Improved Implant Osseointegration in Osteoporosis. Oxid Med Cell Longev. 2020: 2020:6797154. |
| [1] | Cai Yaohao, Lang Lyu, Li Hong. Assessing the bone mass of the residual alveolar ridge in the first molar for implant placement by cone-beam computed tomography [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1572-1577. |
| [2] | Bai Jing, Zhang Xue, Ren Yan, Li Yuehui, Tian Xiaoyu. Effect of lncRNA-TNFRSF13C on hypoxia-inducible factor 1alpha in periodontal cells by modulation of #br# miR-1246 #br# [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(5): 928-935. |
| [3] | Zhao Zengbo, Li Chenxi, Dou Chenlei, Ma Na, Zhou Guanjun. Anti-inflammatory and osteogenic effects of chitosan/sodium glycerophosphate/sodium alginate/leonurine hydrogel [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(4): 678-685. |
| [4] | Yu Shuangqi, Ding Fan, Wan Song, Chen Wei, Zhang Xuejun, Chen Dong, Li Qiang, Lin Zuoli. Effects of polylactic acid-glycolic acid copolymer/lysine-grafted graphene oxide nanoparticle composite scaffolds on osteogenic differentiation of MC3T3 cells [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(4): 707-712. |
| [5] | Liu Haoyang, Xie Qiang, Shen Mengran, Ren Yansong, Ma Jinhui, Wang Bailiang, Yue Debo, Wang Weiguo . Application, research hotspots, and shortcomings of degradable zinc-based alloys in bone defect repair and reconstruction [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(4): 839-845. |
| [6] | Zhou Yang, Liu Kexin, Wang Deli, Sun Zhang. Regenerative effects of engineered extracellular vesicles on repairing bone defects [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(36): 7839-7847. |
| [7] | Zhang Yixuan, Li Dongna, Liu Chunyan. Pathological processes, inflammatory responses, and related biomarkers of periodontitis: a multi-omics analysis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(35): 7601-7610. |
| [8] | Wang Jingshuai, Zhang Xiaotong, Zhang Yange, Wan Zedong, Kong Lingwei, Cao Haiying, Jin Yu. Comparison of Mg-Li-Gd alloy and stainless steel intramedullary nail for fixation of femoral annular hemi-defects in rats [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(34): 7261-7268. |
| [9] | Ren Bo, Tang Yongliang, Li Ni, Liu Bangding. Thermosensitive antibacterial hydrogel for treatment of infected bone defects [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(34): 7269-7277. |
| [10] | Wu Ziwei, Luo Yicai, Wei Yinge, Liao Hongbing. Application of poly(lactic-co-glycolic acid) copolymer in stomatology [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(34): 7393-7404. |
| [11] | Deng Ran, Wei Yi, Ji Xiaowei. Induction of M1/M2 polarization of macrophages by lipopolysaccharides and titanium particles in peri-implant tissues [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(34): 7415-7422. |
| [12] | Deng Guanghui, Xiang Wei, Su Qifan, Chen Xiaoyu, Wang Liangwei, Wan Zhihong, Wu Jiaqi, Chen Xiaojun. Preparation of osteoporotic femoral condylar bone defect model in rabbits and its critical value [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(30): 6426-6433. |
| [13] | Li Shuyuan, Yang Dawen, Zeng Zhanpeng, Cai Qunbin, Zhang Jingtao, Zhou Qishi. Application of induced membrane technique for repairing critical-sized bone defects: advantages and future development [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(28): 6083-6093. |
| [14] | Liu Yang, Yang Jilei, Wang Wenli, Cui Yingying, Sun Qihao, Li Yourui. Application characteristics of thermosensitive hydrogels in bone tissue engineering [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(28): 6094-6100. |
| [15] | Shen Zhen, Huang Ziyue, He Zhijuan, Wang Yiting, Chen Qigang, Geng Chunmei, Huang Yajing, Wu Zugui. Dynamic expression of H-type vessels coupled with bone repair effect in bone induced membrane for massive bone defects [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(28): 5950-5956. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||