Chinese Journal of Tissue Engineering Research ›› 2025, Vol. 29 ›› Issue (12): 2421-2428.doi: 10.12307/2025.393
Linagliptin alleviates wear particle-induced inflammatory osteolysis by regulating macrophage polarization and osteoclast formation
Yang Peng, Zhang Wei, Li Wenming, Li Wenhao, Wu Zebin, Zhou Jun, Geng Dechun
- Department of Orthopedics, First Affiliated Hospital of Soochow University, Suzhou 215006, Jiangsu Province, China
-
Received:2024-03-28Accepted:2024-06-21Online:2025-04-28Published:2024-09-09 -
Contact:Geng Dechun, Researcher, Department of Orthopedics, First Affiliated Hospital of Soochow University, Suzhou 215006, Jiangsu Province, China -
About author:Yang Peng, Master candidate, Department of Orthopedics, First Affiliated Hospital of Soochow University, Suzhou 215006, Jiangsu Province, China -
Supported by:National Natural Science Foundation of China, No. 82272567 (to GDC); Suzhou Municipal Health Commission, No. GSWS2022002 (to GDC); Jiangsu Medical Research Program, No. ZD2022014 (to GDC); Suzhou Science and Technology Project, No. SKJY2021067 (to ZJ); 2023 Special Project of Clinical Key Disease Diagnosis and Treatment Technology in Suzhou City, No. LCZX202302 (to ZJ)
CLC Number:
Cite this article
Yang Peng, Zhang Wei, Li Wenming, Li Wenhao, Wu Zebin, Zhou Jun, Geng Dechun. Linagliptin alleviates wear particle-induced inflammatory osteolysis by regulating macrophage polarization and osteoclast formation[J]. Chinese Journal of Tissue Engineering Research, 2025, 29(12): 2421-2428.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
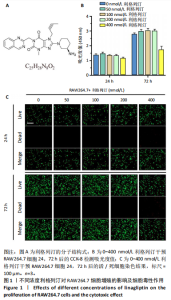
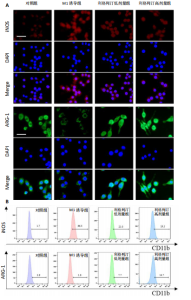
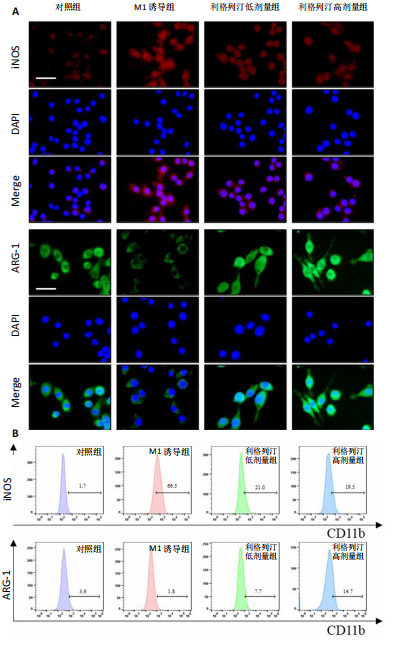
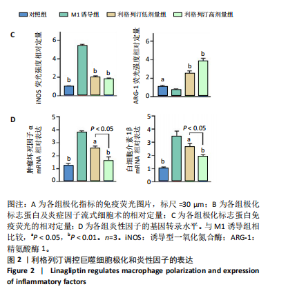
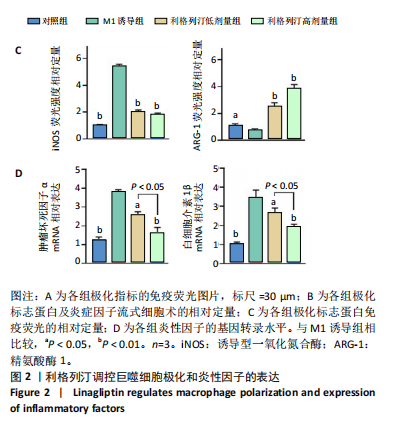
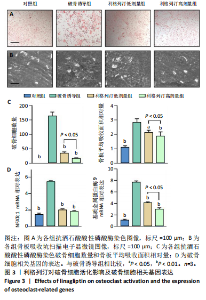
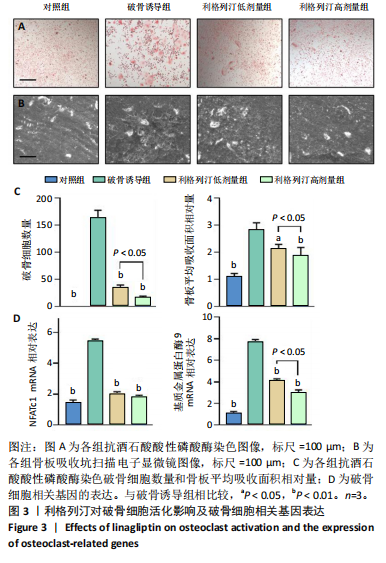
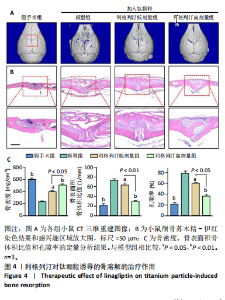
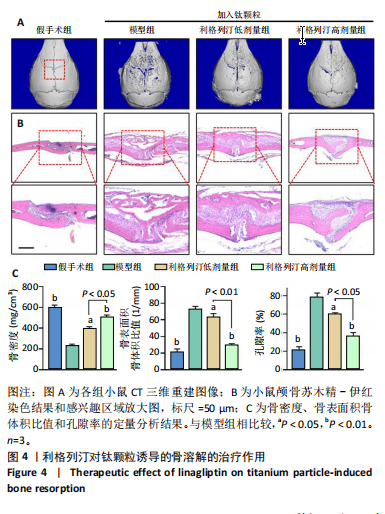
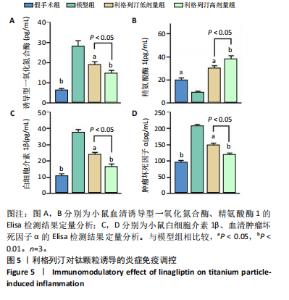
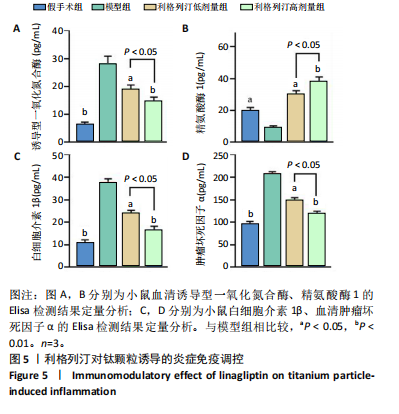
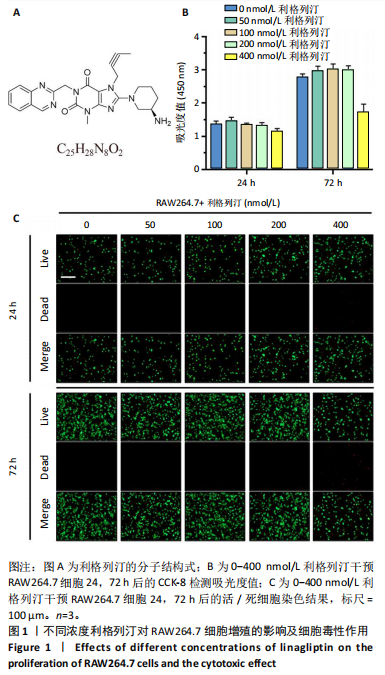
2.1 细胞实验结果 2.1.1 利格列汀对RAW264.7 细胞活性的影响 利格列汀是常见的二肽基肽酶4抑制剂(分子结构式见图1A)。将RAW264.7 细胞分别暴露于浓度为 0-400 nmol/L 的利格列汀中进行了24 h和72 h的干预,并使用CCK-8和活/死细胞染色检测RAW264.7 细胞活力和增殖毒性。结果显示,当利格列汀的浓度低于200 nmol/L时,各组吸光度值均未受到显著影响;当利格列汀浓度达到400 nmol/L时,450 nm处吸光度值急剧降低(图1B)。在利格列汀低于200 nmol/L时,细胞均无明显死亡,当浓度达到400 nmol/L,出现一定数量死细胞;处理72 h后,利格列汀400 nmol/L干预的细胞数量显著低于其他低浓度组,并出现大量死细胞,说明达到400 nmol/L利格列汀抑制细胞增殖并对细胞有毒性作用(图1C)。根据上述结果,实验选择50 nmol/L作为低剂量利格列汀和200 nmol/L作为高剂量利格列汀进行后续实验。 2.1.2 利格列汀对巨噬细胞极化的影响 免疫荧光染色和流式细胞技术的结果显示,在脂多糖(100 ng/mL)和干扰素γ(20 ng/mL)的存在下,与M1诱导组相比,利格列汀干预组M1极化标志蛋白诱导型一氧化氮合酶荧光强度显著下调(P < 0.01),M2极化标志蛋白精氨酸酶1荧光强度显著上调(P < 0.01),但低剂量组和高剂量组差异无显著性意义(图2A-C),提示利格列汀抑制巨噬细胞M1极化和促进巨噬细胞M2极化的途径可能不同;进一步提取各组细胞的总RNA,从基因转录层面检测了巨噬细胞炎症因子的表达水平 (图2D)。结果显示,与M1诱导组相比,利格列汀干预组炎症因子肿瘤坏死因子α、白细胞介素1β mRNA显著下调(P < 0.01),且利格列汀高剂量组低于利格列汀低剂量组(P < 0.05),表明利格列汀调控巨噬细胞:抑制M1极化和促进M2极化,且在0-200 nmol/L范围内具有浓度依赖性。 2.1.3 利格列汀对破骨细胞活化的影响 (1)抗酒石酸酸性磷酸酶染色:在核因子κB受体活化因子配体的刺激下,观察多个巨噬细胞形成的成熟破骨细胞,并可观察到破骨细胞内部红染,与破骨细胞诱导组相比较,利格列汀低剂量组及高剂量组破骨细胞数量显著减少(P < 0.01),且利格列汀高剂量组破骨细胞数量少于利格列汀低剂量组(P < 0.05)(图3A)。 (2)电子显微镜的观察:为了更直观地观察利格列汀抑制破骨细胞骨吸收功能,进行了破骨细胞骨吸收实验。扫描电子显微镜观察发现:与破骨诱导组相比,利格列汀各剂量组的骨片吸收坑的平均面积均显著下降(P < 0.01),并且利格列汀高剂量组的骨片吸收坑面积小于利格列汀低剂量组(P < 0.05)(图3B,C)。 (3)RT-PCR检测:与破骨诱导组相比,利格列汀干预组NFATc1、基质金属蛋白酶9均显著下调(P < 0.01),而利格列汀高剂量组基质金属蛋白酶9较利格列汀低剂量组下调(P < 0.05)(图3D),表明利格列汀以浓度依赖性的方式抑制了破骨细胞活化、减弱了破骨细胞骨吸收能力。此外,利格列汀高剂量组与低剂量组NFATc1的mRNA相对表达无显著差异,提示利格列汀在NFATc1/基质金属蛋白酶9信号通路的传导过程中发挥了重要作用。 2.2 动物实验结果 2.2.1 实验动物数量分析 所有 24 只小鼠均被纳入到实验结果分析。 2.2.2 利格列汀对钛颗粒诱导的骨溶解的影响 对颅骨扫描并进行3D 重建,对颅骨表面手术干预区域进行骨溶解度和骨参数分析。3D 重建图像显示,与假手术组相比,模型组的颅盖广泛侵蚀。模型组小鼠颅盖广泛侵蚀;而利格列汀以浓度依赖性的方式缓解了颅骨的骨质破坏。进一步分析,骨侵蚀参数也证实了利格列汀的保护作用,与模型组相比,利格列汀干预组的小鼠颅骨骨密度显著升高 (P < 0.01)、骨表面积骨体积比值和颅骨孔隙率显著降低 (P < 0.01)。苏木精-伊红染色还显示利格列汀组骨质溶解减弱,骨侵蚀减少。以上结果表明,利格列汀在钛颗粒诱导的小鼠颅骨骨溶解模型中显著缓解了骨质破坏。见图4。 2.2.3 利格列汀对钛颗粒诱导的炎症的免疫调控 基于利格列汀对巨噬细胞极化和破骨细胞的作用,拟探究其对假体周围骨溶解的治疗效果。为此,使用钛颗粒构建了小鼠骨溶解模型,并在造模后的3周内,采取每日灌胃利格列汀进行干预治疗。随后收集小鼠血清样本,并利用Elisa检测了其中极化标志蛋白及炎性因子的表达水平。结果显示,与假手术组相比,体内植入钛颗粒显著提高了小鼠血清中诱导型一氧化氮合酶、肿瘤坏死因子α、白细胞介素1β的表达水平(P < 0.01,P < 0.05),同时降低了M2极化标志蛋白精氨酸酶1的表达水平。而利格列汀的干预则不同程度地逆转了钛颗粒诱导的炎性因子表达,且高剂量利格列汀对小鼠炎性因子的逆转作用优于利格列汀低剂量组(均P < 0.05)。见图5。"
| [1] KATZ JN, ARANT KR, LOESER RF. Diagnosis and Treatment of Hip and Knee Osteoarthritis: A Review. JAMA. 2021;325(6):568-578. [2] CONNOLLY P, COOMBS S, SCHWARZKOPF R. Mechanical complications after total knee arthroplasty. Expert Rev Med Devices. 2023;20(12): 1105-1117. [3] RODRÍGUEZ-MERCHÁN EC, GÓMEZ-CARDERO P, ENCINAS-ULLÁN CA. Management of bone loss in revision total knee arthroplasty: therapeutic options and results. EFORT Open Rev. 2021;6(11):1073-1086. [4] HODGES NA, SUSSMAN EM, STEGEMANN JP. Aseptic and septic prosthetic joint loosening: Impact of biomaterial wear on immune cell function, inflammation, and infection. Biomaterials. 2021;278:121127. [5] MAZZELLA FM, ZHANG Y, BAUER TW. Update on the role of pathology and laboratory medicine in diagnosing periprosthetic infection. Hum Pathol. 2024;147:5-14. [6] MA TL, CHEN JX, KE ZR, et al. Targeting regulation of stem cell exosomes: Exploring novel strategies for aseptic loosening of joint prosthesis. Front Bioeng Biotechnol. 2022;10:925841. [7] NIRWAN N, VOHORA D. Linagliptin in Combination With Metformin Ameliorates Diabetic Osteoporosis Through Modulating BMP-2 and Sclerostin in the High-Fat Diet Fed C57BL/6 Mice. Front Endocrinol (Lausanne). 2022;13:944323. [8] LIU J, LIU Z, LU M, et al. The combination of linagliptin and metformin rescues bone loss in type 2 diabetic osteoporosis. J Drug Target. 2023; 31(6):646-654. [9] WANG SC, WANG XY, LIU CT, et al. The Dipeptidyl Peptidase-4 Inhibitor Linagliptin Ameliorates Endothelial Inflammation and Microvascular Thrombosis in a Sepsis Mouse Model. Int J Mol Sci. 2022;23(6):3065. [10] SINGH TP, VANGAVETI VN, MALABU UH. Dipeptidyl peptidase-4 inhibitors and their potential role in the management of atherosclerosis--A review. Diabetes Metab Syndr. 2015;9(4):223-229. [11] PUIJK R, SIEREVELT IN, PIJLS BGCW, et al. Increased risk of aseptic loosening for posterior stabilized compared with posterior cruciate-retaining uncemented total knee replacements: a cohort study of 13,667 knees from the Dutch Arthroplasty Registry. Acta Orthop. 2023;94:600-606. [12] SUN Z, KANG J, YANG S, et al. CD73 inhibits titanium particle-associated aseptic loosening by alternating activation of macrophages. Int Immunopharmacol. 2023;122:110561. [13] BAO S, YU D, TANG Z, et al. Conformationally regulated “nanozyme-like” cerium oxide with multiple free radical scavenging activities for osteoimmunology modulation and vascularized osseointegration. Bioact Mater. 2023;34:64-79. [14] MA T, CHEN S, WANG J, et al. Enhanced Osteolysis Targeted Therapy through Fusion of Exosomes Derived from M2 Macrophages and Bone Marrow Mesenchymal Stem Cells: Modulating Macrophage Polarization. Small. 2024;20(7):e2303506. [15] OUYANG Z, XU J, LIU T, et al. STING/TBK1 Regulates Inflammation in Macrophages and Titanium Particles-Induced Osteolysis. ACS Biomater Sci Eng. 2023;9(6):3273-3284. [16] WANG Z, TAO H, CHU M, et al. Byakangelicol suppresses TiPs-stimulated osteoclastogenesis and bone destruction via COX-2/NF-κB signaling pathway. Regen Biomater. 2023;11:rbad092. [17] VASCONCELOS DP, JABANGWE C, LAMGHARI M, et al. The Neuroimmune Interplay in Joint Pain: The Role of Macrophages. Front Immunol. 2022;13:812962. [18] SONG B, JIANG C, LUO H, et al. Macrophage M1 Plays a Positive Role in Aseptic Inflammation-Related Graft Loosening After Anterior Cruciate Ligament Reconstruction Surgery. Inflammation. 2017;40(6):1815-1824. [19] LI F, HUANG K, WANG J, et al. A dual functional Ti-Ga alloy: inhibiting biofilm formation and osteoclastogenesis differentiation via disturbing iron metabolism. Biomater Res. 2023;27(1):24. [20] YU X, WU Q, REN Z, et al. Kaempferol attenuates wear particle-induced inflammatory osteolysis via JNK and p38-MAPK signaling pathways. J Ethnopharmacol. 2024;318(Pt B):117019. [21] CHAN KL, POLLER WC, SWIRSKI FK, et al. Central regulation of stress-evoked peripheral immune responses. Nat Rev Neurosci.2023;24(10): 591-604. [22] XIONG Y, MI BB, LIN Z, et al. The role of the immune microenvironment in bone, cartilage, and soft tissue regeneration: from mechanism to therapeutic opportunity. Mil Med Res. 2022;9(1):65. [23] ZREIQAT H, KUMAR RK, MARKOVIC B, et al. Macrophages at the skeletal tissue-device interface of loosened prosthetic devices express bone-related genes and their products. J Biomed Mater Res A. 2003; 65(1):109-117. [24] PENG X, LI Y, CHENG C, et al. Research on the inhibition for aseptic loosening of artificial joints by Sr-doped calcium polyphosphate (SCPP)in vivo. Biomed Mater. 2021;16(6). doi: 10.1088/1748-605X/ac2492. [25] LIU X, DIAO L, ZHANG Y, et al. Piperlongumine Inhibits Titanium Particles-Induced Osteolysis, Osteoclast Formation, and RANKL-Induced Signaling Pathways. Int J Mol Sci. 2022;23(5):2868. [26] GU M, PAN B, CHEN W, et al. SPHK Inhibitors and Zoledronic Acid Suppress Osteoclastogenesis and Wear Particle-Induced Osteolysis. Front Pharmacol. 2022;12:794429. [27] NAPOLI N, CHANDRAN M, PIERROZ DD, et al. Mechanisms of diabetes mellitus-induced bone fragility. Nature reviews. Nat Rev Endocrinol. 2017;13(4):208-219. [28] JOSSE RG, MAJUMDAR SR, ZHENG Y, et al. Sitagliptin and risk of fractures in type 2 diabetes: Results from the TECOS trial. Diabetes Obes Metab. 2017;19(1):78-86. [29] KOHLER S, KASPERS S, SALSALI A, et al. Analysis of Fractures in Patients With Type 2 Diabetes Treated With Empagliflozin in Pooled Data From Placebo-Controlled Trials and a Head-to-Head Study Versus Glimepiride. Diabetes Care. 2018;41(8):1809-1816. [30] YUAN S, WAN ZH, CHENG SL,et al. Insulin-like Growth Factor-1, Bone Mineral Density, and Fracture: A Mendelian Randomization Study. J Clin Endocrinol Metab. 2021;106(4):e1552-e1558. [31] ZHUGE F, NI Y, NAGASHIMADA M, et al. DPP-4 Inhibition by Linagliptin Attenuates Obesity-Related Inflammation and Insulin Resistance by Regulating M1/M2 Macrophage Polarization. Diabetes. 2016;65(10):2966-2979. [32] ISHIDA M, SHEN WR, KIMURA K, et al. DPP-4 inhibitor impedes lipopolysaccharide-induced osteoclast formation and bone resorption in vivo. Biomed Pharmacother. 2019;109:242-253. [33] OHLENDIECK K, SWANDULLA D. Complexity of skeletal muscle degeneration: multi-systems pathophysiology and organ crosstalk in dystrophinopathy. Pflugers Arch. 2021;473(12):1813-1839. [34] YAMADERA S, NAKAMURA Y, INAGAKI M, et al. Linagliptin inhibits lipopolysaccharide-induced inflammation in human U937 monocytes.Inflamm Regen. 2018;38:13. |
| [1] | Wang Wentao, Hou Zhenyang, Wang Yijun, Xu Yaozeng. Apelin-13 alleviates systemic inflammatory bone loss by inhibiting macrophage M1 polarization [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1548-1555. |
| [2] | Chang Jinxia, Liu Yufei, Niu Shaohui, Wang Chang, Cao Jianchun. Visualization analysis of macrophage polarization in tissue repair process [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1486-1496. |
| [3] | Wang Sifan, He Huiyu, Yang Quan, Han Xiangzhen. miRNA-378a overexpression of macrophage cell line composite collagen sponge: anti-inflammation and tissue repair promotion [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(4): 789-799. |
| [4] | Zhao Jianwei, Li Xunsheng, Lyu Jinpeng, Zhou Jue, Jiang Yidi, Yue Zhigang, Sun Hongmei. Deer antler stem cell exosome composite hydrogel promotes the repair of burned skin [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(34): 7344-7352. |
| [5] | Yao Lanxuan, Wang Xuefei, Liu Yang, Yang Yujia, Zhao Yi, Qi Fangfang, Li Yinghui . Mesenchymal stem cells and their derived extracellular vesicles target macrophages to intervene in autoimmune diseases [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(31): 6772-6781. |
| [6] | Chen Yixin, Lu Yan, Zhang Xuan, Chen Xiaoli, Tan Liangyuan, Xu Zhangjie, Chen Wanglong, Su Shaoting, Liang Jiyao, Zhou Honghai. Mechanism by which Tongan Decoction regulates synovial macrophage polarization in rats with knee osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(26): 5621-5631. |
| [7] | Zou Shunyi, Chai yuan, Li Kunjian. Involvement of macrophage polarization in osteoarticular diseases: a visual analysis based on SCI-Expanded information [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(24): 5245-5253. |
| [8] | Cao Panxia, Peng Zining, Liu Shanshan, Fei Tiantian, Liang Tengyun, Zhang Mengwen, Wu Hong. AMP-activated protein kinase mediates macrophage fatty acid oxidation: an approach to prevent and treat atherosclerosis with traditional Chinese medicine [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(18): 3906-3914. |
| [9] | Zhao Yuqing, Wang Wei, You Huijuan, Chen Liyuan, Chen Yan, Wang Qinglu, Yang Fengying. Relationship between macrophage subtypes in obese adipose tissue and metabolic diseases [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(13): 2832-2841. |
| [10] | Ge Ruiyang, Ni Can, Yang Kun, Yan Fuhua. The role of macrophage polarization in the pathogenesis and treatment of periodontitis [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(20): 3246-3251. |
| [11] | Dong Hongfei, Huang Xi, Li Xianhui, Zhang Yanbiao, Wang Xuyang, Wang Bing, Sun Hongyu. Placenta-derived mesenchymal stem cells in promoting acute skin wound healing in rats [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(13): 2047-2053. |
| [12] | Bei Ying, Li Wenjing, Li Meiyun, Su Meng, Zhang Jin, Huang Yu, Zhu Yanzhao, Li Jiali, Wu Yan. Prussian blue nanoparticles promote wound healing of diabetic skin [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(10): 1526-1532. |
| [13] | Zhang Wei, Yu Lei, Yang Peng, Geng Dechun. Tabersonine alleviates wear particle-induced inflammatory osteolysis by inhibiting osteoclast activation [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(10): 1519-1525. |
| [14] | Deng Rui, Huang Keming, Luo Jian, Chen Gong, Feng Jian, Huang Weiyi, Wei Gang. Effect of heme oxygenase-1-mediated atorvastatin on macrophage polarization and cholesterol accumulation [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(1): 62-67. |
| [15] | Li Li, Li Xiao, Li Duchenhui, Zhang Jie, Xiao Tianjiao, Kang Jiabing, Tian Ai. Regulation of interleukin-4 on osteoclast differentiation during bone regeneration guided by bone replacement materials [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(34): 5455-5461. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||