Chinese Journal of Tissue Engineering Research ›› 2025, Vol. 29 ›› Issue (13): 2832-2841.doi: 10.12307/2025.506
Previous Articles Next Articles
Relationship between macrophage subtypes in obese adipose tissue and metabolic diseases
Zhao Yuqing1, Wang Wei2, You Huijuan1, Chen Liyuan1, Chen Yan1, Wang Qinglu1, Yang Fengying1
- 1College of Sports and Health, Shandong Sport University, Jinan 250102, Shandong Province, China; 2Shandong Qingdao Sports Training Center, Qingdao 266023, Shandong Province, China
-
Received:2024-03-28Accepted:2024-05-29Online:2025-05-08Published:2024-09-12 -
Contact:Yang Fengying, MD, College of Sports and Health, Shandong Sport University, Jinan 250102, Shandong Province, China -
About author:Zhao Yuqing, Master candidate, College of Sports and Health, Shandong Sport University, Jinan 250102, Shandong Province, China -
Supported by:Shandong Provincial Natural Science Foundation, No. ZR2022MH163 (to YFY)
CLC Number:
Cite this article
Zhao Yuqing, Wang Wei, You Huijuan, Chen Liyuan, Chen Yan, Wang Qinglu, Yang Fengying. Relationship between macrophage subtypes in obese adipose tissue and metabolic diseases[J]. Chinese Journal of Tissue Engineering Research, 2025, 29(13): 2832-2841.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
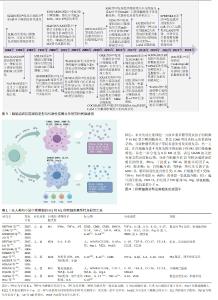
2.1 脂肪组织巨噬细胞亚型与代谢性疾病关系研究的时间脉络 140年前免疫学家ELIE首次发现巨噬细胞[5],并将其作为清除体内受损细胞的“清道夫”,自此,学者们开始对巨噬细胞的作用、分型及其相关信号通路开展广泛研究[6]。初期学者们根据巨噬细胞的炎症特点,将其简单划分为促炎型经典活化巨噬细胞[7]、抗炎型替代激活巨噬细胞[8],2000年MILLS团队[9]提出将巨噬细胞范式化表达,即M1/M2巨噬细胞分型。随着研究的不断深入[10-11],EDWARDS等[12]和WANG等[13]根据巨噬细胞诱导因子、分泌因子及功能等不同将M2型巨噬细胞进一步划分为M2a、M2b、M2c、M2d 4种亚型,巨噬细胞的基本分型得到进一步探索。实际上巨噬细胞具有明显的组织异质性,即便是同一组织中,在不同微环境下其表型特点也会发生变化。2011年ORTEGA团队[14]发现在棕色和白色脂肪组织中巨噬细胞存在异质性,后续学者又发现了与肥胖相关的新的脂肪组织巨噬细胞亚型,例如M3[15]、MMe[16]、CD9+[17]、LAM[18]、DARC+[19]、MFehi亚型[20],极大丰富了脂肪组织巨噬细胞亚型库,同时开启了对脂肪组织巨噬细胞亚型与相关代谢性疾病之间的关系研究[21-27]。具体时间脉络见图3。 2.2 巨噬细胞的基本表型特点 巨噬细胞是一种重要的免疫细胞,分布在包括脂肪组织在内的全身各组织和器官中,通过吞噬病原体及病理细胞残片来维持组织稳态以及组织的修复和重塑。巨噬细胞容易受到外界环境的刺激表现出显著的可塑性和多样性,根据活化状态、标志分子及功能不同,巨噬细胞主要可分为M1型(经典活化)巨噬细胞和M2型(替代激活)巨噬细胞[28],见图4。 在脂多糖、干扰素γ和肿瘤坏死因子α等促炎因子的刺激下,诱导未分化的巨噬细胞向M1型极化,主要表达组织相容性复合物(major histocompatibility complex,MHC)Ⅱ类、CD68、CD80/CD86、CD38、CD11c及诱导型一氧化氮合酶等标志分子,并分泌白细胞介素6、白细胞介素1β、肿瘤坏死因子α等炎性细胞因子和趋化因子配体2(chemokine ligand2,CCL2)等,参与抗原提呈及免疫应答,促进脂肪组织炎症发生发展[29-30]。而M2型巨噬细胞最初是在蠕虫感染期间发现的,这促进了M2极化反应,M2可由白细胞介素4、白细胞介素10、白细胞介素13和转化生长因子β等诱导,通常以M2标志物的表达为特征,例如精氨酸酶1、重组小鼠YM1蛋白、FIZZ1、CD206、CD209和CD301等,分泌白细胞介素10、转化生长因子β、CXCL13、CCL17等抗炎递质,主要发挥抗炎和参与组织修复重塑功能[31-33]。根据功能和表型标记物的表达,M2型巨噬细胞又可以进一步划分M2a、M2b、M2c、M2d等亚型,见表1[32,34-43]。"

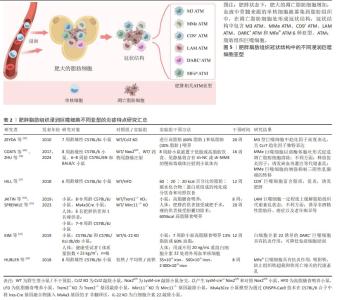
2.3 不同脂肪组织中巨噬细胞的分布特点 巨噬细胞是正常和病理条件下维持组织稳态的关键参与者,不同组织中的巨噬细胞具有各自的组织特异性。例如目前发现在肝脏中的肝巨噬细胞,称为库普弗细胞,参与多种肝功能的调节,与非酒精性脂肪性肝炎、病毒性肝炎、酒精性肝硬化和纤维化等疾病密切相关[44]。肺泡巨噬细胞是气道和肺部的常驻巨噬细胞,可吞噬清除肺泡和肺间质中的细菌异物[45],其功能障碍与慢性阻塞性肺疾病、肺纤维化和肺泡蛋白沉积症等关系密切。最新研究发现,骨骼肌Galectin-3+巨噬细胞的增加是造成肌营养不良和肌萎缩的重要原因[46-47]。巨噬细胞是脂肪组织的重要组成部分,不仅调控脂肪组织的炎症稳态,在脂肪组织的修复重塑过程中亦发挥重要作用[48]。脂肪组织巨噬细胞的数量和表型因脂肪组织的类型及代谢特点不同发生明显变化,进而影响脂肪组织炎症稳态[29]。 2.3.1 棕色脂肪组织巨噬细胞分布特点 脂肪组织是一种多功能器官,除了储存脂质外,其在调控代谢和内分泌方面亦发挥重要作用[49]。脂肪组织内除了脂肪细胞外还有大量内皮细胞、成纤维细胞和单核细胞/巨噬细胞等,其中巨噬细胞是脂肪组织的主要免疫细胞,在维持脂肪组织炎症稳态中发挥重要作用[29]。脂肪组织分为棕色脂肪组织和白色脂肪组织。一般认为,棕色脂肪组织是瘦脂质的健康表型,在维持能量平衡和产热代谢方面发挥重要作用。GALLERAND等[50]通过对小鼠棕色脂肪组织免疫细胞的单细胞测序发现棕色脂肪组织中存在多个巨噬细胞亚群(CD45、CD64、CD11b、Ly6C)和单核细胞亚群(Ly6Clow、Ly6Cint、 Ly6Chigh),表明单核/巨噬细胞在Podoplanin蛋白依赖性机制下,有助于棕色脂肪组织扩增和基质重塑。ROSINA等[51]研究发现棕色脂肪组织中常驻巨噬细胞可主动清除线粒体氧化受损的细胞外囊泡,促进棕色脂肪组织的高效产热。另外也有研究发现棕色脂肪组织衍生的maresin 2(MaR2)通过靶向常驻巨噬细胞促进脂质的分解[52],引起嗜酸性粒细胞浸润增强和巨噬细胞向M2型极化[53],在对抗肥胖引起的炎症方面发挥作用。因此,棕色脂肪组织巨噬细胞各个亚型均表现出抗炎特点以及发挥促进棕色脂肪组织扩增和基质重塑的作用,进而利于能量和脂质代谢。 2.3.2 白色脂肪组织巨噬细胞分布特点 白色脂肪组织是人体最大的能量储存库,同时具有重要的内分泌和免疫功能。HILDRETH团队[54]通过单细胞测序技术发现人类白色脂肪组织存在常驻巨噬细胞和浸润巨噬细胞。脂肪组织常驻巨噬细胞来源于胚胎造血干细胞,其主要作用是重塑受损脂肪组织、调节脂肪组织稳态及胰岛素敏感性[55]。这些常驻巨噬细胞表型为F4/80hiCD11b+CD169+,可进一步细分为3种亚型:MHCIIlow、MHCII+CD11c-和MHCII+CD11c+,前2种表现M2型抗炎特点,而MHCII+CD11c+表现M1型促炎特点[56]。脂肪组织常驻巨噬细胞抗炎亚型会分泌多种抗炎因子(例如白细胞介素10、转化生长因子β)并通过胞葬作用清除凋亡脂肪细胞,有助于维持脂肪组织中的胰岛素敏感性。已有研究表明Cx43-巨噬细胞在维持全身葡萄糖代谢及缓解脂肪肝中发挥重要作用[57]。白色脂肪组织内的抗炎M2型常驻巨噬细胞可促进棕色脂肪的分化并限制肥胖[58],而在肥胖状态下白色脂肪组织巨噬细胞主要呈现M1促炎表型,并通过释放粒细胞-巨噬细胞集落刺激因子、肿瘤坏死因子α、白细胞介素1β或白细胞介素6促进炎症,从而导致胰岛素抵抗[59]。肥胖情况下,肥大脂肪细胞凋亡增加,巨噬细胞吞噬能力下降,血液中大量骨髓来源单核细胞在单核细胞趋化因子(如CCL2趋化因子及受体)驱动下浸润入脂肪组织[4],并分化为不同巨噬细胞亚型围绕在凋亡肥大脂肪细胞周围形成冠状结 构[56,60]。目前发现冠状结构中包含6种不同巨噬细胞亚型,其中M3、MMe、CD9+、LAM巨噬细胞属于促炎亚型,DARC+和MFehi巨噬细胞具有抗炎特点。上述浸润巨噬细胞亚型在肥胖脂肪组织中的积累增加将导致脂肪组织局部以及系统性炎症紊乱,进而诱发多种代谢性疾病。此外,肥胖脂肪组织中原有的常驻M2型巨噬细胞在紊乱的炎症微环境中其表型亦发生改变,该微环境中白细胞介素6、单核细胞趋化因子1、肿瘤坏死因子α、脂肪酶、瘦素等因子表达增加,促使脂肪组织巨噬细胞从抗炎的M2型极化为促炎的M1型,进一步加剧脂肪组织炎症损害[14,59,61]。 综上所述,巨噬细胞在不同脂肪组织中表现出的异质性主要与所处的代谢和炎症微环境有关。巨噬细胞在棕色脂肪组织中以M2型脂肪组织常驻巨噬细胞为主,可促进棕色脂肪组织的生物活性并发挥抗炎作用。在白色脂肪组织中常驻巨噬细胞亦主要发挥吞噬抗炎作用,但肥胖情况下,白色脂肪组织肥大扩增并伴随脂质和能量代谢紊乱,导致肥大脂肪细胞凋亡增加和炎症紊乱。肥胖本质就是一个慢性炎症过程,各炎性因子及趋化因子增加,诱导循环中的单核细胞浸润入脂肪组织,并聚集在凋亡肥大脂肪组织周围形成冠状结构,曾经被认为是促炎M1型的巨噬细胞实际上可能是多种促炎亚型的集合。近期的多项研究共同提示,冠状结构中的浸润巨噬细胞亚型是诱发肥胖脂肪组织炎症损害和代谢性疾病的最关键因素,对其进行深入探索将有助于进一步理解肥胖脂肪组织炎症反应的机制。 2.4 肥胖脂肪组织浸润巨噬细胞不同亚型的炎症特点 肥胖情况下,循环中的单核细胞浸润入脂肪组织,并聚集在凋亡肥大脂肪组织周围形成冠状结构,在冠状结构中存在多种脂肪组织巨噬细胞亚型,见图5,它们有各自的炎性特点,见表2[15,17-18,22,24-25,62-63],共同影响脂肪组织局部以及系统炎症反应[56,60]。"

2.4.1 促炎型肥胖脂肪组织浸润巨噬细胞炎症特点 研究发现,冠状结构中存在以CD11c- CD206- MGL1-为标记的巨噬细胞,以独特表达趋化因子(C-C基元)受体7为特点,脂质含量高,并表现出较强浸润能力,被称为M3型巨噬细胞[14,64]。MMe是最常见的肥胖诱导的脂肪组织巨噬细胞表型,MMe巨噬细胞具有促炎效应但不表达经典的M1型巨噬细胞标记物,而过表达ATP 结合盒转运蛋白、CD36和脂滴包被蛋白2;与经典促炎M1型巨噬细胞不同的是,MMe巨噬细胞表面标志物与Ⅰ型干扰素通路和Toll样受体激活无关[15],而受过氧化物酶体增殖物激活受体γ调控[65],此外,饱和的游离脂肪酸亦会促进MMe巨噬细胞的激活[15,66]。MMe巨噬细胞的作用表现出两面性[62],肥胖早期,MMe巨噬细胞通过上调炎症标志物(如肿瘤坏死因子α、白细胞介素6和白细胞介素1β)以及参与脂质代谢的基因来促进脂肪组织炎症和诱发胰岛素抵抗;而长期高脂膳食诱导MMe巨噬细胞以溶酶体胞吐形式参与凋亡肥大脂肪细胞的清除,从而缓解凋亡脂肪细胞聚集的恶化效应[62] 。 CD9+巨噬细胞亚型亦定位于小鼠和人类的冠状结构中,其类似于MMe巨噬细胞可胞吐大量溶酶体,与MMe巨噬细胞不同的是,CD9+巨噬细胞包含传统的M1/M2标记物,如CD206和CD11b,且富含脂质,高表达溶酶体关联膜蛋白2、溶酶体关联膜蛋白3、脂蛋白酯酶、脂滴包被蛋白2、CD16等[15,67]。研究表明,CD9+巨噬细胞移植至瘦小鼠会导致肥胖相关基因上调,从而诱发肥胖和代谢紊乱[15]。此外,CD9+巨噬细胞分泌的外泌体携带的miRNA会影响细胞胰岛素敏感性,例如肥胖小鼠脂肪组织巨噬细胞来源外泌体含有的miR-155、miR-29a可以导致胰岛素抵抗[20,68]。随着研究的深入,在小鼠和人脂肪组织中发现了一种新的巨噬细胞亚型,称为LAM,以表达髓系细胞特异触发受体2(triggering receptor expressed on myeloid cells 2,TREM2)为典型特征,还表达脂肪酸结合蛋白、脂肪酶、组织蛋白酶、CD9、CD36等[17,69]。在肥胖早期,LAM巨噬细胞一定程度上可以缓解脂肪组织代谢紊乱,体现出一定程度的有益性[17],而长期营养过剩,多种促炎性细胞聚集,导致TREM2降解,致使TREM2依赖的胞葬作用受阻,凋亡脂肪细胞异常堆积进一步加重炎症浸润[70]。 2.4.2 抗炎型肥胖脂肪组织浸润巨噬细胞炎症特点 在冠状结构中,最新发现一种丰富表达趋化因子达菲抗原受体(duffy antigen receptors for chemokines,DARC)的巨噬细胞亚型,对多种C-C和C-X-C趋化因子具有结合能力,在红细胞、小脑浦肯野细胞和内皮细胞中表达[71-72]。有研究表明,白细胞介素22诱导的DARC+巨噬细胞丰富表达白细胞介素22受体以及M2型抗炎因子,在肥胖条件下表现出调节性M2特征,发挥抗炎作用,并且在高脂膳食喂养期间,DARC+巨噬细胞调节小鼠的代谢功能和饮食诱导的肥胖[18,21]。此外,在细胞间隙还存在一种富铁巨噬细胞亚型(MFehi),不同于其他募集来源的巨噬细胞,是脂肪组织常驻巨噬细胞的一种亚型,具有较高的细胞铁含量和铁循环基因表达谱[63,73]。除了铁含量丰富外,MFehi巨噬细胞还表达M2型标记物如CD163和单酰甘油脂肪酶,而M1型标志物减少。MFehi巨噬细胞通过吸附组织中过量的铁以及调控铁代谢相关基因,如细胞膜铁转运蛋白FP1、转铁蛋白受体、轻肽铁蛋白1、重肽铁蛋白1等的表达,以防止铁超载和铁死亡相关的代谢紊乱[19,63,74]。因此,MFehi巨噬细胞可能是脂肪组织常驻M2型巨噬细胞吸附铁之后的特征性表现。 综合上述,脂肪组织巨噬细胞亚型特点大部分属于促炎型,对机体产生炎性损害,但其中的ZMMe和CD9+巨噬细胞亚型具备溶酶体胞吐功能,有利于凋亡细胞的清除,体现出一定的有益性。值得注意的是MFehi、DARC+巨噬细胞两种亚型不仅具有抗炎特点而且还能发挥各自特有的抵抗代谢紊乱作用。因此,合理发挥脂肪组织巨噬细胞亚型的有益作用,探讨促进抗炎脂肪组织巨噬细胞亚型转化的方法,对缓解肥胖引起的代谢性疾病具有重要意义。 2.5 肥胖脂肪组织浸润巨噬细胞不同亚型及其相关的代谢性疾病 2.5.1 促炎脂肪组织浸润巨噬细胞亚型及其相关的代谢性疾病 肥胖会导致慢性炎症,有研究表明肥胖受试者的脂肪组织中以表达趋化因子(C-C基元)受体7为特点的M3型巨噬细胞表达增加,对脂肪组织表现出较强的浸润能力,进而导致脂肪组织功能障碍[14,64]。促炎型MMe巨噬细胞积聚在肥胖小鼠和人类的乳腺脂肪组织中,以烟酰胺腺嘌呤二核苷酸磷酸氧化酶 2依赖性方式释放白细胞介素6,其通过三阴性乳腺癌细胞上的糖蛋白130发出信号,以促进干细胞样特性和三阴性乳腺癌肿瘤的形成[75-76]。研究表明,脂肪组织MMe巨噬细胞的激活与2型糖尿病患者血清中的棕榈酸酯水平也密切相关,棕榈酸酯水平升高促进了巨噬细胞的代谢激活[15,77]。研究显示,在肥胖小鼠中CD9+巨噬细胞分泌含有miR-155的外泌体升高,从而引发葡萄糖耐受不良和胰岛素抵抗;而瘦小鼠的脂肪组织巨噬细胞来源外泌体可治疗肥胖小鼠的胰岛素抵抗,使糖耐量接近正常化,并改善全身胰岛素敏感性[20]。研究表明,过氧化物酶体增殖物激活受体γ可以限制代谢活化巨噬细胞和促进脂质代谢,因此,激活过氧化物酶体增殖物激活受体γ被认为是治疗2型糖尿病以及肥胖诱导的胰岛素抵抗和葡萄糖耐受不良的重要治疗靶点[65,78]。 脂肪组织中TREM2触发受体是一种在阿尔茨海默病、代谢性疾病和癌症中病理诱导的免疫信号转导[79]。研究发现,脂肪组织中的LAM基因特征与患有神经退行性疾病的小鼠和人类大脑中的“疾病相关小胶质细胞”脂肪组织巨噬细胞以及动脉粥样硬化期间的主动脉巨噬细胞表达了高度相似的基因谱[80-81],因此调控LAM可能是神经系统疾病和动脉粥样硬化等疾病的一个重要治疗靶点。另外,在肝脏脂肪组织中,通过单细胞RNA测序检查肝脏CD45细胞,同样这些巨噬细胞具有高脂膳食喂养条件下产生的LAM巨噬细胞基因特征,因此肥胖脂肪组织LAM巨噬细胞的积累与人类非酒精性脂肪性肝病之间密切相关[17]。肥胖状态下,TREM2的缺失减少了LAM巨噬细胞向肥大脂肪细胞的募集,导致大量脂肪细胞肥大、全身高胆固醇血症、炎症和葡萄糖不耐受。因此,TREM2信号传导可以驱动脂肪组织冠状结构中LAM巨噬细胞的形成,防止肥胖条件下脂肪细胞肥大和全身脂质稳态的丧失,但是TREM2过表达同样也会加剧高脂膳食诱导的肥胖、胰岛素抵抗、非酒精性脂肪肝以及老年痴呆等[17]。靶向TREM2信号通路亦将成为治疗肥胖相关代谢疾病的重要免疫信号传导枢纽。 促炎巨噬细胞在肥胖脂肪组织纤维化发展中发挥着重要作用。促炎巨噬细胞诱导型C型凝集素受体(Mincle),是一种在肥胖脂肪组织中发现的新型炎症调节因子,Mincle信号传导激活脂肪组织成纤维细胞,从而导致脂肪组织纤维化[82]。研究表明,肥胖状态下低氧诱导因子1α分子活性活跃,抑制脂肪前体细胞的分化,导致脂肪细胞肥大;同时,低氧诱导因子1α的激活也促使脂肪前体细胞产生大量细胞外基质分子(如胶原蛋白分子),从而促进脂肪组织纤维化[83]。此外,促炎性巨噬细胞过度产生一氧化氮,从而增加低氧诱导因子1α的积累并促进前脂肪细胞的促纤维化反应,导致脂肪组织纤维化[84]。以上综述均表明了不同亚型促炎巨噬细胞对脂肪组织功能产生负面影响,包括脂质代谢异常、慢性炎症疾病、胰岛素抵抗和脂肪组织纤维化等。 2.5.2 抗炎脂肪组织浸润巨噬细胞亚型及其相关的代谢性疾病 肥胖应激会诱导DARC单核细胞亚群浸润脂肪组织,DARC+巨噬细胞在肥胖条件下表现出调节性M2特征[21]。BENSON等[19]报道,DARC敲除小鼠的脂肪组织炎症增加、葡萄糖耐量和胰岛素敏感性受损,导致葡萄糖耐受不良、胰岛素抵抗甚至体内代谢紊乱。另外,白细胞介素22诱导的DARC+巨噬细胞可逆转许多代谢症状(例如高血糖和胰岛素抵抗),调节肝脏和脂肪组织中的脂质代谢,减少慢性炎症[85]。因此,DARC+巨噬细胞在调节全身代谢和脂肪组织炎症方面发挥重要的抗炎作用。作为脂肪组织常驻巨噬细胞的MFe hi具有双重作用,一方面巨噬细胞合成的转铁蛋白以旁分泌方式支持淋巴细胞增殖,从而消除了低铁血症对免疫系统的不利影响[86],另一方面可以清除细胞外血红素来保护脂肪细胞免受氧化应激。有研究表明,在肥胖状态下,脂肪细胞中谷胱甘肽过氧化物酶4的活性降低,而谷胱甘肽过氧化物酶4活性受损会自发诱导脂肪组织中活性氧的过度分泌和巨噬细胞浸润,引起铁积累和铁死亡,从而导致胰岛素抵抗、线粒体功能障碍甚至全身代谢紊乱[22,87-88]。因此,谷胱甘肽过氧化物酶4可视为对抗铁死亡的核心抗氧化因子。ORR等[20]研究表明,高脂膳食诱导的肥胖小鼠会导致异位脂质积累并伴随着组织铁分布的变化,表现为脂肪细胞与肝脏具有相近的铁浓度,发生内脏脂肪萎缩,并伴有肝脂质积累的急剧增加,诱发脂肪肝。值得注意的是,已有研究表明MFehi巨噬细胞可以通过吸附铁,防止组织铁超载和铁死亡[74],对缓解脂肪组织炎症、预防高脂膳食引起的代谢紊乱现象具有重要意义,见表3[15,18,21-26,75,89-90]。"

| [1] CHYLIKOVA J, DVORACKOVA J, TAUBER Z, et al. M1/M2 macrophage polarization in human obese adipose tissue. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2018;162(2):79-82. [2] PRIEUR X, MOK CY, VELAGAPUDI VR, et al. Differential lipid partitioning between adipocytes and tissue macrophages modulates macrophage lipotoxicity and M2/M1 polarization in obese mice. Diabetes. 2011;60(3): 797-809. [3] HERRADA AA, OLATE-BRIONES A, ROJAS A, et al. Adipose tissue macrophages as a therapeutic target in obesity-associated diseases. Obes Rev. 2021;22(6):e13200. [4] LONGO M, ZATTERALE F, NADERI J, et al. Adipose tissue dysfunction as determinant of obesity-associated metabolic complications. Int J Mol Sci. 2019;20(9):2358. [5] CAVAILLON JM. The historical milestones in the understanding of leukocyte biology initiated by Elie Metchnikoff. J Leukoc Biol. 2011;90(3):413-424. [6] MACKANESS GB. The influence of immunologically committed lymphoid cells on macrophage activity in vivo. J Exp Med. 1969;129(5):973-992. [7] NATHAN CF, MURRAY HW, WIEBE ME, et al. Identification of interferon-gamma as the lymphokine that activates human macrophage oxidative metabolism and antimicrobial activity. J Exp Med. 1983;158(3):670-689. [8] STEIN M, KESHAV S, HARRIS N, et al. Interleukin 4 potently enhances murine macrophage mannose receptor activity: a marker of alternative immunologic macrophage activation. J Exp Med. 1992;176(1):287-292. [9] MILLS CD, KINCAID K, ALT JM, et al. M-1/M-2 macrophages and the Th1/Th2 paradigm. J Immunol. 2000;164(12):6166-6173. [10] GERBER JS, MOSSER DM. Reversing lipopolysaccharide toxicity by ligating the macrophage Fc gamma receptors. J Immunol. 2001;166(11):6861-6868. [11] MANTOVANI A, SICA A, SOZZANI S, et al. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004; 25(12):677-686. [12] EDWARDS JP, ZHANG X, FRAUWIRTH KA, et al. Biochemical and functional characterization of three activated macrophage populations. J Leukoc Biol. 2006;80(6):1298-1307. [13] WANG Q, NI H, LAN L, et al. Fra-1 protooncogene regulates IL-6 expression in macrophages and promotes the generation of M2d macrophages. Cell Res. 2010;20(6):701-712. [14] ORTEGA MT, XIE L, MORA S, et al. Evaluation of macrophage plasticity in brown and white adipose tissue. Cell Immunol. 2011;271(1):124-133. [15] ZEYDA M, GOLLINGER K, KRIEHUBER E, et al. Newly identified adipose tissue macrophage populations in obesity with distinct chemokine and chemokine receptor expression. Int J Obes. 2010;34 (12):1684-1694. [16] KRATZ M, COATS BR, HISERT KB, et al. Metabolic dysfunction drives a mechanistically distinct proinflammatory phenotype in adipose tissue macrophages. Cell Metab. 2014;20(4):614-625. [17] HILL DA, LIM HW, KIM YH, et al. Distinct macrophage populations direct inflammatory versus physiological changes in adipose tissue. Proc Natl Acad Sci U S A. 2018;115(22):E5096-E5105. [18] JAITIN DA, ADLUNG L, THAISS CA, et al. Lipid-Associated Macrophages Control Metabolic Homeostasis in a Trem2-Dependent Manner. Cell. 2019; 178(3):686-698.e14. [19] BENSON TW, WEINTRAUB DS, CROWE M, et al. Deletion of the Duffy antigen receptor for chemokines (DARC) promotes insulin resistance and adipose tissue inflammation during high fat feeding. Mol Cell Endocrinol. 2018;473:79-88. [20] ORR JS, KENNEDY A, ANDERSON-BAUCUM EK, et al. Obesity alters adipose tissue macrophage iron content and tissue iron distribution. Diabetes. 2014;63(2):421-432. [21] YING W, RIOPEL M, BANDYOPADHYAY G, et al. Adipose Tissue Macrophage-Derived Exosomal miRNAs Can Modulate In Vivo and In Vitro Insulin Sensitivity. Cell. 2017;171(2):372-384.e12. [22] KIM EY, NOH HM, CHOI B, et al. Interleukin-22 Induces the Infiltration of Visceral Fat Tissue by a DiscreteSubset of Duffy Antigen Receptor for Chemokine-Positive M2-Like Macrophages in Response to a High Fat Diet. Cells. 2019;8(12):1587. [23] SCHWÄRZLER J, MAYR L, RADLINGER B, et al. Adipocyte GPX4 protects against inflammation, hepatic insulin resistance and metabolic dysregulation. Int J Obes (Lond). 2022;46(5):951-959. [24] ZHU Z, WANG H, QIAN X, et al. Inhibitory Impact Of Cinobufagin In Triple-Negative Breast Cancer Metastasis: Involvements Of Macrophage Reprogramming Through Upregulated MME and Inactivated FAK/STAT3 Signaling. Clin Breast Cancer. 2024;26:S1526-8209(24)00025-9. doi: 10.1016/j.clbc.2024.01.014. [25] SPRENKLE NT, WINN NC, BUNN KE, et al. The miR-23-27-24 clusters drive lipid-associated macrophage proliferation in obese adipose tissue. Cell Rep. 2023;42(8):112928. [26] COCHAIN C, VAFADARNEJAD E, ARAMPATZI P, et al. Single-Cell RNA-Seq Reveals the Transcriptional Landscape and Heterogeneity of Aortic Macrophages in Murine Atherosclerosis. Circ Res. 2018;122(12):1661-1674. [27] WANG ZL, YUAN L, LI W, et al. Ferroptosis in Parkinson’s disease: glia-neuron crosstalk. Trends Mol Med. 2022;28(4):258-269. [28] YANG D, YANG L, CAI J, et al. A sweet spot for macrophages: Focusing on polarization. Pharmacol Res. 2021;167:105576. [29] 胡伟,庄然.脂肪组织巨噬细胞研究进展[J].中国免疫学杂志,2017, 33(11):1723-1725,1730. [30] 闫文勇,贺昭昭,庞卫军.脂肪组织中巨噬细胞在肥胖过程中的作用及其调控机制[J].中国生物化学与分子生物学报,2023,39(5):638-647. [31] BI C, FU Y, LI B. Brain-derived neurotrophic factor alleviates diabetes mellitus-accelerated atherosclerosis by promoting M2 polarization of macrophages through repressing the STAT3 pathway. Cell Signal. 2020;70:109569. [32] RŐSZER T. Understanding the mysterious M2 macrophage through activation markers and effector mechanisms. Mediators Inflammation. 2015;2015:816460. [33] YAO J, WU D, QIU Y. Adipose tissue macrophage in obesity-associated metabolic diseases. Front Immunol. 2022;13:977485. [34] BERTANI FR, MOZETIC P, FIORAMONTI M, et al. Classification of M1/M2-polarized human macrophages by label-free hyperspectral reflectance confocal microscopy and multivariate analysis. Sci Rep. 2017;7(1):8965. [35] ZONG Z, ZOU J, MAO R, et al. M1Macrophages Induce PD-L1 Expression in Hepatocellular Carcinoma Cells ThroughIL-1β Signaling. Front Immunol. 2019;10:1643. [36] MOSCHEN AR, TILG H, RAINE T. IL-12, IL-23 and IL-17 in IBD: immunobiology and therapeutic targeting. Nat Rev Gastroenterol Hepatol. 2019;16(3): 185-196. [37] JIA Q, MORGAN-BATHKE ME, JENSEN MD. Adipose tissue macrophage burden, systemic inflammation, and insulin resistance. Am J Physiol Endocrinol Metab. 2020;319(2):E254-E264. [38] LIU X, LIU J, ZHAO S, et al. Interleukin-4 Is Essential for Microglia/Macrophage M2 Polarization and Long-Term Recovery After Cerebral Ischemia. Stroke. 2016;47(2):498-504. [39] MOSSER DM, EDWARDS JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8(12):958-969. [40] ASAI A, NAKAMURA K, KOBAYASHI M, et al. CCL1 released from M2b macrophages is essentially required for the maintenance of their properties. JLeukoc Biol. 2012;92(4):859-867. [41] HUANG X, LI Y, FU M, et al. Polarizing Macrophages In Vitro. Methods Mol Biol. 2018;1784:119-126. [42] ZHANG Q, SIOUD M. Tumor-Associated Macrophage Subsets: Shaping Polarization and Targeting. Int J Mol Sci. 2023;24(8):7493. [43] HOU Y, SHI J, GUO Y, et al. Inhibition of angiogenetic macrophages reduces disc degeneration-associated pain. Front Bioeng Biotechnol. 2022;10: 962155. [44] ELCHANINOV A, VISHNYAKOVA P, MENYAILO E, et al. An Eye on Kupffer Cells: Development, Phenotype and the Macrophage Niche. Int J Mol Sci. 2022;23(17):9868. [45] WOO YD, JEONG D, CHUNG DH. Development and Functions of Alveolar Macrophages. Mol Cells. 2021;44(5):292-300. [46] CHAZAUD B. Inflammation and Skeletal Muscle Regeneration: Leave It to the Macrophages! Trends Immunol. 2020;41(6):481-492. [47] COULIS G, JAIME D, GUERRERO-JUAREZ C, et al. Single-cell and spatial transcriptomics identify a macrophage population associated with skeletal muscle fibrosis. Sci Adv. 2023;9(27):eadd9984. [48] CHAVAKIS T, ALEXAKI VI, FERRANTE AW JR. Macrophage function in adipose tissue homeostasis and metabolic inflammation. Nat Immunol. 2023;24(5):757-766. [49] ROSEN ED, SPIEGELMAN BM. What we talk about when we talk about fat. Cell. 2014;156(1-2):20-44. [50] GALLERAND A, STUNAULT MI, MERLIN J, et al. Brown adipose tissue monocytes support tissue expansion. Nat Commun. 2021;12(1):5255. [51] ROSINA M, CECI V, TURCHI R, et al. Ejection of damaged mitochondria and their removal by macrophages ensure efficient thermogenesis in brown adipose tissue. Cell Metab. 2022;34(4):533-548.e12. [52] SUGIMOTO S, MENA HA, SANSBURY BE, et al. Brown adipose tissue-derived MaR2 contributes to cold-induced resolution of inflammation. Nat Metab. 2022;4(6):775-790. [53] SUÁREZ-ZAMORANO N, FABBIANO S, CHEVALIER C, et al. Microbiota depletion promotes browning of white adipose tissue and reduces obesity. Nat Med. 2015;21(12):1497-1501. [54] HILDRETH AD, MA F, WONG YY, et al. Single-cell sequencing of human white adipose tissue identifies new cell states in health and obesity. Nat Immunol. 2021;22(5):639-653. [55] CHEN Q, RUEDL C. Obesity retunes turnover kinetics of tissue-resident macrophages in fat. J Leukoc Biol. 2020;107(5):773-782. [56] LI X, REN Y, CHANG K, et al. Adipose tissue macrophages as potential targets for obesity and metabolic diseases. Front Immunol. 2023;14:1153915. [57] TIROSH A, TUNCMAN G, CALAY ES, et al. Intercellular Transmission of Hepatic ER Stress in Obesity Disrupts Systemic Metabolism. Cell Metab. 2021;33(2):319-333.e6. [58] FANG W, DENG Z, BENADJAOUD F, et al. Regulatory T cells promote adipocyte beiging in subcutaneous adipose tissue. FASEB J. 2020;34(7): 9755-9770. [59] OSORIO-CONLES Ó, OLBEYRA R, MOIZÉ V, et al. Positive Effects of a Mediterranean Diet Supplemented with Almonds on Female Adipose Tissue Biology in Severe Obesity. Nutrients. 2022;14(13):2617. [60] FARIA SS, CORRÊA LH, HEYN GS, et al. Obesity and Breast Cancer: The Role of Crown-Like Structures in Breast Adipose Tissue in Tumor Progression, Prognosis, and Therapy. J Breast Cancer. 2020;23(3):233-245. [61] ZHANG Y, MEI H, CHANG X, et al. Adipocyte-derived microvesicles from obese mice induce M1 macrophage phenotype throughsecreted miR-155. J Mol Cell Biol. 2016;8(6):505-517. [62] COATS BR, SCHOENFELT KQ, BARBOSA-LORENZI VC, et al. Metabolically activated adipose tissue macrophages perform detrimental and benefificial functions during diet-induced obesity. Cell Rep. 2017;20(13): 3149-3161. [63] HUBLER MJ, ERIKSON KM, KENNEDY AJ, et al. MFehi adipose tissue macrophages compensate for tissue iron perturbations in mice. Am J Physiol Cell Physiol. 2018;315(3):C319-C329. [64] MORRIS DL, SINGER K, LUMENG CN. Adipose tissue macrophages: phenotypic plasticity and diversity in lean and obese states. Curr Opin Clin Nutr Metab Care. 2011;14(4):341-346. [65] CHAKRABORTY S, ONG WK, YAU WWY, et al. CD10 marks non-canonical PPARγ-independent adipocyte maturation and browning potential of adipose-derived stem cells. Stem Cell Res Ther. 2021;12(1):109. [66] HOU Y, WEI D, ZHANG Z, et al. FABP5 controls macrophage alternative activation and allergic asthma by selectively programming long-chain unsaturated fatty acid metabolism. Cell Rep. 2022;41(7):111668. [67] XU X, GRIJALVA A, SKOWRONSKI A, et al. Obesity activates a program of lysosomal-dependent lipid metabolism in adipose tissue macrophages independently of classic activation. Cell Metab. 2013;18(6):816-830. [68] LIU T, SUN YC, CHENG P, et al. Adipose tissue macrophage-derived exosomal miR-29a regulates obesity-associated insulin resistance. Biochem Biophys Res Commun. 2019;515(2):352-358. [69] HARASYMOWICZ NS, RASHIDI N, SAVADIPOUR A, et al. Single-cell RNA sequencing reveals the induction of novel myeloid and myeloid-associated cell populations in visceral fat with long-term obesity. FASEB J. 2021;35(3):e21417. [70] WANG X, HE Q, ZHOU C, et al. Prolonged hypernutrition impairs TREM2-dependent efferocytosis to license chronic liver inflammation and NASH development. Immunity. 2023;56(1):58-77.e11. [71] JINNA N, RIDA P, SU T, et al. The DARC Side of Inflamm-Aging: Duffy Antigen Receptor for Chemokines (DARC/ACKR1) as a Potential Biomarker of Aging, Immunosenescence, and Breast Oncogenesis among High-Risk Subpopulations. Cells. 2022;11(23):3818. [72] XU L, ASHKENAZI A, CHAUDHURI A. Duffy antigen/receptor for chemokines (DARC) attenuates angiogenesis by causing senescence in endothelial cells. Angiogenesis. 2007;10(4):307-318. [73] Gabrielsen JS, Gao Y, Simcox JA, et al. Adipocyte iron regulates adiponectin and insulin sensitivity. J Clin Invest. 2012;122(10):3529-3540. [74] JOFFIN N, GLINIAK CM, FUNCKE JB, et al. Adipose tissue macrophages exert systemic metabolic control by manipulating local iron concentrations. Nat Metab. 2022;4(11):1474-1494. [75] TIWARI P, BLANK A, CUI C, et al. Metabolically activated adipose tissue macrophages link obesity to triple-negative breast cancer. J Exp Med. 2019;216(6):1345-1358. [76] PE KCS, SAETUNG R, YODSURANG V, et al. Triple-negative breast cancer influences a mixed M1/M2 macrophage phenotype associated with tumor aggressiveness. PLoS One. 2022;17(8):e0273044. [77] NI Y, NI L, ZHUGE F, et al. Adipose Tissue Macrophage Phenotypes and Characteristics: The Key to Insulin Resistance in Obesity and Metabolic Disorders. Obesity (Silver Spring). 2020;28(2):225-234. [78] TAKADA I, MAKISHIMA M. Peroxisome proliferator-activated receptor agonists and antagonists: a patent review (2014-present). Expert Opin Ther Pat. 2020;30(1):1-13. [79] DECZKOWSKA A, WEINER A, AMIT I. The physiology, pathology, and potential therapeutic applications of the TREM2 signaling pathway. Cell. 2020;181(6):1207-1217. [80] KEREN-SHAUL H, SPINRAD A, WEINER A, et al. A Unique Microglia Type Associated with Restricting Development of Alzheimer’s Disease. Cell. 2017;169(7):1276-1290.e17. [81] KIM K, PARK SE, PARK JS, et al. Characteristics of plaque lipid-associated macrophages and their possible roles in the pathogenesis of atherosclerosis. Curr Opin Lipidol. 2022;33(5):283-288. [82] TANAKA M. Molecular mechanism of obesity-induced adipose tissue inflammation; the role of Mincle in adipose tissue fibrosis and ectopic lipid accumulation. Endocr J. 2020;67(2):107-111. [83] SHAO M, HEPLER C, ZHANG Q, et al. Pathologic HIF1α signaling drives adipose progenitor dysfunction in obesity. Cell Stem Cell. 2021;28(4): 685-701.e7. [84] JANG JE, KO MS, YUN JY, et al. Nitric oxide produced by macrophages inhibits adipocyte differentiation and promotes profibrogenic responses in preadipocytes to induce adipose tissue fibrosis. Diabetes. 2016;65(9): 2516-2528. [85] WANG X, OTA N, MANZANILLO P, et al. Interleukin-22 alleviates metabolic disorders and restores mucosal immunity in diabetes. Nature. 2014; 514(7521):237-241. [86] DJEHA A, PÉREZ-ARELLANO JL, BROCK JH. Transferrin synthesis by mouse lymph node and peritoneal macrophages: iron content and effect on lymphocyte proliferation. Blood. 1993;81:1046-1050. [87] ZHANG S, SUN Z, JIANG X, et al. Ferroptosis increases obesity: Crosstalk between adipocytes and the neuroimmune system. Front Immunol. 2022; 13:1049936. [88] JIANG X, STOCKWELL BR, CONRAD M. Ferroptosis: mechanisms, biology and role in disease. Nat Rev Mol Cell Biol. 2021;22(4):266-282. [89] AMEKA MK, BEAVERS WN, SHAVER CM, et al. An Iron Refractory Phenotype in Obese Adipose Tissue Macrophages Leads to Adipocyte Iron Overload. Int J Mol Sci. 2022;23(13):7417. [90] SEIDMAN JS, TROUTMAN TD, SAKAI M, et al. Niche-Specific Reprogramming of Epigenetic Landscapes Drives Myeloid Cell Diversity in Nonalcoholic Steatohepatitis. Immunity. 2020;52(6):1057-1074.e7. [91] ARGÜELLO RJ, COMBES AJ, CHAR R, et al. SCENITH: A Flow Cytometry-Based Method to Functionally Profile Energy Metabolism with Single-Cell Resolution. Cell Metab. 2020;32(6):1063-1075.e7. |
| [1] | Wang Wentao, Hou Zhenyang, Wang Yijun, Xu Yaozeng. Apelin-13 alleviates systemic inflammatory bone loss by inhibiting macrophage M1 polarization [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1548-1555. |
| [2] | Yu Ting, Lyu Dongmei, Deng Hao, Sun Tao, Cheng Qian. Icariin pretreatment enhances effect of human periodontal stem cells on M1-type macrophages [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1328-1335. |
| [3] | Huang Ting, Zheng Xiaohan, Zhong Yuanji, Wei Yanzhao, Wei Xufang, Cao Xudong, Feng Xiaoli, Zhao Zhenqiang. Effects of macrophage migration inhibitory factor on survival, proliferation, and differentiation of human embryonic stem cells [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1380-1387. |
| [4] | Chang Jinxia, Liu Yufei, Niu Shaohui, Wang Chang, Cao Jianchun. Visualization analysis of macrophage polarization in tissue repair process [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1486-1496. |
| [5] | Liu Lingyun, He Guixin, Qin Weibin, Song Hui, Zhang Liwen, Tang Weizhi, Yang Feifei, Zhu Ziyi, Ou Yangbin . Improvement of myocardial injury by traditional Chinese medicine: mitochondrial calcium homeostasis mediates macrophage autophagy and pyroptosis pathway [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(6): 1276-1284. |
| [6] | Wang Sifan, He Huiyu, Yang Quan, Han Xiangzhen. miRNA-378a overexpression of macrophage cell line composite collagen sponge: anti-inflammation and tissue repair promotion [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(4): 789-799. |
| [7] | Zhao Jianwei, Li Xunsheng, Lyu Jinpeng, Zhou Jue, Jiang Yidi, Yue Zhigang, Sun Hongmei. Deer antler stem cell exosome composite hydrogel promotes the repair of burned skin [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(34): 7344-7352. |
| [8] | Deng Ran, Wei Yi, Ji Xiaowei. Induction of M1/M2 polarization of macrophages by lipopolysaccharides and titanium particles in peri-implant tissues [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(34): 7415-7422. |
| [9] | Xu Biao, Dong Yuzhen, Lu Tan. Effect of dihydroquercetin on the expression of inflammatory response markers in rats with spinal cord injury [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(32): 6843-6850. |
| [10] | Yan Laijun, Ge Haiya, Wang Zhengming, Yang Zongrui, Niu Lifeng, Zhan Hongsheng. Mechanism by which Tongdu Huoxue Decoction inhibits macrophage inflammation to delay intervertebral disc degeneration in rats [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(32): 6851-6857. |
| [11] | Liu Chenchen, Liu Ruize, Bao Mengmeng, Fang Li, Cao Liquan, Wu Jiangbo. Blood flow restriction training intervention in the elderly with sarcopenic obesity [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(32): 6963-6970. |
| [12] | Yao Lanxuan, Wang Xuefei, Liu Yang, Yang Yujia, Zhao Yi, Qi Fangfang, Li Yinghui . Mesenchymal stem cells and their derived extracellular vesicles target macrophages to intervene in autoimmune diseases [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(31): 6772-6781. |
| [13] | Zhu Menghan, Yang Xuetao, Sun Yimin, Wang Chenglin. Anti-inflammatory peptides for oral inflammatory diseases: regulation of inflammatory response to reduce tissue destruction and structural loss [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(30): 6529-6537. |
| [14] | Liu Haowei, Tian Haodong, Huang Li, Yu Hanglin, Peng Li. Acute effects of blood flow restriction resistance exercise on serum metabolites in obese young men [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(29): 6249-6259. |
| [15] | Sun Hailiang, Pang Jian, Shi Wanzhong, Shi Ying. Protective effect of Huaizhen Yanggan Capsule on knee osteoarthritis induced by sodium iodoacetate in model mice [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(26): 5579-5587. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||