Chinese Journal of Tissue Engineering Research ›› 2024, Vol. 28 ›› Issue (22): 3502-3508.doi: 10.12307/2024.487
Previous Articles Next Articles
Physicochemical properties and cytocompatibility of biomimetically precipitated nanocrystalline calcium phosphate granules
Chen Mingxue1, Niu Jianhua2, Lin Haiyan3, Wu Gang3, Wan Ben1, 3, 4
- 1Beijing Jishuitan Hospital, Capital Medical University, Beijing 100035, China; 2Shanghai Huangpu District Dental Disease Prevention and Control Institute, Shanghai 200020, China; 3Hangzhou Medical College, Hangzhou 310059, Zhejiang Province, China; 4Hangzhou Huibo Science and Technology Co., Ltd., Hangzhou 311217, Zhejiang Province, China
-
Received:2023-07-05Accepted:2023-09-25Online:2024-08-08Published:2024-01-20 -
Contact:Wan Ben, Beijing Jishuitan Hospital, Capital Medical University, Beijing 100035, China; Hangzhou Medical College, Hangzhou 310059, Zhejiang Province, China; Hangzhou Huibo Science and Technology Co., Ltd., Hangzhou 311217, Zhejiang Province, China -
About author:Chen Mingxue, MD, Physician, Beijing Jishuitan Hospital, Capital Medical University, Beijing 100035, China Niu Jianhua, Physician, Shanghai Huangpu District Dental Disease Prevention and Control Institute, Shanghai 200020, China -
Supported by:National Natural Science Foundation of China (Youth Science Foundation Project), No. 82202784 (to CMX); Key Research & Development Plan Project in Zhejiang Province, No. 2021C04013 (to WG); Zhejiang Province “Leading Wild Goose” Research & Development Project, No. 2022C03G1363463 (to LHY)
CLC Number:
Cite this article
Chen Mingxue, Niu Jianhua, Lin Haiyan, Wu Gang, Wan Ben. Physicochemical properties and cytocompatibility of biomimetically precipitated nanocrystalline calcium phosphate granules[J]. Chinese Journal of Tissue Engineering Research, 2024, 28(22): 3502-3508.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
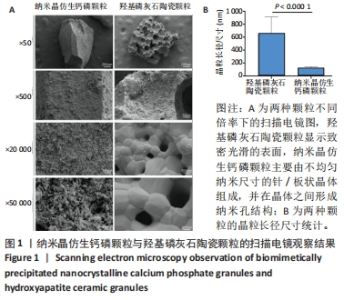
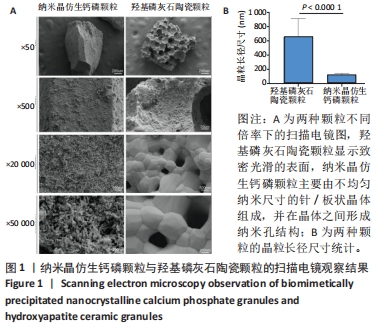
2.1 两种颗粒的形貌 从宏观上看,BpNcCaP和羟基磷灰石陶瓷颗粒均为不规则的颗粒状,而羟基磷灰石陶瓷颗粒具有一些大孔,这些孔是由双氧水发泡造孔而形成的[20]。 低倍率(×500)的扫描电镜图像显示,BpNcCaP和羟基磷灰石陶瓷颗粒都具有略微粗糙的表面形态,并伴有微小颗粒存在;高倍率(×20 000)扫描电镜图像显示,羟基磷灰石陶瓷颗粒显示致密光滑的表面,BpNcCaP颗粒主要由不均匀纳米尺寸的针/板状晶体组成,并在晶体之间形成纳米孔结构,见图1。高倍率下,使用Image J软件测量颗粒表面晶体尺寸,BpNcCaP颗粒的平均晶粒为(116.9±27.13) nm,羟基磷灰石陶瓷颗粒的平均晶粒(655.6±256.6) nm,组间比较差异有显著性意义(P < 0.000 1)。结果表明高温处理会让颗粒表面结构发生显著改变,高温烧结破坏了羟基磷灰石陶瓷颗粒表面的纳米结构,而低温沉积的BpNcCaP颗粒保留有微纳米孔隙。"
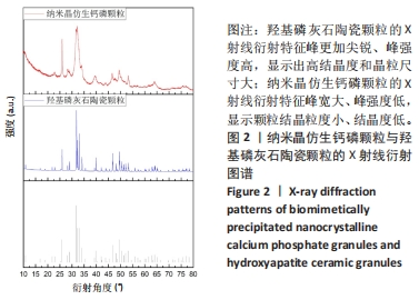
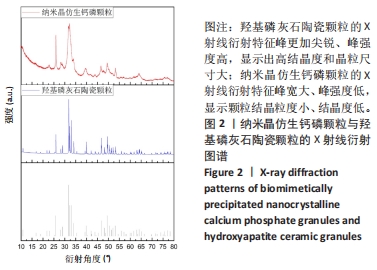
2.2 两种颗粒的X射线衍射分析结果 为了确定两种颗粒的物相组成和结晶度,对BpNcCaP和羟基磷灰石陶瓷颗粒进行了X射线衍射分析(图2)。BpNcCaP和羟基磷灰石陶瓷颗粒的衍射峰位置分别出现在25.96°,29.09°,31.90°,32.22°,33.90°,39.55°,46.58°,49.23°,53.29°和64.06°附近,这些峰对应于羟基磷灰石的特征晶面(002)(210)(211)(112)(202)(130)(222)(213)(004)和(510),所测试衍射峰的位置与ICSD数据库中羟基磷灰石标准PDF卡(09-0432)基本相符,无多余的杂相出现。羟基磷灰石陶瓷经过高温烧结,X射线衍射特征峰更加尖锐、峰强度高,显示出高结晶度和晶粒尺寸大,而BpNcCaP颗粒的X射线衍射特征峰宽大、峰强度低,显示颗粒结晶粒度小、结晶度低。X射线衍物相分析显示BpNcCaP颗粒的物相成分是羟基磷灰石,非煅烧的BpNcCaP颗粒结晶度更低,有助于材料在体内降解。"
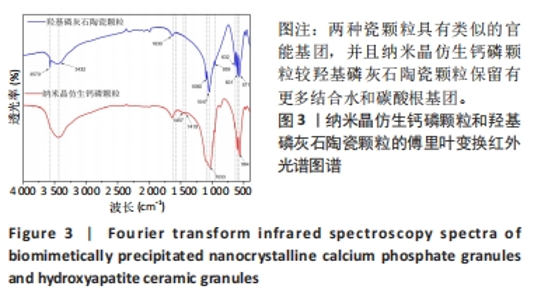
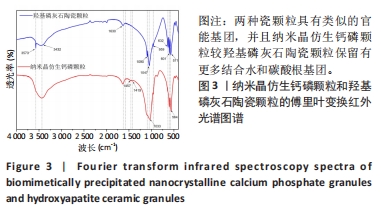
2.3 两种颗粒的傅里叶变换红外光谱分析结果 为了研究两种颗粒化学官能结构的差异,进行了傅里叶变换红外光谱分析。如图3所示,BpNcCaP和羟基磷灰石陶瓷颗粒均在3 573 cm-1波长处出现羟基吸收峰,PO43-的吸收峰在1 090,1 047,959,601和571 cm-1附近,符合羟基磷灰石化学基团特征。BpNcCaP颗粒在波长632 cm-1处缺少羟基吸收峰,在1 033 cm-1和564 cm-1出现P-O基团的伸缩振动峰,并且与羟基磷灰石陶瓷颗粒相比峰型宽大、圆钝。图中1 630 cm-1与3 432 cm-1处是H2O的特征吸收峰,1 457 cm-1和1 419 cm-1为CO32-的特征吸收峰。这些结果显示BpNcCaP与羟基磷灰石陶瓷颗粒具有类似的官能基团,并且BpNcCaP颗粒较羟基磷灰石陶瓷颗粒保留有更多结合水和碳酸根基团。"
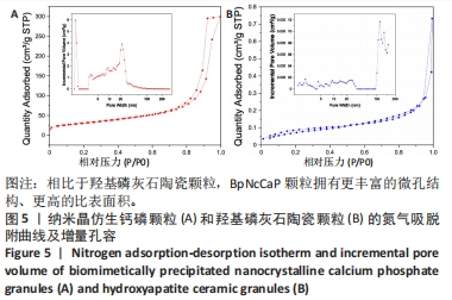
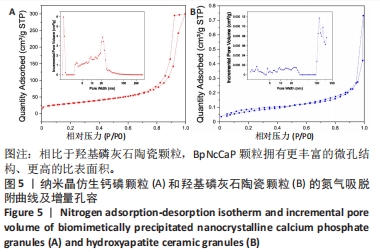
2.5 两种颗粒的比表面积和孔容分布 图5显示了BpNcCaP和羟基磷灰石陶瓷颗粒的氮气吸脱附曲线和不同孔径对孔容贡献的分布曲线。如两种颗粒的氮气吸脱附曲线所示,BpNcCaP颗粒的相对压力(P/P0)在0.70-1范围内,羟基磷灰石陶瓷颗粒的P/P0在0.94-1.0范围内,氮气的吸脱附曲线呈闭环,说明两种颗粒中存在不规则的介孔。此外,在不同P/P0下,BpNcCaP颗粒的吸脱氮气体量明显多于羟基磷灰石陶瓷颗粒。这些结果表明相比于羟基磷灰石陶瓷颗粒,BpNcCaP颗粒拥有更丰富的微孔结构。BpNcCaP颗粒的孔容增量分布曲线呈现出2个峰值,一个在约1.3 nm处,另一个在约21.7 nm处,这两种尺寸的孔隙对孔容的贡献最大。结果表明BpNcCaP颗粒的孔隙结构主要以纳米孔为主。羟基磷灰石陶瓷颗粒的孔隙度分布曲线呈现出多峰,主要为100 nm以上的孔为主(117.23,147.6 nm),而一些更小的纳米孔隙(1.59,2.16,4.32,9.31,13.67,23.39 nm)对颗粒整体孔容积的贡献小。使用BET模型可分别计算得出BpNcCaP颗粒比表面积为110.026 8 m2/g,羟基磷灰石陶瓷颗粒的比表面积为0.290 6 m2/g。结果显示具有微纳米表面的BpNcCaP颗粒比表面积更高,可以为蛋白吸附提供更多的吸附位点,可以作为生物活性因子的缓释载体。"
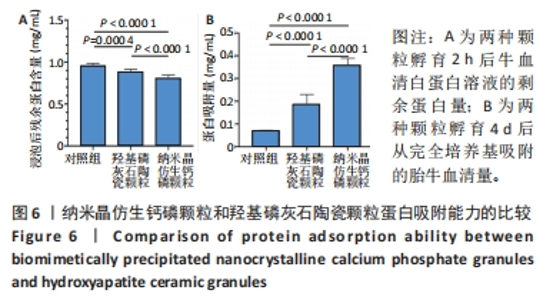
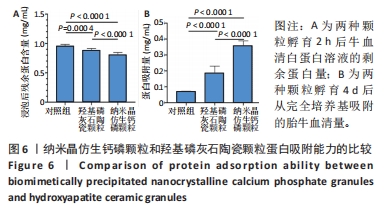
2.6 两种颗粒的蛋白吸附能力比较 如图6所示,采用BCA法测量了两种颗粒对牛血清白蛋白和胎牛血清的吸附能力。首先,将两种颗粒与牛血清白蛋白溶液孵育2 h,检测孵育液中残余的牛血清白蛋白,结果显示BpNcCaP颗粒组未被吸附的蛋白量显著少于羟基磷灰石陶瓷颗粒组[(0.81±0.03),(0.89±0.03) mg/mL],表明BpNcCaP颗粒比HAP陶瓷颗粒吸附蛋白更加快速。另一方面,采用完全培养基对两种颗粒进行孵育,4 d后利用表面活性剂对颗粒表面黏附的蛋白进行洗脱定量检测,BpNcCaP颗粒的洗脱液中蛋白量比羟基磷灰石陶瓷颗粒更多[(0.36±0.03),(0.19±0.04) mg/mL]。结果显示BpNcCaP颗粒吸附蛋白的能力更强,有助于植入后细胞早期攀爬、长入。"
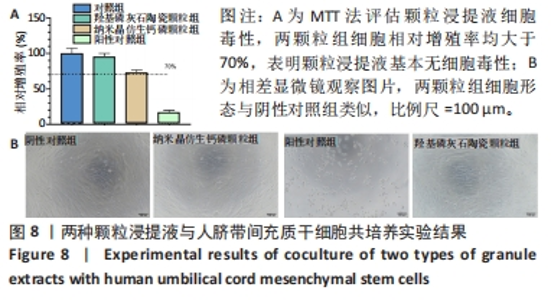
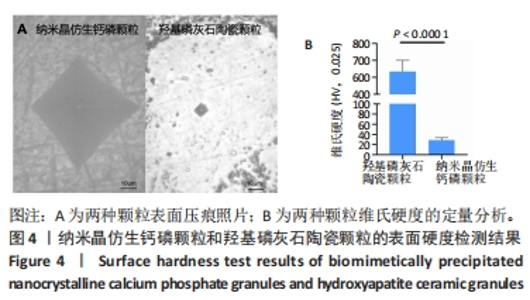
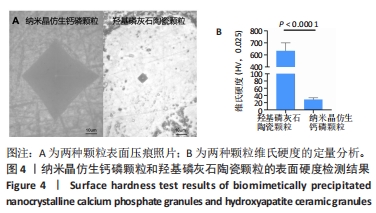
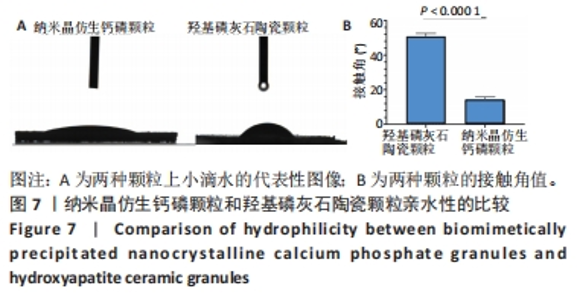
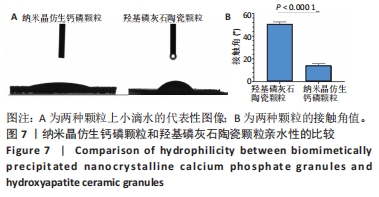
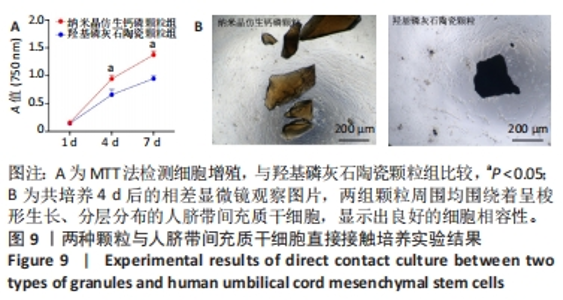
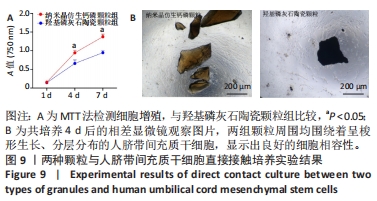
2.8 两种颗粒的细胞相容性 图8A是按照国标GB/T16886实验标准进行的细胞毒性实验,采用浸提液的方法,与人脐带间充质干细胞进行间接培养。阴性对照组的细胞相对增殖率为1,明显高于两实验组。BpNcCaP颗粒组的细胞相对增殖率(0.730 2±0.036 9)低于羟基磷灰石陶瓷颗粒组(0.951 3±0.048 8),但都高于阳性对照组(0.164 3±0.033 7),两实验组的细胞相对增殖率均大于70%,表明颗粒浸提液基本无细胞毒性。图8B相差显微镜观察结果显示,阳性对照细胞皱缩成球,大部分细胞脱离培养皿底部,而BpNcCaP和羟基磷灰石陶瓷颗粒组细胞形态与阴性对照组类似,未见明显变化,呈现出梭形贴壁生长。"

| [1] POH PS, LINGNER T, KALKHOF S, et al. Enabling technologies towards personalization of scaffolds for large bone defect regeneration. Curr Opin Biotechnol. 2022;74:263-270. [2] SHI H, ZHOU Z, LI W, et al. Hydroxyapatite based materials for bone tissue engineering: A brief and comprehensive introduction. Crystals. 2021;11(2):149. [3] BALDWIN P, LI D J, AUSTON DA, et al. Autograft, allograft, and bone graft substitutes: clinical evidence and indications for use in the setting of orthopaedic trauma surgery. J Orthop Trauma. 2019;33(4):203-213. [4] WANG W, YEUNG KW. Bone grafts and biomaterials substitutes for bone defect repair: A review. Bioact Mater. 2017;2(4):224-247. [5] BALDINI N, DE SANCTIS M, FERRARI M. Deproteinized bovine bone in periodontal and implant surgery. Dent Mater. 2011;27(1):61-70. [6] PERIĆ KAČAREVIĆ Z, KAVEHEI F, HOUSHMAND A, et al. Purification processes of xenogeneic bone substitutes and their impact on tissue reactions and regeneration. Int J Artif Organs. 2018;41(11):789-800. [7] RODRÍGUEZ-LUGO V, KARTHIK T, MENDOZA-ANAYA D, et al. Wet chemical synthesis of nanocrystalline hydroxyapatite flakes: effect of pH and sintering temperature on structural and morphological properties. R Soc Open Sci. 2018;5(8):180962. [8] SARTORI S, SILVESTRI M, FORNI F, et al. Ten‐year follow‐up in a maxillary sinus augmentation using anorganic bovine bone (Bio‐Oss). A case report with histomorphometric evaluation. Clin Oral Implants Res. 2003;14(3):369-372. [9] MATHINA M, SHINYJOY E, KAVITHA L, et al. A comparative study of naturally and synthetically derived bioceramics for biomedical applications. Mater Today Proc. 2020;26:3600-3603. [10] 李忠杰,李绍波.磷酸钙人工骨修复骨缺损的研究进展[J].生物骨科材料与临床研究,2021,18(4): 87-91. [11] GEORGE SM, NAYAK C, SINGH I, et al. Multifunctional hydroxyapatite composites for orthopedic applications: a review. ACS Biomater Sci Eng. 2022;8(8):3162-3186. [12] YUAN H, VAN BLITTERSWIJK C, DE GROOT K, et al. A comparison of bone formation in biphasic calcium phosphate (BCP) and hydroxyapatite (HA) implanted in muscle and bone of dogs at different time periods. J Biomed Mater Res A. 2006;78(1):139-147. [13] SCALERA F, GERVASO F, SANOSH K, et al. Influence of the calcination temperature on morphological and mechanical properties of highly porous hydroxyapatite scaffolds. Ceram Int. 2013;39(5):4839-4846. [14] ZHENG Y, WU G, LIU T, et al. A novel BMP2‐coprecipitated, layer‐by‐layer assembled biomimetic calcium phosphate particle: A biodegradable and highly efficient osteoinducer. Clin Implant Dent Relat Res. 2014;16(5):643-654. [15] LIU T, ZHENG Y, WU G, et al. BMP2-coprecipitated calcium phosphate granules enhance osteoinductivity of deproteinized bovine bone, and bone formation during critical-sized bone defect healing. Sci Rep. 2017;7(1):1-12. [16] XU G, SHEN C, LIN H, et al. Development, in-vitro characterization and in-vivo osteoinductive efficacy of a novel biomimetically-precipitated nanocrystalline calcium phosphate with internally-incorporated bone morphogenetic protein-2. Front Bioeng Biotechnol. 2022;10: 920696. [17] WAN B, RUAN Y, SHEN C, et al. Biomimetically precipitated nanocrystalline hydroxyapatite. Nano TransMed. 2022;1(2-4):e9130008. [18] DUAN R, BARBIERI D, LUO X, et al. Variation of the bone forming ability with the physicochemical properties of calcium phosphate bone substitutes. Biomater Sci. 2018; 6(1):136-145. [19] ZHU X, FAN H, XIAO Y, et al. Effect of surface structure on protein adsorption to biphasic calcium-phosphate ceramics in vitro and in vivo. Acta Biomater. 2009;5(4):1311-1318. [20] LI X, DENG Y, CHEN X, et al. Gelatinizing technology combined with gas foaming to fabricate porous spherical hydroxyapatite bioceramic granules. Mater Lett. 2016;185: 428-431. [21] XU G, WANG T, SHEN C, et al. In-vitro physicochemical characterization of a novel type of bone-defect-filling granules-BpNcCaP in comparison to deproteinized bovine bone (Bio-Oss®). Nano TransMed. 2023;2(1):e9130016. [22] ŚLÓSARCZYK A, PASZKIEWICZ Z, PALUSZKIEWICZ C. FTIR and XRD evaluation of carbonated hydroxyapatite powders synthesized by wet methods. J Mol Struct. 2005;744:657-661. [23] 陈鹏.结晶度对羟基磷灰石成骨和骨诱导性影响的研究[D].广州:华南理工大学, 2019. [24] DALL’ARA E, ÖHMAN C, BALEANI M, et al. The effect of tissue condition and applied load on Vickers hardness of human trabecular bone. J Biomech. 2007;40(14):3267-3270. [25] ZHU W, IMAMURA H, MARIN E, et al. Effects of annealing in air on microstructure and hardness of hydroxyapatite ceramics. J Phys D Appl Phys. 2021;54(31):315301. [26] XU Z, LIU C, WEI J, et al. Effects of four types of hydroxyapatite nanoparticles with different nanocrystal morphologies and sizes on apoptosis in rat osteoblasts. J Appl Toxicol. 2012;32(6):429-435. [27] PATEL N, GIBSON IR, KE S, et al. Calcining influence on the powder properties of hydroxyapatite. J Mater Sci Mater Med. 2001;12:181-188. [28] ONI OP, HU Y, TANG S, et al. Syntheses and applications of mesoporous hydroxyapatite: a review. Mater Chem Front. 2023;7:9-43. [29] CHAKRABORTY PK, ADHIKARI J, SAHA P. Variation of the properties of sol–gel synthesized bioactive glass 45S5 in organic and inorganic acid catalysts. Mater Adv. 2021;2(1):413-425. [30] NAGASAKI T, NAGATA F, SAKURAI M, et al. Effects of pore distribution of hydroxyapatite particles on their protein adsorption behavior. J Asian Ceram Soc. 2017;5(2):88-93. [31] WANG K, ZHOU C, HONG Y, et al. A review of protein adsorption on bioceramics. Interface Focus. 2012;2(3):259-277. [32] KLIMEK K, BELCARZ A, PAZIK R, et al. “False” cytotoxicity of ions-adsorbing hydroxyapatite—Corrected method of cytotoxicity evaluation for ceramics of high specific surface area. Mater Sci Eng C. 2016;65:70-79. |
| [1] | Wang Wu, Fan Xiaolei, Xie Jie, Hu Yihe, Zeng Min. Hydroxyapatite-polyvinyl alcohol/collagen-chitosan-gelatin composite hydrogel for repairing rabbit osteochondral defect [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(5): 682-689. |
| [2] | Han Xia, Zhao Ruidong, Yang Junli. Targeted induction of human umbilical cord mesenchymal stem cells cultured with human peripheral blood serum into neural stem cells [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(25): 4000-4004. |
| [3] | Li Xingyu, Zhou Jie, Li Shasha, Zhang Tianxi, Guo Guoning, Yu Anyong, Deng Jiang, Ye Peng. Repair of infected osteochondral defect with sustained release vancomycin three-dimensional scaffold in rabbits [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(22): 3509-3516. |
| [4] | Liu Yan, Zheng Xuexin. Performance of 3D-printed polylactic acid-nano-hydroxyapatite/chitosan/doxycycline antibacterial scaffold [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(22): 3532-3538. |
| [5] | Wu Yaru, Mi Yan, Wei Kaiyue, Gao Heping, Zhang Dingyu, Wang Caili. Regulatory effect of human umbilical cord mesenchymal stem cells on intestinal barrier function in diabetic nephropathy rats [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(19): 2967-2973. |
| [6] | Xie Ting, Liu Tingting, Zeng Xuehui, Li Yamin, Zhou Panghu, Yi Nianhua. Vitamin D3 attenuates high-glucose exposure-induced oxidative stress to promote osteogenic differentiation of human umbilical cord mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(19): 2981-2987. |
| [7] | Bian Lu, Xia Dandan, Qian Yuan, Shi Wen, Que Yunduan, Lyu Long, Xu Aihua, Shi Wentao. Effects of nano-zirconium dioxide on osteogenic differentiation of ectomesenchymal stem cells in nasal mucosa [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(15): 2346-2350. |
| [8] | Liu Zhong, Li Kewei, Wang Min, Liu Wenhui, Zhang Leilei, Guo Song, Qian Hui, Fu Qiang. Effects of micro-electric field on proliferation and osteogenic differentiation of human umbilical cord mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(13): 1983-1988. |
| [9] | Yuan Yuanyuan, Pan Lu, Zhou Shaolan, Liang Yan, Xu Jianwei, Xu Wen. Effect of miR-26b on proliferation, migration and osteogenic differentiation of stem cells from human exfoliated deciduous teeth and human umbilical cord mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(13): 2017-2023. |
| [10] | Lyu Shangyi, He Huiyu, Wufanbieke · Baheti, Yang Quan, Ma Lisha, Han Xiangzhen. Physicochemical properties and biocompatibility of graphene oxide-hydroxyapatite composite coating materials [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(10): 1477-1483. |
| [11] | Qi Junqiang, Wang Haotian, Xiao Bing, Liu Jia, Liu Yifei, Xu Guohua. Characteristics and problems of hydroxyapatite/polymer bone repair material [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(10): 1592-1598. |
| [12] | Zheng Mingkui, Xue Chenhui, Guan Xiaoming, Ma Xun. Human umbilical cord mesenchymal stem cell-derived exosomes reduce the permeability of blood-spinal cord barrier after spinal cord injury [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(1): 50-55. |
| [13] | Zhang Tingting, Liu Juan, Zhang Xu. Bioactivity of phase-transition lysozyme for surface modification of zirconia all-ceramic implant material mediating hydroxyapatite coating [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(7): 1043-1049. |
| [14] | Liu Zixuan, Li Yan, Ji Lin, Xia Delin. Biological properties of nano-hydroxyapatite-zinc oxide composite scaffolds and their effects on the behavior of MC3T3-E1 osteoblasts [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(34): 5441-5447. |
| [15] | Zhao Kang, Lin Si, Ge Xiaotian, Du Xinrui, Han Yingchao. Biosafety evaluation of poly(lactic acid)/nano-hydroxyapatite composite microspheres [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(34): 5497-5504. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||