Chinese Journal of Tissue Engineering Research ›› 2024, Vol. 28 ›› Issue (19): 2967-2973.doi: 10.12307/2024.158
Previous Articles Next Articles
Regulatory effect of human umbilical cord mesenchymal stem cells on intestinal barrier function in diabetic nephropathy rats
Wu Yaru1, Mi Yan2, Wei Kaiyue1, Gao Heping1, Zhang Dingyu1, Wang Caili2
- 1Baotou Medical College of Inner Mongolia University of Science and Technology, Baotou 014000, Inner Mongolia Autonomous Region, China; 2Department of Nephrology, First Affiliated Hospital of Baotou Medical College, Inner Mongolia University of Science and Technology, Baotou 014000, Inner Mongolia Autonomous Region, China
-
Received:2023-03-21Accepted:2023-05-19Online:2024-07-08Published:2023-09-25 -
Contact:Wang Caili, Professor, Chief physician, Department of Nephrology, First Affiliated Hospital of Baotou Medical College, Inner Mongolia University of Science and Technology, Baotou 014000, Inner Mongolia Autonomous Region, China -
About author:Wu Yaru, Master, Attending physician, Baotou Medical College of Inner Mongolia University of Science and Technology, Baotou 014000, Inner Mongolia Autonomous Region, China -
Supported by:National Natural Science Foundation of China, No. 82160141 (to WCL)
CLC Number:
Cite this article
Wu Yaru, Mi Yan, Wei Kaiyue, Gao Heping, Zhang Dingyu, Wang Caili. Regulatory effect of human umbilical cord mesenchymal stem cells on intestinal barrier function in diabetic nephropathy rats[J]. Chinese Journal of Tissue Engineering Research, 2024, 28(19): 2967-2973.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
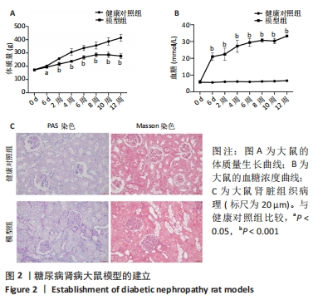
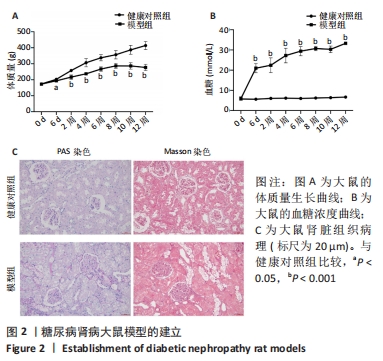
2.2 糖尿病肾病大鼠的一般情况 SD大鼠腹腔注射35 mg/kg链脲佐菌素后,从第6天开始到12周大鼠体质量增加明显低于健康对照组,见图2A。同样,模型组大鼠血糖水平在链脲佐菌素注射后从第6天开始到12周均显著高于健康对照组,见图2B。此外,在链脲佐菌素注射第12周时,健康对照组大鼠肾脏组织PAS染色和Masson染色显示肾小球、肾小管、肾间质及小血管均结构正常;而模型组大鼠肾脏PAS染色和Masson染色显示肾小球系膜细胞和系膜基质轻中度弥漫性增生,基底膜轻中度均匀增厚,肾小管上皮细胞小灶性空泡变性,肾间质小灶性纤维化,小血管管壁轻度增厚,管腔轻度狭窄,肾小球体积增大,见图2C。这些结果说明糖尿病肾病大鼠模型成功建立。"
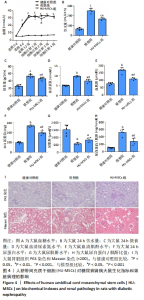
2.4 HU-MSCs对糖尿病肾病大鼠肾脏功能的影响 在糖尿病肾病大鼠建模过程中观察到模型组大鼠的饮水量和摄食量明显高于健康对照组。与模型组相比,HU-MSCs组大鼠饮水和摄食量下降,血糖浓度增加不明显,且在第2次注射后1周血糖水平明显下降,见图4A-C。末次治疗后1周,与健康对照组相比,模型组大鼠尿素氮和血肌酐水平均明显增加,HU-MSCs组大鼠尿素氮和血肌酐水平显著降低,见图4D-E。与模型组比较,HU-MSCs组大鼠24 h尿蛋白水平和尿白蛋白/肌酐比值明显下降,尿肌酐水平上升,见图4F-H。同样,HU-MSCs治疗后大鼠病理改变得到改善,肾小球系膜细胞和系膜基质轻度弥漫性增生,基底膜轻度均匀增厚,肾小管上皮细胞空泡变性数量减少,肾间质纤维化减轻,小血管病变也得到改善,见图4I。"
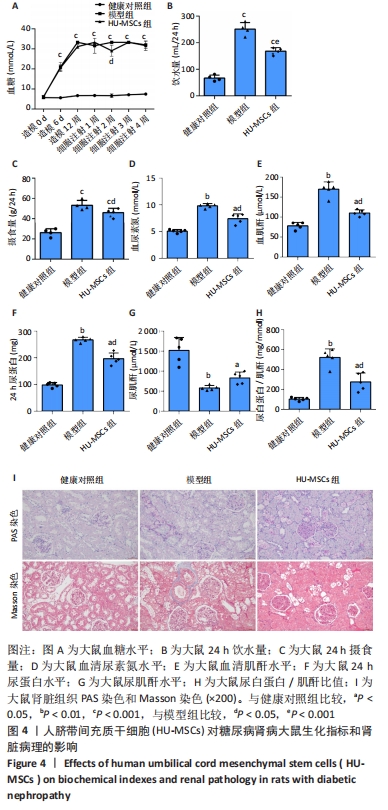
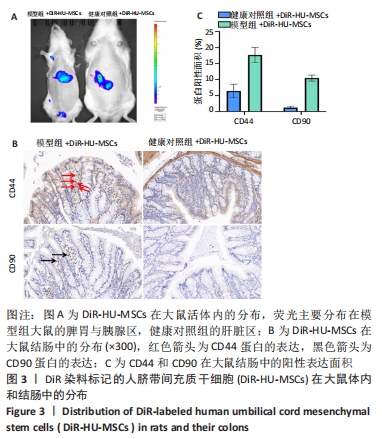
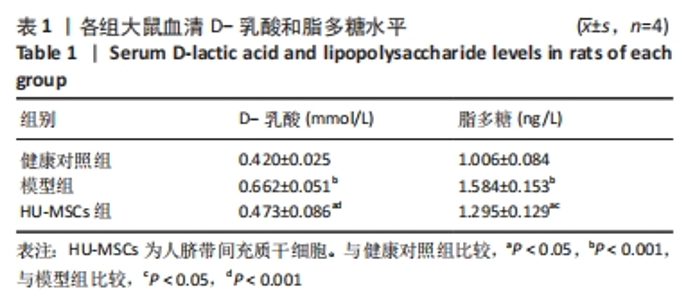
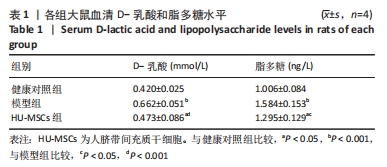
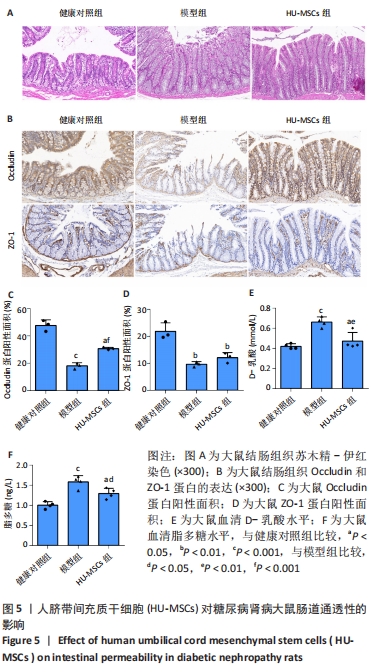
2.5 HU-MSCs移植改善糖尿病肾病大鼠肠道通透性 健康对照组大鼠结肠切片显示黏膜结构正常,而模型组大鼠结肠组织可见黏膜脱落,HU-MSCs治疗后糖尿病肾病大鼠的结肠组织损伤得到改善,见图5A。为了进一步评估肠道屏障的完整性,通过免疫组化方法检测了肠道屏障中紧密连接蛋白(Occludin和ZO-1)的表达。健康对照组大鼠结肠组织中Occludin蛋白阳性细胞主要分布在黏膜上皮,与健康对照组相比,模型组大鼠结肠组织中Occludin蛋白表达水平明显降低,而HU-MSCs治疗后显著提高了结肠组织中Occludin蛋白的表达水平。健康对照组大鼠结肠组织中ZO-1蛋白阳性细胞主要分布在黏膜间质,与健康对照组相比,模型组大鼠结肠组织中ZO-1蛋白表达水平明显降低,而HU-MSCs治疗后显著提高了结肠组织中ZO-1蛋白的表达水平,见图5B-D。为了进一步观察HU-MSCs治疗对糖尿病肾病大鼠肠道屏障功能的改善,检测了大鼠血清D-乳酸和脂多糖水平,结果发现,与健康对照组相比,模型组大鼠血清中D-乳酸和脂多糖水平显著升高,而HU-MSCs组大鼠血清中D-乳酸和脂多糖水平下降,见图5E,F及表1。因此,HU-MSCs可能通过增强必需紧密连接蛋白的表达水平来保护肠道屏障。"
| [1] NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in diabetes since 1980: a pooled analysis of 751 population-based studies with 4.4 million participants. Lancet. 2016;387(10027):1513-1530. [2] ZIMMET PZ, MAGLIANO DJ, HERMAN WH, et al. Diabetes: a 21st century challenge. Lancet Diabetes Endocrinol. 2014;2(1):56-64. [3] SUN H, SAEEDI P, KARURANGA S, et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract. 2022;183:109119. [4] KATO M, NATARAJAN R. Diabetic nephropathy--emerging epigenetic mechanisms. Nat Rev Nephrol. 2014;10(9):517-530. [5] MENG XM, NIKOLIC-PATERSON DJ, LAN HY. TGF-β: the master regulator of fibrosis. Nat Rev Nephrol. 2016;12(6):325-338. [6] RICO-FONTALVO J, AROCA G, CABRALES J, et al. Molecular Mechanisms of Diabetic Kidney Disease. Int J Mol Sci. 2022;23(15):8668. [7] CHUNG S, BARNES JL, ASTROTH KS. Gastrointestinal Microbiota in Patients with Chronic Kidney Disease: A Systematic Review. Adv Nutr. 2019;10(5): 888-901. [8] MEIJERS B, EVENEPOEL P, ANDERS HJ. Intestinal microbiome and fitness in kidney disease. Nat Rev Nephrol. 2019;15(9):531-545. [9] WANG X, YANG S, LI S, et al. Aberrant gut microbiota alters host metabolome and impacts renal failure in humans and rodents. Gut. 2020; 69(12):2131-2142. [10] WANG Y, ZHAO J, QIN Y, et al. The Specific Alteration of Gut Microbiota in Diabetic Kidney Diseases-A Systematic Review and Meta-Analysis. Front Immunol. 2022;13:908219. [11] HAN S, CHEN M, CHENG P, et al. A systematic review and meta-analysis of gut microbiota in diabetic kidney disease: Comparisons with diabetes mellitus, non-diabetic kidney disease, and healthy individuals. Front Endocrinol. 2022;13:1018093. [12] TANG G, DU Y, GUAN H, et al. Butyrate ameliorates skeletal muscle atrophy in diabetic nephropathy by enhancing gut barrier function and FFA2-mediated PI3K/Akt/mTOR signals. Br J Pharmacol. 2022;179(1):159-178. [13] LIN W, LI HY, YANG Q, et al. Administration of mesenchymal stem cells in diabetic kidney disease: a systematic review and meta-analysis. Stem Cell Res Ther. 2021;12(1):43. [14] YIN S, LIU W, JI C, et al. hucMSC-sEVs-Derived 14-3-3ζ Serves as a Bridge between YAP and Autophagy in Diabetic Kidney Disease. Oxid Med Cell Longev. 2022;2022:3281896. [15] SALA E, GENUA M, PETTI L, et al. Mesenchymal Stem Cells Reduce Colitis in Mice via Release of TSG6, Independently of Their Localization to the Intestine. Gastroenterology. 2015;149(1):163-176. [16] YANG S, LIANG X, SONG J, et al. A novel therapeutic approach for inflammatory bowel disease by exosomes derived from human umbilical cord mesenchymal stem cells to repair intestinal barrier via TSG-6. Stem Cell Res Ther. 2021;12(1):315. [17] BOSI E, MOLTENI L, RADAELLI MG, et al. Increased intestinal permeability precedes clinical onset of type 1 diabetes. Diabetologia. 2006;49(12): 2824-2827. [18] ZHONG HJ, YUAN Y, XIE WR, et al. Type 2 Diabetes Mellitus Is Associated with More Serious Small Intestinal Mucosal Injuries. PloS one. 2016;11(9): e0162354. [19] SCHOULTZ I, KEITA ÅV. The Intestinal Barrier and Current Techniques for the Assessment of Gut Permeability. Cells. 2020;9(8):1909. [20] YAN JT, SUN Q, ZHANG XL, et al. Methods for the evaluation of intestinal mucosal permeability. Sheng Li Xue Bao. 2022;74(4):596-608. [21] MONACO A, OVRYN B, AXIS J, et al. The Epithelial Cell Leak Pathway. Int J Mol Sci. 2021;22(14):7677. [22] SHEN L, WEBER CR, RALEIGH DR, et al. Tight junction pore and leak pathways: a dynamic duo. Annu Rev Physiol. 2011;73:283-309. [23] DENG L, YANG Y, XU G. Empagliflozin ameliorates type 2 diabetes mellitus-related diabetic nephropathy via altering the gut microbiota. Biochim Biophys Acta Mol Cell Biol Lipids. 2022;1867(12):159234. [24] 霍丽霞,朱鸣,王霄一,等. 早期糖尿病肾病患者肠道菌群结构特征及菌群移位的研究[Z]. 湖州市第一人民医院. 2020. [25] FRIDENSHTEĬN AIA, PETRAKOVA KV, KURALESOVA AI, et al. Precursor cells for osteogenic and hemopoietic tissues. Analysis of heterotopic transplants of bone marrow. Tsitologiia. 1968;10(5):557-567. [26] LI T, XIA M, GAO Y, et al. Human umbilical cord mesenchymal stem cells: an overview of their potential in cell-based therapy. Expert Opin Biol Ther. 2015;15(9):1293-1306. [27] EL OMAR R, BEROUD J, STOLTZ JF, et al. Umbilical cord mesenchymal stem cells: the new gold standard for mesenchymal stem cell-based therapies? Tissue Eng Part B Rev. 2014;20(5):523-544. [28] CHEN L, XIANG E, LI C, et al. Umbilical Cord-Derived Mesenchymal Stem Cells Ameliorate Nephrocyte Injury and Proteinuria in a Diabetic Nephropathy Rat Model. J Diabetes Res. 2020;2020:8035853. [29] SZYDLAK R. Biological, chemical and mechanical factors regulating migration and homing of mesenchymal stem cells. World J Stem Cells. 2021;13(6):619-631. [30] ULLAH M, LIU DD, THAKOR AS. Mesenchymal Stromal Cell Homing: Mechanisms and Strategies for Improvement. iScience. 2019;15:421-438. [31] WANG S, BAO L, FU W, et al. Protective effect of exosomes derived from bone marrow mesenchymal stem cells on rats with diabetic nephropathy and its possible mechanism. Am J Transl Res. 2021;13(6):6423-6430. [32] MAO R, SHEN J, HU X. BMSCs-derived exosomal microRNA-let-7a plays a protective role in diabetic nephropathy via inhibition of USP22 expression. Life Sci. 2021;268:118937. [33] LIN Y, YANG Q, WANG J, et al. An overview of the efficacy and signaling pathways activated by stem cell-derived extracellular vesicles in diabetic kidney disease. Front Endocrinol (Lausanne). 2022;13:962635. [34] YUE Y, YEH JN, CHIANG JY, et al. Intrarenal arterial administration of human umbilical cord-derived mesenchymal stem cells effectively preserved the residual renal function of diabetic kidney disease in rat. Stem Cell Res Ther. 2022;13(1):186. [35] YU S, CHENG Y, ZHANG L, et al. Treatment with adipose tissue-derived mesenchymal stem cells exerts anti-diabetic effects, improves long-term complications, and attenuates inflammation in type 2 diabetic rats. Stem Cell Res Ther. 2019;10(1):333. [36] RAFIEE Z, ORAZIZADEH M, NEJAD DEHBASHI F, et al. Mesenchymal stem cells derived from the kidney can ameliorate diabetic nephropathy through the TGF-β/Smad signaling pathway. Environ Sci Pollut Res Int. 2022;29(35): 53212-53224. [37] LIU Q, LV S, LIU J, et al. Mesenchymal stem cells modified with angiotensin-converting enzyme 2 are superior for amelioration of glomerular fibrosis in diabetic nephropathy. Diabetes Res Clin Pract. 2020;162:108093. [38] LEE SE, JANG JE, KIM HS, et al. Mesenchymal stem cells prevent the progression of diabetic nephropathy by improving mitochondrial function in tubular epithelial cells. Exp Mol Med. 2019;51(7):1-14. [39] ZHANG F, WANG C, WEN X, et al. Mesenchymal stem cells alleviate rat diabetic nephropathy by suppressing CD103+ DCs-mediated CD8+ T cell responses. J Cell Mol Med. 2020;24(10):5817-5831. [40] LV W, GRAVES DT, HE L, et al. Depletion of the diabetic gut microbiota resistance enhances stem cells therapy in type 1 diabetes mellitus. Theranostics. 2020;10(14):6500-6516. |
| [1] | Xie Ting, Liu Tingting, Zeng Xuehui, Li Yamin, Zhou Panghu, Yi Nianhua. Vitamin D3 attenuates high-glucose exposure-induced oxidative stress to promote osteogenic differentiation of human umbilical cord mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(19): 2981-2987. |
| [2] | Liu Zhong, Li Kewei, Wang Min, Liu Wenhui, Zhang Leilei, Guo Song, Qian Hui, Fu Qiang. Effects of micro-electric field on proliferation and osteogenic differentiation of human umbilical cord mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(13): 1983-1988. |
| [3] | Yuan Yuanyuan, Pan Lu, Zhou Shaolan, Liang Yan, Xu Jianwei, Xu Wen. Effect of miR-26b on proliferation, migration and osteogenic differentiation of stem cells from human exfoliated deciduous teeth and human umbilical cord mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(13): 2017-2023. |
| [4] | Zheng Mingkui, Xue Chenhui, Guan Xiaoming, Ma Xun. Human umbilical cord mesenchymal stem cell-derived exosomes reduce the permeability of blood-spinal cord barrier after spinal cord injury [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(1): 50-55. |
| [5] | Zhao Lu, Zhao Yifei, Gao Da, Liu Yanfang, Fu Tingting, Xu Jiangyan. Expression of suppressor of Zeste 12 in kidney tissues of rats with diabetic nephropathy [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(8): 1179-1186. |
| [6] | Zhang Yuwei, Liu Chuanchuan, Mao Jiaqi, Zhang Qingqing, Liu Hong, Chen Ying, Ma Lan. Umbilical cord mesenchymal stem cell-derived exosomes treated with hypoxic preconditioning inhibits proliferation of pulmonary artery smooth muscle cells [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(19): 2986-2992. |
| [7] | Yang Xu, Wang Feiqing, Zhao Jianing, Zhang Chike, Liang Huiling, Qi Shasha, Wu Dan, Liu Yanqing, Tang Dongxin, Liu Yang, Li Yanju. Effects of multiple myeloma cell conditioned medium on proliferation and differentiation of human umbilical cord mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(10): 1514-1520. |
| [8] | Liu Siqi, Wu Mingrui, Qiao Lingran, Xie Liying, Chen Siyu, Han Zhibo, Zuo Lin. Effects of hydrogel loaded with human umbilical cord mesenchymal stem cells on diabetic wound repair in mice [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(1): 21-27. |
| [9] | Wang Qin, Shen Cheng, Liao Jing, Yang Ye. Dapagliflozin improves renal injury in diabetic nephropathy rats [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(8): 1216-1222. |
| [10] | Zhu Bingbing, Deng Jianghua, Chen Jingjing, Mu Xiaoling. Interleukin-8 receptor enhances the migration and adhesion of umbilical cord mesenchymal stem cells to injured endothelium [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1045-1050. |
| [11] | Xie Liying, Liu Siqi, Wu Mingrui, Gao Erhe, Han Zhibo, Zuo Lin. Human umbilical cord mesenchymal stem cell transplantation for myocardial hypertrophy in mice [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(30): 4826-4833. |
| [12] | Shi Yao, Han Shufeng, Yuan Yitong, Du Ruochen, Jing Zhijie, Zhao Bichun, Zhang Ruxin, Zhang Yujuan, Wang Chunfang. Efficacy and safety of human umbilical cord mesenchymal stem cells in the treatment of spinal cord injury: a meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(25): 4093-4100. |
| [13] | Liu Xingyu, Hu Xiaofang, Xu Guangli, Wang Rui, Li Peiyao, Wang Mengyuan, Peng Li, Zhu Xiangying. Human umbilical cord mesenchymal stem cells in endometrial injury repair [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(24): 3921-3927. |
| [14] | Shan Zhengming, Tao Shuchun, Hu Chunmei, Zhang Zhiyuan, Ding Yinan, He Mengcheng, Tang Qiusha. Extraction, identification and proteomic analysis of exosomes derived from human umbilical cord mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(19): 3036-3042. |
| [15] | Wang Shuyun, Xie Junhui, Yu Xuefeng. Effect and mechanism of mesenchymal stem cells in the treatment of diabetic nephropathy [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(1): 148-152. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||