Chinese Journal of Tissue Engineering Research ›› 2024, Vol. 28 ›› Issue (15): 2391-2397.doi: 10.12307/2024.376
Previous Articles Next Articles
Comparison and analysis of modeling heart valve bracket based on magnetic resonance imaging
Cui Yiwen, Yuan Quan, Liu Jikai
- National Demonstration Center for Experimental Mechanical Engineering Education of Shandong University, Key Laboratory of High Efficiency and Clean Mechanical Manufacture, Ministry of Education, Jinan 250061, Shandong Province, China
-
Received:2023-03-16Accepted:2023-06-08Online:2024-05-28Published:2023-09-23 -
Contact:Yuan Quan, Professor, National Demonstration Center for Experimental Mechanical Engineering Education of Shandong University, Key Laboratory of High Efficiency and Clean Mechanical Manufacture, Ministry of Education, Jinan 250061, Shandong Province, China -
About author:Cui Yiwen, Master candidate, National Demonstration Center for Experimental Mechanical Engineering Education of Shandong University, Key Laboratory of High Efficiency and Clean Mechanical Manufacture, Ministry of Education, Jinan 250061, Shandong Province, China -
Supported by:Shandong Natural Science Foundation Project, No. ZR2020ME143 (to YQ)
CLC Number:
Cite this article
Cui Yiwen, Yuan Quan, Liu Jikai. Comparison and analysis of modeling heart valve bracket based on magnetic resonance imaging[J]. Chinese Journal of Tissue Engineering Research, 2024, 28(15): 2391-2397.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
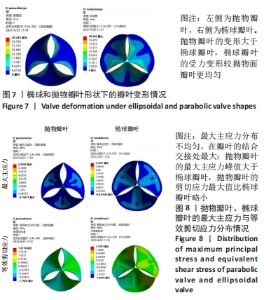
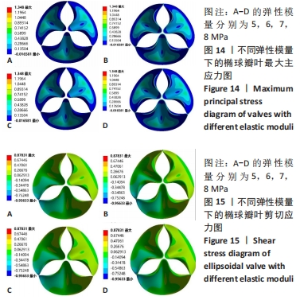
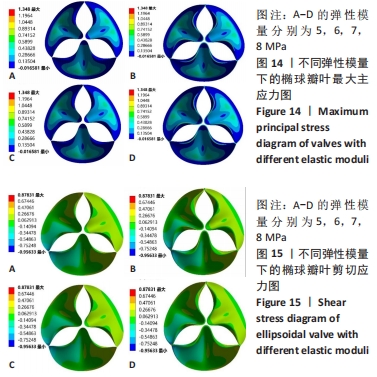
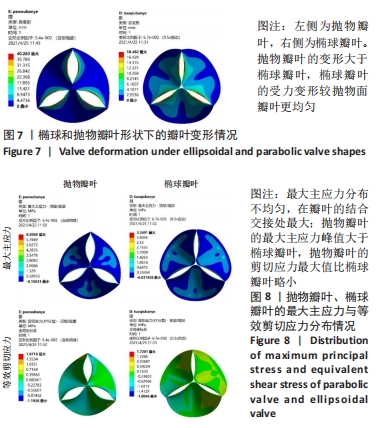
2.1 心脏瓣膜叶力学性能分析 基于ANSYWORKBENCH平台,设定不同的参数后对两种类型的瓣膜叶进行力学分析。结合相应文献假定瓣叶的厚度为0.4-0.6之间[16-17],弹性模量为7 MPa,瓣叶的密度为1 200 kg/m3,泊松比为0.45;模拟血压设定血压为12 kPa,同时设定瓣叶环边缘与瓣架为固定支撑。生物瓣膜瓣叶性能优化及瓣架成型加工方法研究在力学分析过程中严格利用控制变量法进行分析瓣叶的总变形和瓣叶的最大主应力,分别只改变瓣叶的形状、瓣叶的厚度、泊松比和弹性模量来分析对瓣叶力学性能的影响。 2.1.1 不同瓣叶形状下的瓣叶变形及力学分析 只改变瓣叶的形状下对比抛物面瓣叶和椭球瓣叶的总变形情况,如图7所示,抛物瓣叶的变形大于椭球瓣叶,也可见椭球瓣叶的受力变形较抛物面瓣叶来讲较为均匀。由图8可知,最大主应力分布不均匀,在瓣叶的结合交接处最大;抛物瓣叶的最大主应力峰值大于椭球瓣叶;对比抛物瓣叶和椭球瓣叶的等效剪切应力,抛物瓣叶的剪切应力最大值比椭球瓣叶略小。"
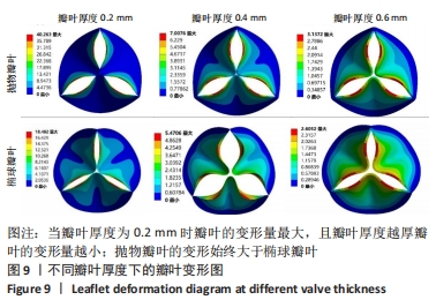
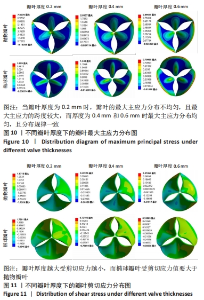
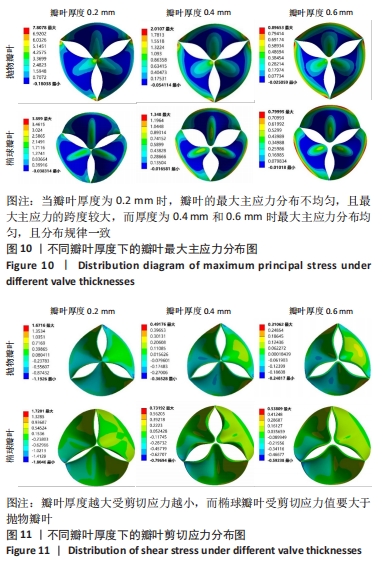
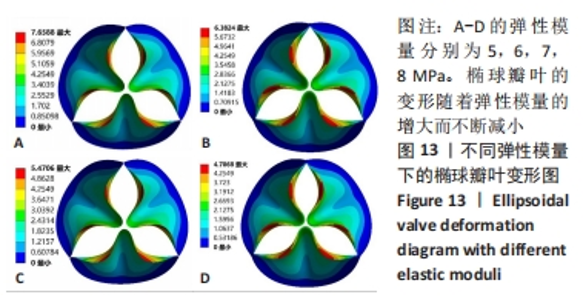
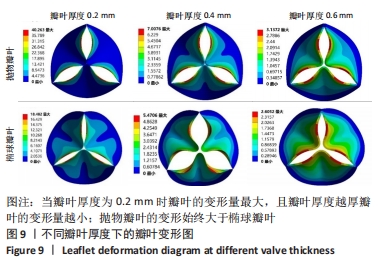
2.1.2 不同瓣叶厚度下的瓣叶变形及力学分析 为探求最合适的瓣叶厚度,观察瓣叶厚度0.2,0.4,0.6 mm对抛物瓣叶和椭球瓣叶的影响。由图9可知,当瓣叶厚度为0.2 mm时瓣叶的变形量最大,且瓣叶厚度越厚瓣叶的变形量越小;抛物瓣叶的变形始终大于椭球瓣叶。其他条件不变,在瓣叶受压一侧施加12 kPa静压力,重新对瓣叶进行分析计算,对比瓣叶最大主应力和剪切应力的受力情况得到图10,11结果。当瓣叶厚度为0.2 mm时,瓣叶的最大主应力分布不均匀,且最大主应力的跨度较大,而厚度为0.4 mm和0.6 mm时最大主应力分布均匀,且分布规律一致。瓣叶厚度越大受剪切应力越小,而椭球瓣叶受剪切应力值要大于抛物瓣叶;抛物瓣叶的最大剪切应力方向为正向,且集中在瓣叶与瓣架结合的边缘处;椭球瓣叶的最大剪切应力方向为负向,同样也集中在结合的边缘处;由此以上分析可知边缘处出现应力集中现象,瓣叶与瓣架的结合边缘为此后优化设计瓣架构型的重点。"
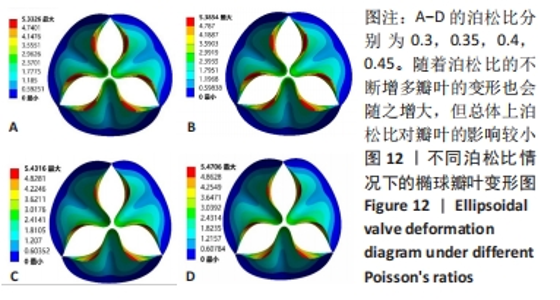
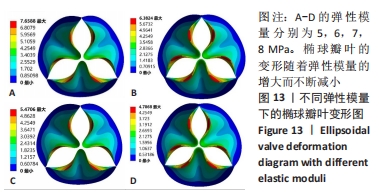
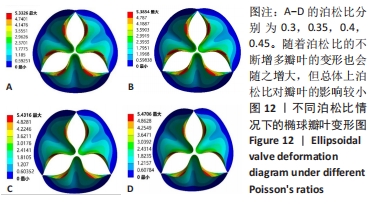
2.1.3 不同泊松比下的瓣叶变形及力学分析 一般采用牛或猪的心切包片材料制作瓣膜,其泊松比为0.45[17]。接下来将材料的泊松比分别改为0.3,0.35,0.4,0.45进行对比。为保证单一变量的原则规定瓣膜的厚度为0.4 mm、弹性模量为7×106 Pa的椭球瓣叶进行分析。 由图12可知,随着泊松比的不断增多瓣叶的变形也会随之增大,但总体上泊松比对瓣叶的影响较小。由于瓣叶的变形影响瓣叶有效开口面积,所以在瓣叶没有失效的情况下选择变形较大的瓣叶。总体来看泊松比对瓣叶变形影响较小,最大的变形和最小的变形分别是5.470 6 mm和5.332 6 mm,相差为0.138 mm。总体来讲泊松比选择0.45有效开口面积更大。"
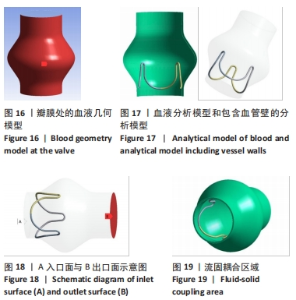
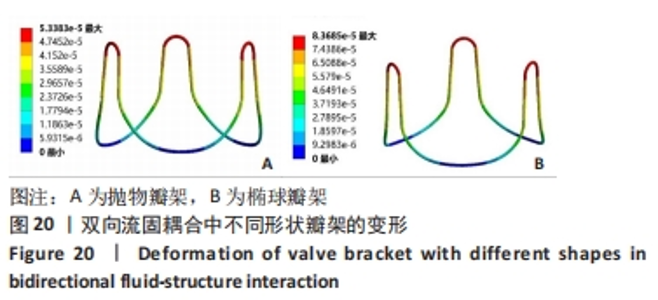
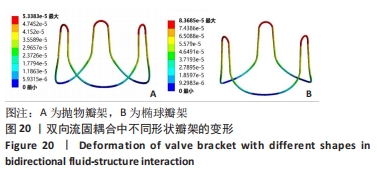
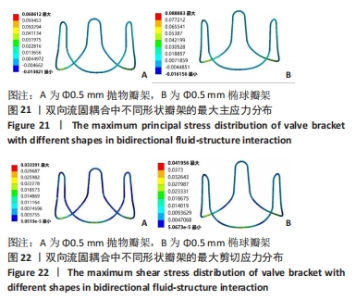
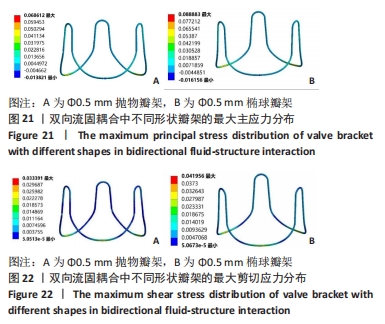
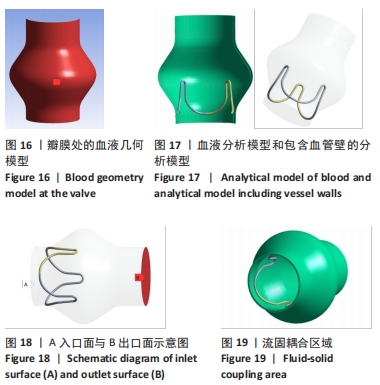
2.2 心脏瓣膜瓣架双向流固耦合分析 双向流固耦合分析是指固体域与流体域相互耦合进行并行计算,两个域之间有压力等参数的数据相互传输[6]。在瓣架进行力学分析时采用双向流固耦合分析,通过计算后即可以分析出瓣架的变形、受力情况,还可以分析出血液流场因瓣架受到的影响。 首先基于医学知识和基于MRI数据模拟出瓣膜处的血液模型如图16所示,利用所得数据进行三维建模,再将绘制出的瓣架与血液模型进行装配如图17。查阅大量医学资料可知,定义血液密度1.105 g/cm3,黏度为0.004 66 Pa?s,弹性模量为3×108 N/m2。定义血液流场的性质,在瓣膜附近的流场环境为:血液由心室流经瓣膜处,通过瓣膜后流至主动脉。设定固定区域的流场属性,定义输入面和输出面,根据血液流动的相关数据设定输入面血液瞬时流速为 0.8 m/s,输出口用压力定义边界,设置出口的压强为0 Pa,如图18所示。流固耦合的区域为瓣架所有面和血液流场区域如图19所示。"
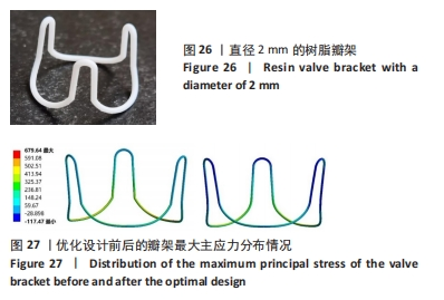
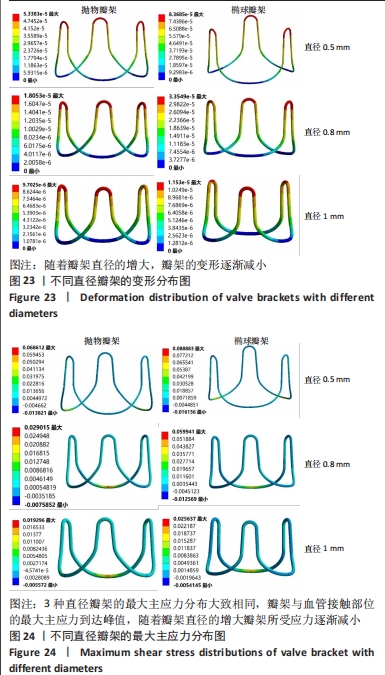
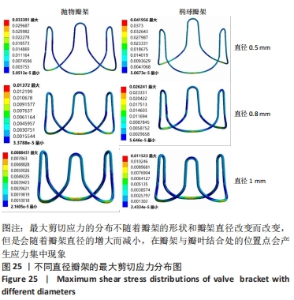
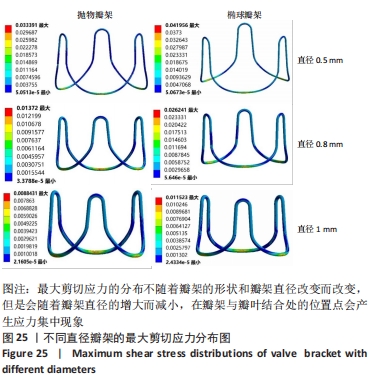
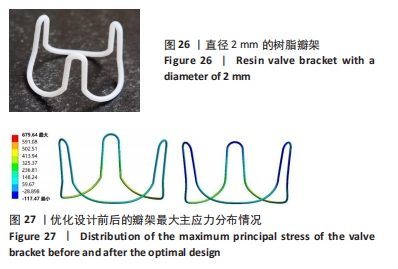
总结:抛物瓣架与椭球瓣架的变形、最大主应力和最大剪切应力的分布规律基本相同。椭球瓣架的变形和所受应力都大于抛物瓣架,二者的最大主应力和最大剪切应力分布规律相同,都在瓣架与瓣叶结合处产生了应力集中现象,且瓣架的总变形、最大主应力和最大剪切应力都随着瓣架直径的增大而减小,抛物瓣架的减小规律是非线性的,椭球瓣架的变化规律是线性的;瓣架直径的改变从总体上来说对整个心脏瓣膜的影响较小,说明瓣架起到一定的支撑作用即可满足相应的物理条件。 2.3 心脏瓣膜瓣架的3D打印 在3D打印材料的选取方面,最初采用聚乳酸-聚乙二醇-聚乳酸三嵌段共聚物材料作为打印材料,其具有良好的生物相容性;目前考虑到经济性和瓣架的力学性能,要求瓣架具有一定的刚度,可以有一定的变形,但总体型要保持不变,同时要具有一定的强度和韧性,所以最终选择高强度树脂Pro 10,该材料是一种介于高韧性和高刚度之间的一种材料,高强度树脂材料的拉伸模量可达到2 500 MPa、弯曲模量2 300 MPa、冲击性能52 J/m、弯曲强度达到90 MPa。后续研究将打印出聚乳酸-聚乙二醇-聚乳酸三嵌段共聚物材料的心脏瓣架与已有的瓣架进行对比[32-39]。 使用Rayshape Shape 1系列3D打印机利用数字光处理打印技术成功打印出直径2 mm的瓣架,如图26所示,但打印完成后发现瓣架的相贯线部分与拟合直线部分没有圆滑过渡连接,产生了顶角。为探究顶角对瓣架的影响对瓣架进行了静力学分析,分别在瓣架的3个圆弧拟合部分施加法线方向的外力,设定瓣架最低点处的面为固定支撑,加入圆角的瓣架和原型瓣架的最大主应力分布如图27所示。"
| [1] 罗乐,许贤玉,郭慧尔,等.人工心脏瓣膜的研究进展[J].合肥工业大学学报(自然科学版),2008,31(2):305-308+312. [2] 孙慧萍,王来成,唐会卓,等.人工机械瓣膜置换术后的抗凝药物治疗分析[J].北方药学,2019,16(3):189-191. [3] 徐志云.人工心脏瓣膜的进展[J].继续医学教育,2006,20(10):63-65. [4] 崔永春,刘晓鹏,张宏,等.不同种类人工心脏瓣膜的比较及其生物学评价[J].中国医疗器械信息,2016,22(1):2-7+14. [5] 田子朴,罗传兴,黄旭中,等.双叶机械瓣的研制和临床应用初步报告[J].中华胸心血管外科杂志,1992,8(1):1. [6] STARR A, EDWARDS ML. Mitral Replacement: Clinical Experience with a Ball-Valve Prosthesis. Ann Surg. 1961;154(4):726-740. [7] SHINOKA T, BREUER CK, TANEL RE, et al. Tissue engineering heart valves: valve leaflet replacement study in a lamb model. Ann Thorac Surg. 1995;60:S513-S516. [8] ROTH GA, JOHNSON C, ABAJOBIR A, et al. Global, Regional, and National Burden of Cardiovascular Diseases for 10 Causes, 1990 to 2015[. J Am Coll Cardiol. 2017;70(1):1-25. [9] 高家红,雷皓,陈群,等.磁共振成像发展综述[J].中国科学:生命科学,2020,50(11):1285-1295. [10] 宋宏宁,郭瑞强.基于医学影像学的3D打印技术在心血管疾病诊疗中的应用现状及研究进展[J].中国医学影像技术,2017,33(3): 375-380. [11] GREIL GF, WOLF I, KUETTNER A, et al. Stereolithographic reproduction of complex cardiac morphology based on high spatial resolution imaging. Clin Res Cardiol. 2007;96(3):176-185. [12] 杜海瑞.仿生人工心脏瓣膜材料多元耦合设计、制备与性能研究[D].长春:吉林大学,2022. [13] 孟丑拴,洪洋,姜华,等.心脏瓣膜生物力学及相关建模方式的研究进展[J].北京生物医学工程,2023,42(2):212-216. [14] 袁泉.生物瓣膜瓣叶性能优化及瓣架成型加工方法研究[D].济南:山东大学,2008. [15] 黄旭.基于ANSYS的生物瓣膜流固耦合力学性能分析[D].济南:山东大学,2014. [16] 袁泉,张承瑞.生物瓣支架几何造型CAD系统[J].南京航空航天大学学报,2005,37(Supp1):141-143. [17] 刘长安.生物瓣膜支架数字化设计与制造[D].济南:山东大学,2009. [18] 董磊.4D flow MRI在心脏瓣膜病临床应用的研究进展[J].中国医学创新,2021,18(10):185-188. [19] 朱海燕.基于ANSYS/LS-DYNA的生物瓣膜动态力学性能分析[D].济南:山东大学,2011. [20] 申炳申.基于FLUENT及LS-DYNA的生物瓣膜流固耦合分析[D].济南:山东大学,2017. [21] 林金利,董柱,郑燕纯,等.3D打印技术在瓣膜性心脏病诊疗中的应用[J].中国胸心血管外科临床杂志, 2022,29(2):267-271. [22] 邓欣,沈雳,葛均波.3D打印技术在心血管疾病中的应用进展[J].中国误诊学杂志,2018,13(6):266-269. [23] 王璐,胡为杰,聂昊,等.3D打印与组织工程心肌、心脏瓣膜、大血管及血管网的构建[J].中国组织工程研究,2015,19(43):7029-7034. [24] 左进富,孙淼,韩宁宁,等.3D生物打印在组织工程中的应用[J].组织工程与重建外科杂志,2019,15(3):201-203. [25] VUKICEVIC M, PUPERI DS, JANE GRANDE-ALLEN K, et al. 3D Printed Modeling of the Mitral Valve for Catheter-Based Structural Interventions. Ann Biomed Eng. 2017;45(2):508-519. [26] 周为.基于模具辅助的心脏主动脉瓣膜3D打印及细胞损伤仿真分析[D].大连:大连理工大学,2019. [27] 韩刘君,孙尧,于晓龙,等.3D打印技术在心血管疾病中的应用现状[J].西南国防医药,2019,29(12):1267-1269. [28] 郭睿霖,白龙,焦轩,等.3D打印在心血管外科的临床应用[J].心血管康复医学杂志,2021,30(3):325-329. [29] SODIAN R, LOEBE M, HEIN A, et al. Application of Stereolithography for Scaffold Fabrication for Tissue Engineered Heart Valves. ASAIO J. 2002;48(1):12-16. [30] DUAN B. State-of-the-Art Review of 3D Bioprinting for Cardiovascular Tissue Engineering. Ann Biomed Eng. 2017;45(1):195-209. [31] MAHMOOD F, OWAIS K, TAYLOR C, et al. Three-Dimensional Printing of Mitral Valve Using Echocardiographic Data. JACC Cardiovasc Imaging. 2015;8(2):227-229. [32] 郭峰.高分子复合材料人工心脏瓣膜制备及其性能研究[D].合肥:中国科学技术大学,2021. [33] 邹明晖,董念国.组织工程心脏瓣膜支架材料的研究与进展[J].中国组织工程研究与临床康复,2010,14(29):5471-5474. [34] 王明玉,令文慧,熊春霞,等.3D ECM凝胶支架对干细胞或祖细胞来源心脏细胞行为的影响[J].中国细胞生物学学报,2019,41(10): 2000-2011. [35] 毛宏理,顾忠伟.生物3D打印高分子材料发展现状与趋势[J].中国材料进展,2018,37(12):949-969+993. [36] 郭双壮.聚乳酸/聚乙二醇纳米凝胶的合成及性能研究[D].天津:天津大学,2008. [37] 龚勇吉.聚乳酸/聚乙二醇嵌段共聚物复合材料的制备与性能研究[D].贵阳:贵州大学,2019. [38] 葛建华,王迎军,郑裕东,等.PLA-PEG-PLA嵌段共聚物的合成及研究[J].材料科学与工程学报,2003(6):817-820. [39] 曹嘉欣.SLA-3D打印光敏树脂的改性及其性能研究[D].西安:西安科技大学,2020. [40] HOCKADAY LA, KANG KH, COLANGELO NW, et al. Rapid 3D printing of anatomically accurate and mechanically heterogeneous aortic valve hydrogel scaffolds. Biofabrication. 2012;4(3):035005. |
| [1] | Kong Xiangyu, Wang Xing, Pei Zhiwei, Chang Jiale, Li Siqin, Hao Ting, He Wanxiong, Zhang Baoxin, Jia Yanfei. Biological scaffold materials and printing technology for repairing bone defects [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(3): 479-485. |
| [2] | Dong Hanqing, Wu Xing, Xu Pengcheng, Wang Qingwen, Zhang Zhisheng, Zhao Jianyong. 3D printing precise positioning guided ulnar groove plasty for treatment of cubital tunnel syndrome [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(18): 2825-2829. |
| [3] | Alimu·Keremu, Liang Zhilin, Pazila·Aila, Maimaitiaili·Abulikemu, Aikebaier·Tuxun. Application of multi-slice spiral CT combined with 3D printing in the treatment of rotationally unstable pelvic fractures [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(27): 4345-4350. |
| [4] | Wu Di, Si Lina, Wu Lizhu, Wang Jianhua, Luo Jinwei, Chang Qiankun, Lyu Yongming, Du Yuanliang. Feasibility of three-dimensional printing technology combined with computer-aided design in total knee arthroplasty for severe knee osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(13): 2051-2057. |
| [5] | Song Yuxin, Zhang Tongtong, Niu Jianxiong, Wang Zengping, Wen Jie, Zhang Qunli, Xue Wen, Liu Lin. Precise screw placement of 3D printing model and orthopedic robot in spinal deformity [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(6): 904-907. |
| [6] | He Guowen, Hu Baijun, Gao Dawei, Chen Liang. Treatment of Schatzker type V and VI tibial plateau fractures with 3D printing preoperative planning combined with double reverse traction device [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(36): 5764-5769. |
| [7] | Li Lu, Tang Wen. Screw placement in posterior route pedicle on subaxial cervical spine: how to improve the therapeutic effect with the development of artificial intelligence [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(3): 474-479. |
| [8] | Jin Herong, Cui Jingbin, Shao Cang. Materials of interbody fusion cage: advantages and focus of clinical application [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(22): 3592-3597. |
| [9] | Li Shan, Liu Chao, Yan Yiguo. Bio-functionalization of medical metal materials in orthopedic application [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(34): 5523-5529. |
| [10] | Yang Jiujie, Wang Tao, Li Zhi, Yang Lifeng, Tian Ye, Bi Zheng, Zeng Yaling. Cervical spondylosis with osteoporosis treated by the new pedicle fixation system through the anterior cervical approach with three-dimensional printing technology: three-dimensional finite element analysis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(33): 5249-5253. |
| [11] | Chen Jie, Liao Chengcheng, Zhao Hongbo, Zhao Wei, Chen Zhiwei, Wang Yan. Application of tissue engineering urethral stent and its preparation technology in urethral reconstruction [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3591-3596. |
| [12] | Yao Lu, Hu Peixin, Liu Wu, Lü Qitao, Nie Zilin, He Zhengdi. Application and prospects of 3D printing technology in dental manufacturing [J]. Chinese Journal of Tissue Engineering Research, 2020, 24(4): 637-642. |
| [13] | Qin Yuxing, Ren Qiangui, Shen Peifeng. Superiority of tissue-engineered bone technology for treating bone defects [J]. Chinese Journal of Tissue Engineering Research, 2020, 24(24): 3877-3882. |
| [14] | Yuan Dechao, Wu Chao, Deng Jiayan, Wang Xiangyu, Li Tao, Tan Lun, Wang Wei, Luo Min . Construction and evaluation of 3D printing simulation model for lumbar surgery [J]. Chinese Journal of Tissue Engineering Research, 2020, 24(12): 1870-1874. |
| [15] | Lin Liulan, Zhou Jianyong. Application status of 3D printed polyetheretherketone and its composite in bone defect repair [J]. Chinese Journal of Tissue Engineering Research, 2020, 24(10): 1622-1628. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||