Chinese Journal of Tissue Engineering Research ›› 2024, Vol. 28 ›› Issue (15): 2364-2370.doi: 10.12307/2024.369
Previous Articles Next Articles
Preparation and characterization of a novel self-assembled polypeptide hydrogel sustainably releasing platelet-rich plasma growth factors
Qi Fengying1, 2, Wang Lei2, Li Dongdong2, Yan Shaoduo2, Liu Kun1, 2, Zheng Yizhe1, 2, He Zixin1, 2, Yi Xiaoyang2, Wang Donggen2, Fu Qiuxia2, Liang Jun1
- 1Department of Light Industry Science and Engineering, Tianjin University of Science and Technology, Tianjin 300457, China; 2Institute of Health Services and Transfusion Medicine, Academy of Military Medical Sciences, Beijing 100850, China
-
Received:2023-04-04Accepted:2023-05-27Online:2024-05-28Published:2023-09-20 -
Contact:Liang Jun, PhD, Professor, Department of Light Industry Science and Engineering, Tianjin University of Science and Technology, Tianjin 300457, China Fu Qiuxia, PhD, Associate researcher, Institute of Health Services and Transfusion Medicine, Academy of Military Medical Sciences, Beijing 100850, China -
About author:Qi Fengying, Master candidate, Department of Light Industry Science and Engineering, Tianjin University of Science and Technology, Tianjin 300457, China; Institute of Health Services and Transfusion Medicine, Academy of Military Medical Sciences, Beijing 100850, China
CLC Number:
Cite this article
Qi Fengying, Wang Lei, Li Dongdong, Yan Shaoduo, Liu Kun, Zheng Yizhe, He Zixin, Yi Xiaoyang, Wang Donggen, Fu Qiuxia, Liang Jun. Preparation and characterization of a novel self-assembled polypeptide hydrogel sustainably releasing platelet-rich plasma growth factors[J]. Chinese Journal of Tissue Engineering Research, 2024, 28(15): 2364-2370.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks

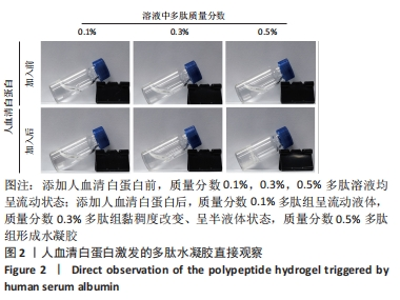
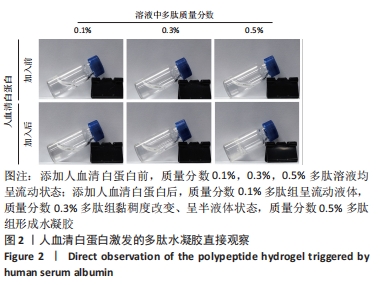
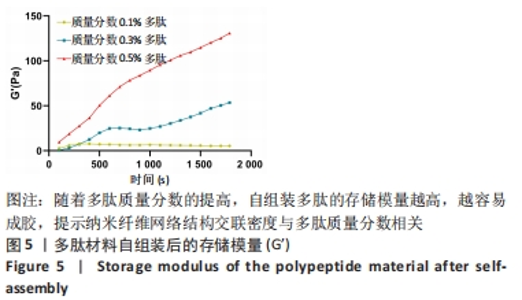
2.1 富血小板血浆对多肽自组装的影响 富血小板血浆纯度达到98%以上,由凝血酶诱导的血小板聚集率达到100%,二磷酸腺苷诱导的血小板聚集率达到30%左右,聚集活性良好。 为了探究富血小板血浆对多肽自组装的影响,首先将贫血小板血浆与自组装多肽溶液混合,直接观察结果显示,血浆可激发多肽溶液自发组装形成多肽水凝胶;富血小板血浆与氯化钙/凝血酶溶液混合,使血浆中丰富的纤维蛋白原形成紧密排列聚合纤维蛋白网络,结果显示成功制备富血小板血浆水凝胶;将不同体积的多肽溶液与富血小板血浆混合均匀后立即添加氯化钙/凝血酶溶液,静置10 min,结果显示形成多肽-富血小板血浆水凝胶外观上与富血小板血浆水凝胶无差异。图1展示了质量分数0.3%多肽-富血小板血浆水凝胶的状态。"


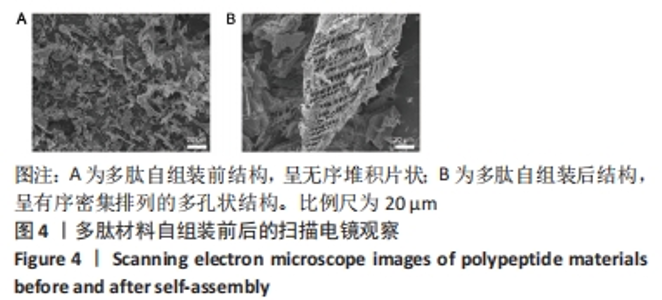
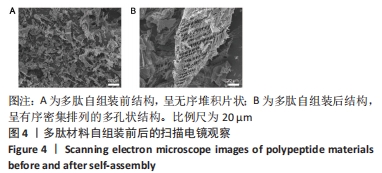
2.3 自组装多肽的微观结构观察 自组装多肽的分析研究多集中于物性力学表征,包括利用透射电镜、扫描电镜等观察其表面形貌[31]。透射电镜观察多肽自组装情况,结果显示:组装前,多肽纳米纤维较短且无序堆积;添加人血清白蛋白后,多肽自组装,多肽纳米纤维明显变长,并且相互交联成束,形成致密的纤维网络结构,见图3。以上结果表明较低质量分数多肽溶液在人血清白蛋白激发下也可自组装,可能由于纳米纤维结合、缠绕的强度不够形成水凝胶。多肽溶液与人血清白蛋白溶液混合,多肽终质量分数为0.1%,干燥后喷金处理,通过扫描电镜进一步观察多肽组装后的微观结构,结果显示,组装前多肽呈无序堆积的片状结构;加入人血清白蛋白后,多肽自组装成相互交联且有序密集排列的多孔状结构,见图4。"

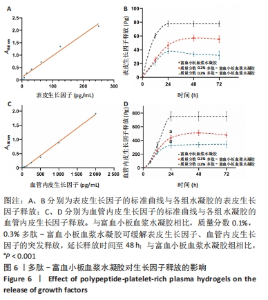
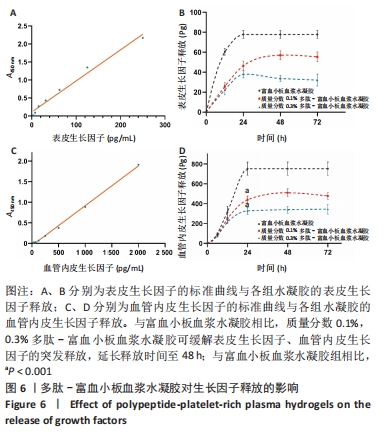
2.5 自组装多肽对富血小板血浆生长因子释放的影响 选择表皮生长因子与血管内皮生长因子为模型蛋白,探究多肽-富血小板血浆水凝胶对富血小板血浆生长因子释放的影响。线性拟合得到表皮生长因子的标准曲线方程为Y=0.008 641X+0.108 0,R2=0.981 7,表明表皮生长因子标准品在0-250 pg/mL范围内具有较好的线性关系。富血小板血浆水凝胶组在12 h时有突释现象,24 h后表皮生长因子的累积释放不再显著增加,释放达到平衡;质量分数0.1%,0.3%多肽-富血小板血浆水凝胶组12 h时的表皮生长因子累积释放量分别为(25.73±3.77),(22.26±4.94) pg,显著低于富血小板血浆水凝胶组的(60.45±3.24) pg(P均< 0.001)。并且质量分数0.1%多肽-富血小板血浆水凝胶组在48 h达到释放平衡,延长了表皮生长因子的释放时间,见图6A、B。 线性拟合得到血管内皮生长因子的标准曲线方程为Y=0.000 966 5X-0.051 82,R2=0.997 5,表明血管内皮生长因子标准品在0-2 000 pg/mL范围内具有较好的线性关系。释放24 h时,质量分数0.1%,0.3%多肽-富血小板血浆水凝胶组血管内皮生长因子累积释放量分别为(439.37±37.84),(327.88±34.96) pg,显著低于富血小板血浆水凝胶组的(752.26±67.11) pg(P均< 0.001),而且质量数0.1%多肽-富血小板血浆水凝胶组延长了血管内皮生长因子的释放时间,在48 h达到释放平衡,见图6C、D。以上结果表明,不同质量分数的自组装多肽(0.1%,0.3%)均可有效延长富血小板血浆中表皮生长因子与血管内皮生长因子的释放时间,对富血小板血浆生长因子具有一定的缓释效果。"
| [1] WILKINSON HN, HARDMAN MJ. Wound healing: cellular mechanisms and pathological outcomes. Open Biol. 2020;10(9):200223. [2] LEGRAND JMD, MARTINO MM. Growth Factor and Cytokine Delivery Systems for Wound Healing. Cold Spring Harb Perspect Biol. 2022;14(8):a041234. [3] YAMAKAWA S, HAYASHIDA K. Advances in surgical applications of growth factors for wound healing. Burns Trauma. 2019;7:10. [4] NIU Y, LI Q, DING Y, et al. Engineered delivery strategies for enhanced control of growth factor activities in wound healing. Adv Drug Deliv Rev. 2019;146:190-208. [5] EVERTS P, OOISHI K, JAYARAM P, et al. Platelet-Rich Plasma: New Performance Understandings and Therapeutic Considerations in 2020. Int J Mol Sci. 2020;21(20):7794. [6] VERMA R, KUMAR S, GARG P, et al. Platelet-rich plasma: a comparative and economical therapy for wound healing and tissue regeneration. Cell Tissue Bank. 2022;12:1-22. [7] 刘宗星,江丽,邹细平,等.自体富血小板凝胶促进创面愈合的临床研究[J].当代医学,2022,28(4):61-64. [8] GODOI TTF, RODRIGUES BL, HUBER SC, et al. Platelet-Rich Plasma Gel Matrix (PRP-GM): Description of a New Technique. Bioengineering (Basel). 2022;9(12):817. [9] ONETO P, ETULAIN J. PRP in wound healing applications. Platelets. 2021;32(2):189-199. [10] ATTILI AR, IACOUCCI C, SERRI E, et al. Antibacterial Properties of Canine Platelet-Rich Plasma and Other Non-Transfusional Hemo-Components: An in vitro Study. Front Vet Sci. 2021;8:746809. [11] SCHAR MO, DIAZ-ROMERO J, KOHL S, et al. Platelet-rich concentrates differentially release growth factors and induce cell migration in vitro. Clin Orthop Relat Res. 2015;473(5):1635-1643. [12] DOHAN EHRENFEST DM, RASMUSSON L, ALBREKTSSON T. Classification of platelet concentrates: from pure platelet-rich plasma (P-PRP) to leucocyte- and platelet-rich fibrin (L-PRF). Trends Biotechnol. 2009; 27(3):158-167. [13] EMING SA, MARTIN P, TOMIC-CANIC M. Wound repair and regeneration: mechanisms, signaling, and translation. Sci Transl Med. 2014;6(265):265sr6. [14] VASILE C, PAMFIL D, STOLERU E, et al. New Developments in Medical Applications of Hybrid Hydrogels Containing Natural Polymers. Molecules. 2020;25(7):1539. [15] HU H, XU FJ. Rational design and latest advances of polysaccharide-based hydrogels for wound healing. Biomater Sci. 2020;8(8):2084-2101. [16] ZHAO W, LI Y, ZHANG X, et al. Photo-responsive supramolecular hyaluronic acid hydrogels for accelerated wound healing. J Control Release. 2020;323:24-35. [17] LIU C, MA Y, GUO S, et al. Topical delivery of chemotherapeutic drugs using nano-hybrid hydrogels to inhibit post-surgical tumour recurrence. Biomater Sci. 2021;9(12):4356-4363. [18] PAN Q, FAN R, CHEN R, et al. Weakly acidic microenvironment of the wound bed boosting the efficacy of acidic fibroblast growth factor to promote skin regeneration. Front Bioeng Biotechnol. 2023;11:1150819. [19] BAHADORAN M, SHAMLOO A, NOKOORANI YD. Development of a polyvinyl alcohol/sodium alginate hydrogel-based scaffold incorporating bFGF-encapsulated microspheres for accelerated wound healing. Sci Rep. 2020;10(1):7342. [20] KUTLU B, TIGLI AYDIN RS, AKMAN AC, et al. Platelet-rich plasma-loaded chitosan scaffolds: preparation and growth factor release kinetics. J Biomed Mater Res B Appl Biomater. 2013;101(1):28-35. [21] SON SR, SARKAR SK, NGUYEN-THUY BL, et al. Platelet-rich plasma encapsulation in hyaluronic acid/gelatin-BCP hydrogel for growth factor delivery in BCP sponge scaffold for bone regeneration. J Biomater Appl. 2015;29(7):988-1002. [22] GROWNEY EA, LINDER HR, GARG K, et al. Bio-conjugation of platelet-rich plasma and alginate through carbodiimide chemistry for injectable hydrogel therapies. J Biomed Mater Res B Appl Biomater. 2020;108(5):1972-1984. [23] CENSI R, CASADIDIO C, DENG S, et al. Interpenetrating Hydrogel Networks Enhance Mechanical Stability, Rheological Properties, Release Behavior and Adhesiveness of Platelet-Rich Plasma. Int J Mol Sci. 2020;21(4):1399. [24] JAIN E, SHETH S, DUNN A, et al. Sustained release of multicomponent platelet-rich plasma proteins from hydrolytically degradable PEG hydrogels. J Biomed Mater Res A. 2017;105(12):3304-3314. [25] 谭婷媛.多肽超分子网络的构建及凝胶性能调控[D].北京:中国科学院大学(中国科学院上海应用物理研究所),2022. [26] 王韵晴,卢婷利,陈婷,等.自组装多肽支架材料的研究进展[J].材料科学与工程学报,2012,30(2):324-328+311. [27] 元亮亮,梁鹏.自组装多肽纳米纤维支架的结构特点及应用优势[J].中国组织工程研究,2013,17(29):5379-5386. [28] LI J, WANG L, YI X, et al. Platelet 3D preservation using a novel biomimetic nanofiber peptide for reduced apoptosis and easy storage. ACS Appl Mater Interfaces. 2021;13(32):38040-38049. [29] 李佳瑶.生物纳米材料在血小板包装储运中的应用研究[D].天津:天津科技大学,2021. [30] WANG P, BERRY D, MORAN A, et al. Controlled growth factor release in 3D-printed hydrogels. Adv Healthc Mater. 2020;9(15):e1900977. [31] ULIJN RV, SMITH AM. Designing peptide based nanomaterials. Chem Soc Rev. 2008;37(4):664-675. [32] KIM HS, SUN X, LEE JH, et al. Advanced drug delivery systems and artificial skin grafts for skin wound healing. Adv Drug Deliv Rev. 2019; 146:209-239. [33] ZHAO X, WU H, GUO B, et al. Antibacterial anti-oxidant electroactive injectable hydrogel as self-healing wound dressing with hemostasis and adhesiveness for cutaneous wound healing. Biomaterials. 2017;122: 34-47. [34] KEIL C, HUBNER C, RICHTER C, et al. Ca-Zn-Ag Alginate Aerogels for Wound Healing Applications: Swelling Behavior in Simulated Human Body Fluids and Effect on Macrophages. Polymers (Basel). 2020; 12(11):2741. [35] ZHENG Z, LI M, SHI P, et al. Polydopamine-modified collagen sponge scaffold as a novel dermal regeneration template with sustained release of platelet-rich plasma to accelerate skin repair: A one-step strategy. Bioact Mater. 2021;6(8):2613-2628. [36] QIU M, CHEN D, SHEN C, et al. Platelet-rich plasma-loaded poly(D,L-lactide)-poly(ethylene glycol)-poly(D,L-lactide) hydrogel dressing promotes full-thickness skin wound healing in a rodent model. Int J Mol Sci. 2016;17(7):1001. [37] CHEN J, ZOU X. Self-assemble peptide biomaterials and their biomedical applications. Bioact Mater. 2019;4:120-131. [38] CALVELO M, GRANJA JR, GARCIA-FANDINO R. Competitive double-switched self-assembled cyclic peptide nanotubes: a dual internal and external control. Phys Chem Chem Phys. 2019;21(37):20750-20756. [39] CAO M, WANG Y, HU X, et al. Reversible thermoresponsive peptide-PNIPAM hydrogels for controlled drug delivery. Biomacromolecule. 2019;20(9):3601-3610. [40] El BACKLY RM, ZAKY SH, MURAGLIA A, et al. A platelet-rich plasma-based membrane as a periosteal substitute with enhanced osteogenic and angiogenic properties: a new concept for bone repair. Tissue Eng Part A. 2013;19(1-2):152-165. [41] ROSADI I, KARINA K, ROSLIANA I, et al. In vitro study of cartilage tissue engineering using human adipose-derived stem cells induced by platelet-rich plasma and cultured on silk fibroin scaffold. Stem Cell Res Ther. 2019;10(1):369. [42] YANG HS, SHIN J, BHANG SH, et al. Enhanced skin wound healing by a sustained release of growth factors contained in platelet-rich plasma. Exp Mol Med. 2011;43(11):622-629. [43] TAMBELLA AM, ATTILI AR, DUPRE G, et al. Del Fabbro M. Platelet-rich plasma to treat experimentally-induced skin wounds in animals: A systematic review and meta-analysis. PLoS One. 2018;13(1):e0191093. [44] CHOI SM, LEE KM, KIM HJ, et al. Effects of structurally stabilized EGF and bFGF on wound healing in type I and type II diabetic mice. Acta Biomater. 2018;66:325-334. [45] APTE RS, CHEN DS, FERRARA N. VEGF in signaling and disease: beyond discovery and development. Cell. 2019;176(6):1248-1264. [46] MARTINO MM, BRIQUEZ PS, RANGA A, et al. Heparin-binding domain of fibrin(ogen) binds growth factors and promotes tissue repair when incorporated within a synthetic matrix. Proc Natl Acad Sci U S A. 2013; 110(12):4563-4568. |
| [1] | Yang Yufang, Yang Zhishan, Duan Mianmian, Liu Yiheng, Tang Zhenglong, Wang Yu. Application and prospects of erythropoietin in bone tissue engineering [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(9): 1443-1449. |
| [2] | Chen Kaijia, Liu Jingyun, Cao Ning, Sun Jianbo, Zhou Yan, Mei Jianguo, Ren Qiang. Application and prospect of tissue engineering in treatment of osteonecrosis of the femoral head [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(9): 1450-1456. |
| [3] | Mei Jingyi, Liu Jiang, Xiao Cong, Liu Peng, Zhou Haohao, Lin Zhanyi. Proliferation and metabolic patterns of smooth muscle cells during construction of tissue-engineered blood vessels [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(7): 1043-1049. |
| [4] | Wang Shanshan, Shu Qing, Tian Jun. Physical factors promote osteogenic differentiation of stem cells [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(7): 1083-1090. |
| [5] | Yin Tong, Yang Jilei, Li Yourui, Liu Zhuoran, Jiang Ming. Application of core-shell structured nanofibers in oral tissue regeneration [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(5): 766-770. |
| [6] | Chen Xiaofang, Zheng Guoshuang, Li Maoyuan, Yu Weiting. Preparation and application of injectable sodium alginate hydrogels [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(5): 789-794. |
| [7] | Wang Jiani, Chen Junyu. Angiogenesis mechanism of metal ions and their application in bone tissue engineering [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(5): 804-812. |
| [8] | Shen Ziqing, Xia Tian, Shan Yibo, Zhu Ruijun, Wan Haoxin, Ding Hao, Pan Shu, Zhao Jun. Vascularized tracheal substitutes constructed by exosome-load hydrogel-modified 3D printed scaffolds [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(5): 697-705. |
| [9] | Zhu Liwei, Wang Jiangyue, Bai Ding. Application value of nanocomposite gelatin methacryloyl hydrogels in different bone defect environments [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(5): 753-758. |
| [10] | Maisituremu·Heilili, Zhang Wanxia, Nijiati·Nuermuhanmode, Maimaitituxun·Tuerdi. Effect of intraarticular injection of different concentrations of ozone on condylar histology of rats with early temporomandibular joint osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(4): 505-509. |
| [11] | Yang Yuqing, Chen Zhiyu. Role and application of early transient presence of M1 macrophages in bone tissue engineering [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(4): 594-601. |
| [12] | Ma Sicong, Chen Jing, Li Yunqing. Functions and roles of connective tissue growth factor in nervous systems [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(4): 615-620. |
| [13] | Liu Xueli, Shen Li, Bi Wenguang, Mou Yang, Li Sen. Effect and mechanism of low intensity pulsed ultrasound on early angiogenesis in rats with acute tendon injury [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(32): 5097-5103. |
| [14] | Xiong Yang, Zhou Shibo, Yu Xing, Bi Lianyong, Yang Jizhou, Wang Fengxian, Qu Yi, Yang Yongdong, Zhao Dingyan, Zhao He, Qiu Ziye, Jiang Guozheng. Molecular biological mechanism of acquired heterotopic ossification [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(30): 4881-4888. |
| [15] | Gu Mingxi, Wang Changcheng, Tian Fengde, An Ning, Hao Ruihu, Guo Lin. Preparation and in vitro evaluation of a three-dimensional porous cartilage scaffold made of silk fibroin/gelatin/chitosan [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(3): 366-372. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||