Chinese Journal of Tissue Engineering Research ›› 2024, Vol. 28 ›› Issue (30): 4881-4888.doi: 10.12307/2024.647
Previous Articles Next Articles
Molecular biological mechanism of acquired heterotopic ossification
Xiong Yang, Zhou Shibo, Yu Xing, Bi Lianyong, Yang Jizhou, Wang Fengxian, Qu Yi, Yang Yongdong, Zhao Dingyan, Zhao He, Qiu Ziye, Jiang Guozheng
- Department of Orthopedics, Dongzhimen Hospital, Beijing University of Chinese Medicine, Beijing 100700, China
-
Received:2023-08-07Accepted:2023-10-07Online:2024-10-28Published:2023-12-28 -
Contact:Yu Xing, Professor, Doctoral supervisor, Chief physician, Department of Orthopedics, Dongzhimen Hospital, Beijing University of Chinese Medicine, Beijing 100700, China -
About author:Xiong Yang, MD, Physician, Department of Orthopedics, Dongzhimen Hospital, Beijing University of Chinese Medicine, Beijing 100700, China Zhou Shibo, MD, Department of Orthopedics, Dongzhimen Hospital, Beijing University of Chinese Medicine, Beijing 100700, China -
Supported by:National Natural Science Foundation of China, No. 81973882 (to YX); National Natural Science Foundation of China, No. 82305273 (to XY); New Teacher Initiation Fund Project of Beijing University of Chinese Medicine, No. 2023-BUCMXJKY007 (to XY)
CLC Number:
Cite this article
Xiong Yang, Zhou Shibo, Yu Xing, Bi Lianyong, Yang Jizhou, Wang Fengxian, Qu Yi, Yang Yongdong, Zhao Dingyan, Zhao He, Qiu Ziye, Jiang Guozheng. Molecular biological mechanism of acquired heterotopic ossification[J]. Chinese Journal of Tissue Engineering Research, 2024, 28(30): 4881-4888.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
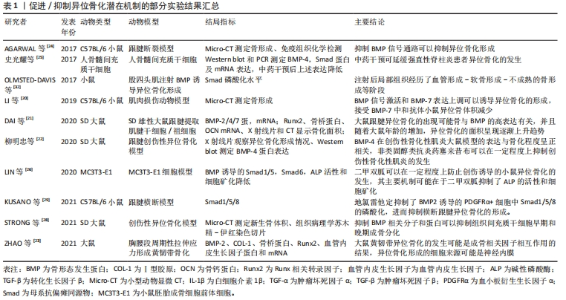
2.1 获得性异位骨化相关信号通路 2.1.1 骨形态发生蛋白信号通路 骨形态发生蛋白是转化生长因子超家族中最大的分支,在人类已发现近30种亚型。自21世纪初,骨形态发生蛋白通路在促骨与软骨分化及骨代谢疾病领域得到了最广泛的关注。在异位骨化的发生发展过程中,骨形态发生蛋白起到重要作用。以骨形态发生蛋白2/4/7为代表,诱导多种前体/干细胞成骨向分化,促进异位骨化的形成。肌腱、韧带、长骨在受到创伤或反复机械刺激后,组织细胞分泌骨形态发生蛋白水平显著增加,导致跟腱、脊柱周围韧带及大关节出现异位骨组织[7,11,16-23]。相反,抑制或阻断骨形态发生蛋白通路表达则可显著缓解异位骨化的形成[17,24-30]。在肌肉间隙、周围神经内膜组织中存在着大量多潜能细胞,自然状态下表现出成脂、成肌或保护神经作用,但在骨形态发生蛋白2刺激后则表现出成骨活性[31-32]。局部注射骨形态发生蛋白2制剂或植入肌袋,被广泛应用于异位骨化动物模型的制备[33-34]。在临床长骨骨折、关节置换或脊柱融合等手术中,骨形态发生蛋白2被用于加速新骨生成促进骨愈合,部分研究获得了显著的疗效[35-36]。但另有大量研究显示,递送骨形态发生蛋白2的可吸收胶原海绵或植骨块常不能控制骨形态发生蛋白在局部大量突释,导致融合率上升的同时也增加了异位骨化的发生率[37-44],甚至引发严重不良事件,极大限制了外源性骨形态发生蛋白2在临床的推广应用[45]。部分学者尝试在有效性与安全性之间探索可维持平衡的适宜剂量[46-47],另有学者尝试研发新型生物材料以改善骨形态发生蛋白2的缓释性能及靶点分布,包括人羊膜[48]、肝素基微粒或多电解质复合物[49-51]、骨形态发生蛋白2/壳聚糖/硫酸葡聚糖复合微球、海藻酸水凝胶、载骨形态发生蛋白2糖胺聚糖/多聚赖氨酸复合材料等[52-54],但新型材料的安全性及有效性仍有待进一步研究。 骨形态发生蛋白信号的表达具有环境依赖性或时空特异性,与其他多种信号因子形成交叉信号网络,相互作用,共同调节细胞局部微环境以干预异位骨化的发生发展[55-58]。DAVIS等[59]向大鼠肌肉中注射骨形态发生蛋白2后并未诱导形成异位骨化,由此认为过低剂量骨形态发生蛋白2并不能独立诱导异位骨化的产生。KAN等[60]在创伤性异位骨化动物模型中发现,骨形态发生蛋白4与IHH (Indian hedgehog)可通过反馈和非细胞自治机制共同调节干细胞局部微环境,激发下游成骨级联反应导致异位骨化的形成。AGARWAL 等[61]认为,肌肉损伤后骨形态发生蛋白联合哺乳动物雷帕霉素靶蛋白(Mammalian target of rapamycin,mTOR)共同作用促进了异位骨化的形成,应用雷帕霉素阻断mTOR可消除损伤相关纤维化和异位骨化形成,同时协同骨形态发生蛋白促进肌肉损伤的愈合。创伤可导致血清中具有更高浓度的白细胞介素和肿瘤坏死因子α炎性因子,它们可促进骨形态发生蛋白2的产生[62]。然而肿瘤坏死因子亦可抑制骨形态发生蛋白表达而起到限制异位骨化的作用[16]。近年有研究报道了骨形态发生蛋白/Smad可能联合WNT/β-catenin信号通路介导内皮间充质转化过程,并参与结肠癌术后患者异位骨化的形成过程[63-64]。FU等[65]发现,骨形态发生蛋白7可通过GSK-3激活Wnt/β-catenin通路加速间充质干细胞的成软骨向分化,而软骨细胞的增殖、肥大是异位骨化形成早期软骨内化骨过程中的重要环节。 局部神经、血管损伤对骨形态发生蛋白介导的异位骨化也起到协同作用。创伤激活感觉神经并释放疼痛介质P物质和降钙素基因相关肽。疼痛介质P物质可调节骨形态发生蛋白的表达并协同诱导环氧合2产生前列腺素,同时可刺激单核细胞、巨噬细胞、肥大细胞产生炎症因子,增强异位骨化早期炎症反应;降钙素基因相关肽可能作为疼痛介质P物质诱导炎症反应的一个调节因子,虽然可上调骨形态发生蛋白2的表达,促进软骨形成,但却延迟软骨细胞肥大和基质矿化,通过上调cAMP水平削弱软骨内化骨[66]。创伤后组织中升高的骨形态发生蛋白诱导局部棕色脂肪组织含量升高,通过消耗氧气和降低氧气溶解度导致周围细胞缺氧,局部低氧微环境诱导缺氧诱导因子1、血管内皮生长因子产生,促进细胞成软骨分化和新血管形成,为异位骨化形成提供条件[67-68];局部肥大细胞募集、脱颗粒,分泌的基质金属蛋白酶9可进一步打开血管、神经屏障,使成骨相关前体/干细胞聚集和细胞因子,包括胰岛素样生长因子1、骨桥蛋白及Runt相关转录因子2(Runx2),表达进一步上调,最终促异位骨化的形成[69-70]。 作者发现,获得性异位骨化的实验研究大多数以动物实验为主,多见大鼠/小鼠跟腱损伤/断裂模型,干预方式多为通过给予异位骨化诱导剂,诱导局部异位骨化的形成,进而给予某种干预措施,干预后可以在一定程度上抑制异位骨化的发生,检测手段方面包括X射线片、Micro-CT等影像学检查,通过影像学观察局部骨形成的二维或三维表现;除此之外,组织病理学切片多观察、检测成骨特异性表达基因如碱性磷酸酶、Runx2及骨桥蛋白为主。除此之外,实验对象还包括骨髓间充质干细胞等,见表1。"
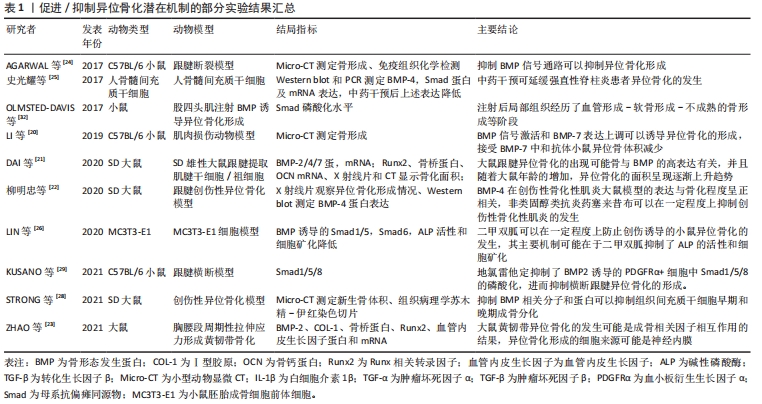

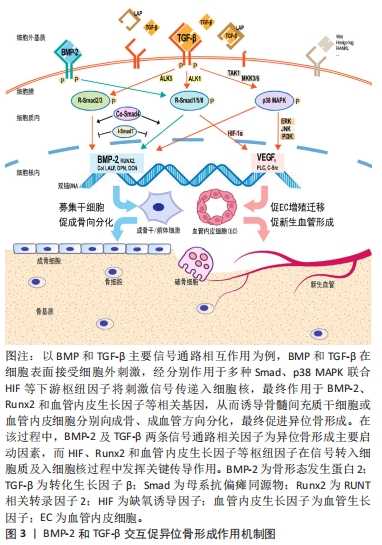
2.1.2 转化生长因子信号通路 高度保守的转化生长因子遍布人体全身大部分细胞,参与了组织生长发育、伤口愈合、骨代谢稳态调节、免疫调节等多种生命活动。在哺乳动物转化生长因子主要包括转化生长因子1、转化生长因子2和转化生长因子3亚型,转化生长因子1含量最丰富,对骨与软骨发育及相关细胞功能表型具有核心作用。在复杂的异位骨化分子信号网络中,转化生长因子通常与多种因子相互作用共同调节异位骨化发生与发展。转化生长因子信号传导入细胞核后作用于转录因子Runx2,与骨形态发生蛋白协同调控间充质干/祖细胞的软骨向分化。YU等[71]认为创伤后异位骨化的形成与周围组织中转化生长因子1、骨形态发生蛋白1、白细胞介素1β、缺氧诱导因子1α及基质金属蛋白酶2基因表达增高有关。ZHANG等[72]研究发现,在创伤局部转化生长因子诱导细胞外基质转化生长因子人克隆基因3 (ig-h3)蛋白表达,增加肌腱源干细胞之间的黏附,最终增强软骨分化促进异位骨化的发展。在机械应力及神经根性痛刺激下,转化生长因子与骨形态发生蛋白经Smad和MAPK通路又协同介导韧带骨化[73]。在神经肌肉联合损伤中,转化生长因子1与神经炎症相关因子P物质、制瘤素M协同促进成纤维-成脂祖细胞向成骨细胞分化,最终促进了异位骨化产生[74]。 转化生长因子对骨代谢调节具有高度的时空特异性。成骨早期,基于经典Smads信号传导,转化生长因子与骨形态发生蛋白可协同促进软骨细胞分化与增殖,但在特定时空环境下,转化生长因子又抑制了骨形成后期成骨细胞向骨细胞分化及基质矿化过程,由此转化生长因子对骨形成存在双向调节作用。前期研究中,基于不同的实验方法和动物模型,转化生长因子调节异位骨化得出不一致甚至相互矛盾的结果,其具体作用机制尚未达成共识。近年研究显示,转化生长因子的异常表达是异位骨化形成的重要因素[75] ,它也成为防治异位骨化的潜在靶点。ZHONG等[76]通过改变转化生长因子受体蛋白结构阻断转化生长因子/Smad7信号传递,显著抑制异位骨化的形成。ZENG 等[77]向大鼠跟腱创伤异位骨化模型中注入高分子聚乙烯颗粒后可显著减少异位骨化形成,组织学检测发现实验组转化生长因子、骨形态发生蛋白2及Runx2等成骨因子表达均低于对照组。MAO等[78]将苦参碱药液按75 mg/kg进行腹腔注射可有效削弱小鼠跟腱创伤异位骨化的形成,其机制在于苦参碱通过抑制转化生长因子下游Smad2/3磷酸化和Runx2、碱性磷酸酶和骨钙蛋白的转录,减少局部微环境中间充质干细胞的迁移和成骨分化以发挥作用。在跟腱创伤性异位骨化局部,破骨细胞介导骨吸收可显著提高活化的转化生长因子含量,后者向局部微环境中募集间充质干/祖细胞促进异位骨化的发展,应用转化生长因子中和性抗体可削弱异位骨的形成,进一步敲除间充质干细胞内转化生长因子Ⅱ型受体,可显著抑制异位骨化的进展[79]。Smad7可竞争性阻碍Samd4与Smad2/3形成复合物,扰乱转化生长因子信号传导。王威等[80]通过正弦交变电磁场促进Smad7表达抑制Smad3磷酸化,从而阻断转化生长因子信号传导抑制大鼠跟腱创伤性异位骨化的进展。除了Smad靶点,阻断下游非经典TAK1信号转导亦可显著抑制间充质祖细胞和成软骨细胞的分化,削弱创伤性异位骨化的形成[81]。由此说明,基于转化生长因子受体复合物或下游信号因子,降低异位骨化微环境中转化生长因子的表达是抑制异位骨化的有效途径,详见图3。"

2.1.3 Hedgehog信号通路 Hedgehog(HH)在物种间具有高度保守性,对肌腱、长骨生长及骨组织修复具有重要作用,HH与骨形态发生蛋白、成纤维细胞生长因子及Wnt等多种信号通路相互作用,参与到生长板及干骺端软骨细胞的增殖、肥大、软骨内化骨及异位骨化的形成中[82]。Mkx基因在肌腱组织稳态中起关键作用,阻断Mkx表达可激活小鼠跟腱组织中HH异常表达,诱导跟腱源细胞进行软骨内化骨最终导致跟腱异位骨化的形成[83]。FENG等[84]利用Ctsk-Cre标记跟腱组织中具有高度自我更新和分化潜能的肌腱源祖细胞,HH信号由于下游Sufu转录因子的功能缺失可介导肌腱源祖细胞命运改变,促进其向软骨和成骨细胞分化,由此促进异位骨化的形成。HH仅在异位骨化病灶周围组织中表达增高,在病灶内部表达减弱,抑制HH信号通路可降低局部Runx2、碱性磷酸酶、Osterix、骨钙蛋白、骨桥蛋白等成骨标志物的水平[85],但并不足以完全阻止软骨内异位骨化进程,说明HH信号通路参与了异位骨化形成,但其作用并不是必须的,这与膜内成骨具有不同的机制[86]。创伤性异位骨化主要通过软骨内成骨形成,HH仅在软骨细胞肥大前作为非必需调节因素参与异位骨化的形成,这导致HH靶向抑制在异位骨化防治中作用有限,对创伤后异位骨化的有效预防需要不同类型HH抑制剂或与其他药物联合应用才能发挥完全阻断作用[86-87]。 2.1.4 Wnt信号通路 Wnt/β-catenin是Wnt信号传导的经典通路,主要作用于骨代谢促进骨重塑,该作用具有精确的时空特异性,其异常表达可导致骨质疏松或高骨量疾病发生,包括异位骨化和肿瘤的形成。 近年Wnt参与异位骨化相关研究大都集中于强直性脊柱炎疾病相关异位骨化形成中。ZOU 等[88]研究发现,与对照组比较,强直性脊柱炎患者髋关节滑膜组织中DKK-1表达下调,一方面促进了成纤维细胞过度增殖和凋亡率降低,另一方面,降低了对Wnt配体与其受体结合的抑制作用,增加Wnt下游β-catenin信号传导促进强直性脊柱炎患者异位骨组织的形成,这可能成为强直性脊柱炎患者异位骨化发展的潜在机制。另有研究发现,雷公藤红素可抑制前列腺素E2的表达,降低其成骨诱导性,同时可增加GSK-3的表达,抑制Wnt/β-catenin信号传导,由此抑制强直性脊柱炎源成纤维细胞的增殖和成骨分化,且抑制作用具有时间剂量依赖性[89],这为治疗强直性脊柱炎患者异位骨化形成提供了潜在靶点。一定浓度补肾舒脊颗粒含药血清作用于骨髓间充质干细胞,可有效下调细胞Wnt5a、β-catenin相应mRNA和蛋白的表达,干预骨髓间充质干细胞成骨,这为延缓强直性脊柱炎患者异位骨化进展提供了新思路[90]。机械载荷在强直性脊柱炎患者异位骨化进展中具有重要作用,ZHANG 等[91]通过尾部悬吊减少强直性脊柱炎小鼠模型骶髂关节的机械负荷,观察发现局部组织中miR103上调,它可抑制MAPK信号通路中机械激酶ROCK1和p-Erk1/2的活性,从而抑制局部组织中Wnt/β-catenin通路,延缓强直性脊柱炎骶髂关节骨化进展。然而,将跟腱源干细胞在低负荷环境下进行长时间培养后,细胞内β-catenin持续累积,最终导致异位骨化的发生和进展[92]。典型的弥漫性特发性骨肥厚症(diffuse idiopathic skeletal hyperostosis,DISH)患者颈椎体前可见到大量异位骨化组织,局部骨髓间充质干细胞中半乳糖凝集素3具有更高表达,体内实验显示,它可激活Wnt/β-catenin的表达,促进异位骨化的形成,体外研究表明半乳糖凝集素3可增加β-catenin在骨髓间充质干细胞细胞核内积累,促进其成骨分化,这说明异位骨化形成与半乳糖凝集素3和Wnt/β-catenin关系密切相关,后者可作为预防DISH患者异位骨化进展的潜在靶点[93]。 2.1.5 mTOR信号通路 mTOR是一种丝氨酸/苏氨酸蛋白激酶,通过mTORC1和mTORC2两个不同蛋白介导多种信号因子传递,与骨形态发生蛋白和Wnt等共同作用,对骨骼发育及内稳态调节起到重要作用,其异常表达可导致多种骨代谢疾病产生。XU等[94]在肌腱炎患者组织中发现Nesfatin-1具有高表达,体内大鼠跟腱损伤模型研究显示,Nesfatin-1主要通过激活mTOR通路,抑制跟腱干细胞肌腱表型,促进跟腱局部异位骨化的产生。将雷帕霉素在跟腱损伤局部缓释,可有效抑制p-mTOR的表达,抑制白细胞介素1诱导的跟腱干细胞的成骨分化,显著缓解异位骨化的进展,当敲除跟腱细胞中mTOR后,局部组织中骨形态发生蛋白、转化生长因子表达亦显著降低[95]。氧化应激可加剧异位骨化早期局部缺氧,雷帕霉素不仅抑制了mTOR的表达[96],同时也削弱了局部微环境中氧化应激过程[97],这使得基于mTOR干预干细胞成骨分化或削弱氧化应激,成为防治异位骨化的有效靶点。 2.2 核心枢纽因子 2.2.1 Runx2信号因子 即Runt相关转录因子2(Runx2),又被称为Cbfa1,PEBP2αA或AML3,是runt同源结构域家族3个成员之一。作为成骨细胞分化的调节剂,Runx2在控制干细胞向成骨细胞分化、软骨/骨骼形成、牙齿发育和血管形成中均发挥重要作用。在复杂的骨形成过程中,Runx2与包括转录因子和辅助因子的多种蛋白质协作并进行翻译后修饰,整合细胞内外多种信号,共同调节成骨细胞的增殖、分化与成骨功能。创伤性异位骨化最常见原因即骨骼及周围肌肉肌腱损伤,Runx2在局部微环境中的表达显著增加[98],由此部分研究者尝试将Runx2作为治疗异位骨化的潜在靶点。林霖等[99]通过特异性siRNA抑制Runx2的表达可抑制成骨细胞分化,相关成骨基因表达也受到抑制,包括Ⅰ型胶原、碱性磷酸酶、骨桥蛋白和骨钙蛋白等。TU等[100]发现联接素43(Cx43)参与到创伤性异位骨化的发展中,下调Cx43的表达可降低细胞外调节激酶(ERK)活性,减少Runx2及相关成骨基因的表达,显著减少异位骨化的形成。单独阻断Runx2表达可有效减少异位骨化异位骨组织的形成,其效果与单独阻断Smad4效果近似[101],联合阻断Runx2和Smad4的表达后,异位骨化的防治疗效进一步增强。对Runx2的抑制主要干扰了成骨细胞的分化及细胞外基质矿化的形成,Smad4是成骨细胞中骨形态发生蛋白和转化生长因子通路传递信号入核过程中重要的协作因子,对Smad4的抑制可能影响了成骨细胞中骨形态发生蛋白和转化生长因子与其他信号因子的作用[102]。另有研究通过慢病毒搭载Runx2和缺氧诱导因子1特异性siRNA,同时抑制Runx2和缺氧诱导因子1的表达亦可显著抑制异位骨化的形成,阻断Runx2可作用于软骨形成整个阶段,而阻断缺氧诱导因子1可协同Runx2抑制软骨内成骨过程,同时也显著抑制了新血管的形成[103]。 在Runx2成骨相关信号网络中,除了膜内外信号蛋白,多种miRNA (miR)与Runx2调节异位骨化发展密切相关。TU等[104]进行体外研究显示上调miR-203的表达可抑制Runx2的表达,削弱成骨细胞活性减少基质矿化;进一步在跟腱横断异位骨化小鼠模型中,miR-203的过表达则直接靶向Runx2抑制异位骨化的形成。有研究显示,miR-29b可抑制转化生长因子3的翻译,促进Runx2的表达,是韧带成纤维细胞向成骨细胞分化形成异位骨的潜在机制[105]。miR-92b-3p高表达上调Runx2同时可抑制DKK1,从而促进Wnt信号通路,最终促进人脐带间充质干细胞的成骨分化,敲除miR-92b-3p则综合抑制了Runx2表达和Wnt信号,可显著削弱异位骨的形成[106]。综合而言,Runx2主要被作为异位骨形成标志或特异性作用靶点参与到多种上游信号因子对异位骨化的调节中[107-112]。 2.2.2 血管内皮生长因子信号因子 血管内皮生长因子在早期又被称为血管通透因子,其参与到机体多种生理病理过程中。一方面,血管内皮生长因子作为目前研究最广泛的促血管形成因子,可显著促进骨折愈合;另一方面,异常表达的血管内皮生长因子也参与到风湿性关节炎、老年性黄斑变性、糖尿病视网膜病变及肿瘤进展中。大量研究显示血管内皮生长因子参与到异位骨化的发生发展中。组织损伤早期,局部形成缺氧微环境,它可诱导缺氧诱导因子1的产生,后者可上调局部组织细胞血管内皮生长因子、SRY家族包含高迁移率族框9(SRY-related HMG box-containing,Sox9)的表达,它们对新血管和软骨形成起到促进作用,进而为异位骨化形成创造条件[113]。肘关节创伤后异位骨化患者局部组织中Cox2表达升高,可引起骨形态发生蛋白2和血管内皮生长因子表达上升促进异位骨组织形成和新血管重建,导致异位骨化的发生和发展[114]。 中枢神经系统损伤是异位骨化发生的高危因素,在神经损伤联合肌肉骨骼创伤后,异位骨化发生率常进一步增加。有研究者在脊髓损伤患者异位骨组织中亦可检测到Cox2与血管内皮生长因子相应mRNA表达较正常组显著升高[115],这说明虽然从病因上将神经源性异位骨化与创伤性异位骨化进行了区别,但在不同的异位骨化病理微环境中存在着,至少部分相似的病理性成骨过程。SUNG等[116]在跟腱损伤小鼠唾液中亦发现了血管内皮生长因子较正常组显著增高,且唾液血管内皮生长因子浓度与血清血管内皮生长因子浓度呈正相关,由此提出唾液内血管内皮生长因子增高可作为异位骨化的早期诊断标志。有研究发现,创伤和炎症诱导早期促血管生成微环境产生,其中形成了大量内皮组织样结构,但微环境中血管内皮生长因子A并不是来源于血管祖细胞,而是源自于局部组织中募集的大量间充质祖细胞及其分化的后代细胞,阻断间充质祖细胞中血管内皮生长因子A基因表达可显著抑制肢体创伤后异位骨化的形成,因此,作者认为间充质来源的血管内皮生长因子A是异位骨化发展的一个关键因子,它也成为延缓异位骨化发展或预防异位骨化发生的一个潜在靶点[117]。基于血管内皮生长因子强烈促血管形成活性,大量血管内皮生长因子相关研究集中在促生理性成骨及预防局部微血管病变及肿瘤治疗中,以血管内皮生长因子为靶点的异位骨化防治仍需要更深入和广泛的探索。 2.2.3 缺氧诱导因子信号因子 该因子是组织细胞适应周围环境的关键调控因子。缺氧诱导因子1对调节骨代谢、促进骨形成起到重要促进作用。缺氧诱导因子参与到了异位骨化的形成中。在大鼠跟腱横断损伤术后10周,异位骨化实验组跟腱组织中缺氧诱导因子1蛋白和基因表达较假手术组均有显著提高[118]。ZHAO等[119]发现牛颞下颌关节损伤局部组织中除了可观察到血管内皮生长因子、Sox9及骨钙蛋白等骨、软骨及血管形成因子的表达,也可观察到缺氧诱导因子1的显著表达,尤其在纤维增殖区和纤维软骨形成区,缺氧诱导因子1主要在局部低氧和炎症微环境中介导间充质干细胞向软骨细胞和成骨细胞分化促进异位骨化的形成。另有研究显示,炎症环境也可激活缺氧诱导因子2介导肌腱干细胞命运向(软)骨细胞分化,促进异位骨化的形成[120]。缺氧诱导因子1在低氧诱导异位骨化过程中起到核心作用,低氧微环境可刺激缺氧诱导因子1产生,后者除了直接作用于成骨前体细胞,也可与缺氧诱导因子1形成复合物增加骨形态发生蛋白、血管内皮生长因子及神经纤毛蛋白1的翻译,促进软骨细胞分化、基质矿化和血管化,为异位骨化创造条件[121-122]。 内皮间充质转化和血管化在烧伤诱导异位骨化过程中发挥着重要作用,烧伤后48 h即可检测到缺氧诱导因子1和血管内皮生长因子的表达,伤后3 d达到高峰,由此被认为是介导内皮间充质转化和血管化促异位骨化的关键启动因子[123]。基于对细胞氧代谢稳态调节和促成骨分化作用,缺氧诱导因子被作为异位骨化防治靶点,缺氧诱导因子相关抑制剂主要包括缺氧诱导因子1和缺氧诱导因子2两类,研究显示抑制缺氧诱导因子1对小鼠烧伤或创伤性异位骨化可起到显著抑制作用[124],而缺氧诱导因子2则更多被用于癌症治疗中[125]。 2.2.4 成纤维细胞生长因子信号因子 成纤维细胞生长因子主要通过旁分泌方式与其特异性受体成纤维细胞生长因子受体结合,可与骨形态发生蛋白/Smads、Wnt/β-catenin成骨信号通路共同调节骨形成。在跟腱创伤模型中,Col2+淋巴内皮细胞中,成纤维细胞生长因子R3的缺失可引起骨形态发生蛋白/Smad1/5信号表达上调,同时淋巴管生成受阻,淋巴引流减少,加重局部炎症,最终促进异位骨化的发展[126]。特异性敲除神经嵴细胞中成纤维细胞生长因子R1可导致小鼠在胚胎期和出生后颅面骨接缝中异位软骨和异位骨的形成,在局部组织中,可检测到骨形态发生蛋白4、Wnt1的表达[127]。由此说明,成纤维细胞生长因子参与到了异位骨化形成信号网络中,与骨形态发生蛋白和Wnt信号共同维持成骨内稳态的平衡。 2.2.5 Sox9信号因子 该因子是SRY家族包含高迁移率族框(SRY-related HMG box-containing,Sox)结构域的转录因子,在多潜能干细胞、软骨细胞和其他组织中表达,对机体软骨生长、性别决定及脏器发育等具有重要作用。基于对软骨的正向及对骨的负向调节, Sox9一方面参与到异位骨化的早期发生过程中,另一方面,过表达的Sox9或可成为防治异位骨化进展潜在靶点,在后纵韧带骨化患者异位骨化局部软骨样细胞中,可直接检测到Sox9的表达[128]。创伤性异位骨化患者组织中也发现miR-1和miR-206的上调,体外研究显示,它们在间充质祖细胞成骨分化早期作用于Sox9促进软骨细胞的增殖与成熟,进而促进异位骨化形成[129]。SUZUKI等[130]发现Mohawk基因缺失可加速小鼠跟腱异位骨化的形成,在肌腱源细胞中过表达则可显著上调Sox5,Sox6及Sox9,促进跟腱组织分化而抑制异位骨化形成,这使Sox9成为Mohawk调控异位骨化的作用靶点。LIAO等[131]亦得出类似的结果,研究发现,Sox9可增强骨形态发生蛋白2诱导的间充质干细胞成软骨分化和软骨形成,而过表达可抑制骨形态发生蛋白2诱导的间充质干细胞成骨分化及软骨细胞肥大,阻断软骨内化骨,这为组织工程防治异位骨化提供了潜在靶点。"

| [1] BEMENDERFER TB, DAVIS WH, ANDERSON B, et al. Heterotopic ossification in total ankle arthroplasty: case series and systematic review. J Foot Ankle Surg. 2020;59(4):716-721. [2] WAHL EP, CASEY PM, RISOLI T, et al. Heterotopic ossification formation after fractures about the elbow. Eur J Orthop Surg Traumatol. 2021;31(6):1061-1067. [3] WONG KR, MYCHASIUK R, O BRIEN TJ, et al. Neurological heterotopic ossification: novel mechanisms, prognostic biomarkers and prophylactic therapies. Bone Res. 2020;8(1):1-14. [4] GONZÁLEZ EH, LUJARDO YG, DELAFE AD, et al. Heterotopic ossification: prevalence and clinical characteristics in spinal cord injured patients treated at CIREN. Ann Orthop Trauma Rehabil. 2022;4(1):1-7. [5] CHAPUT CD, SIDDIQUI M, RAHM MD. Obesity and calcification of the ligaments of the spine: a comprehensive CT analysis of the entire spine in a random trauma population. Spine J. 2019;19(8):1346-1353. [6] YU T, ZHANG J, ZHU W, et al. Chondrogenesis mediates progression of ankylosing spondylitis through heterotopic ossification. Bone Res. 2021;9(1):1-12. [7] ZHANG Q, ZHOU D, WANG H, et al. Heterotopic ossification of tendon and ligament. J Cell Mol Med. 2020;24(10):5428-5437. [8] FUERY MA, LIANG L, KAPLAN FS, et al. Vascular ossification: pathology, mechanisms, and clinical implications. Bone. 2018;109:28-34. [9] DU J, CHEN Y, PENG Y, et al. Calcification of the intervertebral disc and ossification of posterior longitudinal ligament in children. BMC Musculoskelet Disord. 2018; 19(1):1-6. [10] WANG G, KANG Y, CHEN F, et al. Cervical intervertebral disc calcification combined with ossification of posterior longitudinal ligament in an-11-year old girl: case report and review of literature. Childs Nerv Syst. 2016;32(2):381-386. [11] LICINI C, FARINELLI L, CERQUENI G, et al. Heterotopic ossification in a patient with diffuse idiopathic skeletal hyperostosis: input from histological findings. Eur J Histochem. 2020;64(4):317-322. [12] PIGNOLO RJ, HSIAO EC, BAUJAT G, et al. Prevalence of fibrodysplasia ossificans progressiva (FOP) in the United States: estimate from three treatment centers and a patient organization. Orphanet J Rare Dis. 2021;16(1):1-8. [13] BAUJAT G, CHOQUET R, BOUÉE S, et al. Prevalence of fibrodysplasia ossificans progressiva (FOP) in France: an estimate based on a record linkage of two national databases. Orphanet J Rare Dis. 2017;12(1):1-9. [14] ELLI F M, MANTOVANI G. Pseudohypoparathyroidism, acrodysostosis, progressive osseous heteroplasia: different names for the same spectrum of diseases? Endocrine. 2021;72(3):611-618. [15] HAN SR, LEE YA, SHIN C, et al. Clinical and molecular characteristics of GNAS inactivation disorders observed in 18 Korean patients. Exp Clin Endocrinol Diabetes. 2021;129(2):118-125. [16] 罗涛.肱骨骨折后异位骨化形成中BMP-2、BMP-9及TNF-α表达的变化[J].武汉大学学报(医学版),2017,38(1):112-114. [17] 柳明忠,曾荣东,张志珊,等.塞来昔布对创伤性异位骨化模型大鼠的BMP-4影响[J].中外医疗,2017,36(28):18-21. [18] 宁尚龙,陈仲强,马信龙,等. 胸椎黄韧带骨化患者韧带细胞成骨潜能的评估及相关高通量测序分析[J].中华骨科杂志,2017,37(20):1300-1309. [19] LEES-SHEPARD JB, GOLDHAMER DJ. Stem cells and heterotopic ossification: lessons from animal models. Bone. 2018;109:178-186. [20] LI L, JIANG Y, LIN H, et al. Muscle injury promotes heterotopic ossification by stimulating local bone morphogenetic protein-7 production. J Orthop Translat. 2019;18:142-153. [21] DAI G, LI Y, LIU J, et al. Higher BMP expression in tendon stem/progenitor cells contributes to the increased heterotopic ossification in achilles tendon with aging. Front Cell Dev Biol. 2020;8:570605. [22] 柳明忠,曾荣东,施建辉,等.骨形态发生蛋白4在创伤性骨化性肌炎大鼠模型的表达及意义[J].福建医药杂志,2020,42(6):131-134. [23] ZHAO Y, YUAN B, CHENG L, et al. Cyclic tensile stress to rat thoracolumbar ligamentum flavum inducing the ossification of ligamentum flavum. Spine (Phila Pa 1976). 2021;46(17):1129-1138. [24] AGARWAL S, LODER SJ, BREULER C, et al. Strategic targeting of multiple BMP receptors prevents trauma-induced heterotopic ossification. Mol Ther. 2017; 25(8):1974-1987. [25] 史光耀, 鄢泽然, 白雯,等.补肾舒脊颗粒对人骨髓间充质干细胞BMP-4、Smad-4 mrna及蛋白表达的影响[J]. 中国中西医结合杂志,2017,37(7):819-824. [26] LIN H, SHI F, JIANG S, et al. Metformin attenuates trauma-induced heterotopic ossification via inhibition of bone morphogenetic protein signalling. J Cell Mol Med. 2020;24(24):14491-14501. [27] WU J, REN B, SHI F, et al. BMP and mtor signaling in heterotopic ossification: does their crosstalk provide therapeutic opportunities? J Cell Biochem. 2019; 120(8):12108-12122. [28] STRONG AL, SPREADBOROUGH PJ, DEY D, et al. BMP ligand trap ALK3-Fc attenuates osteogenesis and heterotopic ossification in blast-related lower extremity trauma. Stem Cells Dev. 2021;30(2):91-105. [29] KUSANO T, NAKATANI M, ISHIGURO N, et al. Desloratadine inhibits heterotopic ossification by suppression of BMP2-Smad1/5/8 signaling. J Orthop Res. 2021; 39(6):1297-1304. [30] LIANG Q, LU Y, YU L, et al. Disruption of the mouse Bmal1 locus promotes heterotopic ossification with aging via TGF-beta/BMP signaling. J Bone Miner Metab. 2022;40(1):40-55. [31] WOSCZYNA MN, BISWAS AA, COGSWELL CA, et al. Multipotent progenitors resident in the skeletal muscle interstitium exhibit robust BMP‐dependent osteogenic activity and mediate heterotopic ossification. J Bone Miner Res. 2012;27(5):1004-1017. [32] OLMSTED-DAVIS E A, SALISBURY E A, HOANG D, et al. Progenitors in peripheral nerves launch heterotopic ossification. Stem Cells Transl Med. 2017;6(4):1109-1119. [33] CAPPATO S, GAMBERALE R, BOCCIARDI R, et al. Genetic and acquired heterotopic ossification: a translational tale of mice and men. Biomedicines. 2020;8(12):611. [34] ŁĘGOSZ P, DRELA K, PULIK A, et al. Challenges of heterotopic ossification-Molecular background and current treatment strategies. Clin Exp Pharmacol Physiol. 2018;45(12):1229-1235. [35] 张辉,王茜,陶建峰,等.国产多孔钽复合骨形态发生蛋白7植入兔竖脊肌内的生物相容性[J].中国组织工程研究,2016,20(16):2376-2383. [36] PUVANESARAJAH V, JAIN A, CANCIENNE JM, et al. BMP use and the risk of revision surgery after long posterolateral fusions in the elderly. Clin Spine Surg. 2017;30(7):E931-E937. [37] BRANNAN PS, GASTON RG, LOEFFLER BJ, et al. Complications with the use of BMP-2 in scaphoid nonunion surgery. J Hand Surg Am. 2016;41(5):602-608. [38] SHI L, SUN W, GAO F, et al. Heterotopic ossification related to the use of recombinant human BMP-2 in osteonecrosis of femoral head. Medicine (Baltimore). 2017;96(27):e7413. [39] PAPANAGIOTOU M, DAILIANA ZH, KARACHALIOS T, et al. Heterotopic ossification after the use of recombinant human bone morphogenetic protein-7. World J Orthop. 2017;8(1):36-41. [40] NIU S, ANASTASIO AT, FARAJ RR, et al. Evaluation of heterotopic ossification after using recombinant human bone morphogenetic protein-2 in transforaminal lumbar interbody fusion: a computed tomography review of 996 disc levels. Global Spine J. 2020;10(3):280-285. [41] 袁佳滨,缪雄,栗景峰,等.骨形态发生蛋白2临床应用的不良反应[J]. 第二军医大学学报,2019,40(9):1010-1014. [42] SEBASTIAN AS, WANDERMAN NR, CURRIER BL, et al. Prospective evaluation of radiculitis following bone morphogenetic protein-2 use for transforaminal interbody arthrodesis in spine surgery. Asian Spine J. 2019;13(4):544-555. [43] ARZENO A, WANG T, HUDDLESTON JR. Abundant heterotopic bone formation following use of rhbmp-2 in the treatment of acetabular bone defects during revision hip arthroplasty. Arthroplast Today. 2018;4(2):162-168. [44] POLMEAR MM, ANDERSON AB, LANIER PJ, et al. Bone morphogenetic protein in scaphoid nonunion: a systematic review. J Wrist Surg. 2021;10(3):184-189. [45] ELIAS E, NASSER Z, WINEGAN L, et al. Bone morphogenetic protein usage in anterior lumbar interbody fusion: what else can go wrong? World Neurosurg. 2018;111:55-59. [46] RUEHLE MA, KRISHNAN L, VANTUCCI CE, et al. Effects of BMP-2 dose and delivery of microvascular fragments on healing of bone defects with concomitant volumetric muscle loss. J Orthop Res. 2019;37(3):553-561. [47] IHN H, KANG H, IGLESIAS B, et al. Regional gene therapy with transduced human cells: the influence of “cell dose” on bone repair. Tissue Eng Part A. 2021;27(21-22):1422-1433. [48] PRIDDY LB, KRISHNAN L, HETTIARATCHI MH, et al. Amniotic membrane attenuates heterotopic ossification following high-dose bone morphogenetic protein-2 treatment of segmental bone defects. J Orthop Res. 2023;41(1):130-140. [49] HETTIARATCHI MH, KRISHNAN L, ROUSE T, et al. Heparin-mediated delivery of bone morphogenetic protein-2 improves spatial localization of bone regeneration. Sci Adv. 2020;6(1):y1240. [50] WANG M, LAM R, ABBAH SA, et al. Heparin-based polyelectrolyte complex enhances the therapeutic efficacy of bone morphogenetic protein-2 for posterolateral fusion in a large animal model. Spine (Phila Pa 1976). 2016;41(15): 1199-1207. [51] VANTUCCI CE, KRISHAN L, CHENG A, et al. BMP-2 delivery strategy modulates local bone regeneration and systemic immune responses to complex extremity trauma. Biomater Sci. 2021;9(5):1668-1682. [52] 余翔,夏远军,郑晓辉,等.两种壳聚糖载rhbmp-2纳米微球对SD大鼠体内异位成骨的影响[J].中国临床解剖学杂志,2016,34(4):412-418. [53] KRISHNAN L, PRIDDY LB, ESANCY C, et al. Delivery vehicle effects on bone regeneration and heterotopic ossification induced by high dose BMP-2. Acta Biomater. 2017;49:101-112. [54] JEON EY, UM S, PARK J, et al. Precisely localized bone regeneration mediated by marine-derived microdroplets with superior BMP-2 binding affinity. Small. 2022;18(24):e2200416. [55] 胡洋洋,卢海妹,刘亚坤,等.获得性异位骨化的研究进展[J].基础医学与临床,2017,37(11):1634-1639. [56] 龙国赓, 程娇, 蒋练. BMP9介导多种干细胞成骨分化的研究进展[J].医学综述,2020,26(11):2099-2105. [57] 张保良, 陈仲强. 胸椎黄韧带骨化相关分子信号通路的研究进展[J].中华骨科杂志,2020,40(10):680-688. [58] YANG P, TRONCONE L, AUGUR ZM, et al. The role of bone morphogenetic protein signaling in vascular calcification. Bone. 2020;141:115542. [59] DAVIS EL, SONNET C, LAZARD ZW, et al. Location-dependent heterotopic ossification in the rat model: the role of activated matrix metalloproteinase 9. J Orthop Res. 2016;34(11):1894-1904. [60] KAN C, DING N, YANG J, et al. BMP-dependent, injury-induced stem cell niche as a mechanism of heterotopic ossification. Stem Cell Res Ther. 2019;10(1):14. [61] AGARWAL S, CHOLOK D, LODER S, et al. Mtor inhibition and BMP signaling act synergistically to reduce muscle fibrosis and improve myofiber regeneration. JCI Insight. 2016;1(20):e89805. [62] SHAH A, UY M, YAN JR, et al. Heterotopic ossification following total elbow arthroplasty in a patient with Parkinson’s disease: case report and literature review. Case Rep Surg. 2020;2020:2068045. [63] NAGANO H, TOGAWA T, WATANABE T, et al. Heterotopic ossification in lymph node metastasis after rectal cancer resection: a case report and literature review. World J Surg Oncol. 2021;19(1):2. [64] KATONO N, TSUDA M, SUZUKA J, et al. Involvement of BMP and Wnt signals leadingto epithelial-mesenchymal transition in colon adenocarcinoma with heterotopic ossification. Ann Clin Lab Sci. 2021;51(2):271-276. [65] FU HD, WANG HR, LI DH. BMP-7 accelerates the differentiation of rabbit mesenchymal stem cells into cartilage through the Wnt/β-catenin pathway. Exp Ther Med. 2017;14(6):5424-5428. [66] TUZMEN C, VERDELIS K, WEISS L, et al. Crosstalk between substance P and calcitonin gene-related peptide during heterotopic ossification in murine Achilles tendon. J Orthop Res. 2018;36(5):1444-1455. [67] DAVIS EL, DAVIS AR, GUGALA Z, et al. Is heterotopic ossification getting nervous? The role of the peripheral nervous system in heterotopic ossification. Bone. 2018;109:22-27. [68] DROUIN G, COUTURE V, LAUZON MA, et al. Muscle injury-induced hypoxia alters the proliferation and differentiation potentials of muscle resident stromal cells. Skelet Muscle. 2019;9(1):18. [69] GUGALA Z, OLMSTED-DAVIS EA, XIONG Y, et al. Trauma-induced heterotopic ossification regulates the blood-nerve barrier. Front Neurol. 2018;9:408. [70] CROWGEY EL, WYFFELS JT, OSBORN PM, et al. A systems biology approach for studying heterotopic ossification: proteomic analysis of clinical serum and tissue samples. genomics proteomics bioinformatics. 2018;16(3):212-220. [71] YU D, JU J, XUE F, et al. Expression and significance of related genes in the early stage of post-traumatic heterotopic ossification in a rat model of Achilles tenotomy. Acta Orthop Traumatol Turc. 2021;55(2):94-101. [72] ZHANG Q, ZHANG Y, YAN M, et al. Big-h3 enhances chondrogenesis via promoting mesenchymal condensation in rat Achilles tendon heterotopic ossification model. Aging (Albany NY). 2020;12(8):7030-7041. [73] YAN L, GAO R, LIU Y, et al. The pathogenesis of ossification of the posterior longitudinal ligament. Aging Dis. 2017;8(5):570-582. [74] ALEXANDER KA, TSENG H, SALGA M, et al. When the nervous system turns skeletal muscles into bones: how to solve the conundrum of neurogenic heterotopic ossification. Curr Osteoporos Rep. 2020;18(6):666-676. [75] XIONG Y, YANG YL, GAO YS, et al. Histological changes of cervical disc tissue in patients with degenerative ossification. J Korean Neurosurg Soc. 2022;65(2): 186-195. [76] ZHONG B, ZHANG C, GUO S, et al. Rational design of cyclic peptides to disrupt TGF-Β/SMAD7 signaling in heterotopic ossification. J Mol Graph Model. 2017; 72:25-31. [77] ZENG LR, ZHU FB, WANG JY, et al. Local influence of high molecular polyethylene particles on heterotopic ossification. Exp Ther Med. 2017;13(6):2934-2938. [78] MAO D, PAN X, RUI Y, et al. Matrine attenuates heterotopic ossification by suppressing TGF-β induced mesenchymal stromal cell migration and osteogenic differentiation. Biomed Pharmacother. 2020;127:110152. [79] WANG X, LI F, XIE L, et al. Inhibition of overactive TGF-β attenuates progression of heterotopic ossification in mice. Nat Commun. 2018;9(1):551. [80] 王威,刘伟军,黎清波,等.电磁场干预对异位骨化大鼠模型的抗异位骨化效应及机制探讨[J].中华物理医学与康复杂志,2020,42(10):870-876. [81] STRONG AL, SPREADBOROUGH PJ, PAGANI CA, et al. Small molecule inhibition of non-canonical (TAK1-mediated) BMP signaling results in reduced chondrogenic ossification and heterotopic ossification in a rat model of blast-associated combat-related lower limb trauma. Bone. 2020;139:115517. [82] FANG F, SUP M, LUZZI A, et al. Hedgehog signaling underlying tendon and enthesis development and pathology. Matrix Biol. 2022;105:87-103. [83] LIU H, XU J, JIANG R. Mkx-deficient mice exhibit hedgehog signaling-dependent ectopic ossification in the achilles tendons. J Bone Miner Res. 2019;34(3):557-569. [84] FENG H, XING W, HAN Y, et al. Tendon-derived cathepsin K-expressing progenitor cells activate Hedgehog signaling to drive heterotopic ossification. J Clin Invest. 2020;130(12):6354-6365. [85] GAO R, SHI C, YANG C, et al. Cyclic stretch promotes the ossification of ligamentum flavum by modulating the Indian hedgehog signaling pathway. Mol Med Rep. 2020;22(2):1119-1128. [86] KAN C, CHEN L, HU Y, et al. Gli1-labeled adult mesenchymal stem/progenitor cells and hedgehog signaling contribute to endochondral heterotopic ossification. Bone. 2018;109:71-79. [87] CHAKRABORTY A, GVOZDENOVIC-JEREMIC J, WANG F, et al. Hedgehog pathway inhibitors significantly reduce the formation of heterotopic ossification in a direct trauma/burn mouse model. Biorxiv. 2021. doi:10.1101/2021.01.31.429058. [88] ZOU YC, YANG XW, YUAN SG, et al. Downregulation of dickkopf-1 enhances the proliferation and osteogenic potential of fibroblasts isolated from ankylosing spondylitis patients via the Wnt/β-catenin signaling pathway in vitro. Connect Tissue Res. 2016;57(3):200-211. [89] ZOU YC, YANG XW, YUAN SG, et al. Celastrol inhibits prostaglandin E2-induced proliferation and osteogenic differentiation of fibroblasts isolated from ankylosing spondylitis hip tissues in vitro. Drug Des Devel Ther. 2016;10:933-948. [90] 鄢泽然,史光耀,王晓曈,等.补肾舒脊颗粒含药血清对人骨髓间充质干细胞Wnt5a、β-catenin mrna及蛋白表达的影响[J].中医杂志,2018,59(5):415-419. [91] ZHANG Z, ZENG J, LI Y, et al. Tail suspension delays ectopic ossification in proteoglycan-induced ankylosing spondylitis in mice via mir-103/DKK1. Exp Ther Med. 2021;22(3):965. [92] WANG T, CHEN P, CHEN L, et al. Reduction of mechanical loading in tendons induces heterotopic ossification and activation of the β-catenin signaling pathway. J Orthop Translat. 2021;29:42-50. [93] XU L, QIAN Z, WANG S N, et al. Galectin-3 enhances osteogenic differentiation of precursor cells from patients with diffuse idiopathic skeletal hyperostosis via wnt/beta-catenin signaling. J Bone Miner Res. 2022;37(4):724-739. [94] XU K, ZHANG Z, CHEN M, et al. Nesfatin-1 promotes the osteogenic differentiation of tendon-derived stem cells and the pathogenesis of heterotopic ossification in rat tendons via the mtor pathway. Front Cell Dev Biol. 2020;8:547342. [95] CHEN Y, SHEN W, TANG C, et al. Targeted pathological collagen delivery of sustained-release rapamycin to prevent heterotopic ossification. Sci Adv. 2020; 6(18):y9526. [96] YAO L, SUN B, WANG B, et al. The mtorc1 signaling pathway inhibitor rapamycin inhibits leptin induced osteogenic differentiation of tdscs and ectopic ossification of achilles tendon. Acta Medica Mediterranea. 37(1):115-121. [97] HU Y, WANG Z. Rapamycin prevents heterotopic ossification by inhibiting the mtor pathway and oxidative stress. Biochem Biophys Res Commun. 2021;573: 171-178. [98] MOLLIGAN J, MITCHELL R, SCHON L, et al. Influence of bone and muscle injuries on the osteogenic potential of muscle progenitors: contribution of tissue environment to heterotopic ossification. Stem Cells Transl Med. 2016;5(6):745-753. [99] 林霖,陈连旭,王海军,等.Runx2特异性sirna筛选和功能鉴定[J].中国运动医学杂志, 2009,28(5):530-533. [100] TU B, LIU S, LIU G, et al. Inhibition of connexin 43 prevents trauma-induced heterotopic ossification. Sci Rep. 2016;6:37184. [101] 薛涛,林霖,魏学磊,等.Sirna抑制Smad4或Runx2基因表达治疗大鼠异位骨化效果比较[J].中国运动医学杂志,2011,30(7):644-649. [102] XUE T, MAO Z, LIN L, et al. Non-virus-mediated transfer of sirnas against Runx2 and Smad4 inhibit heterotopic ossification in rats. Gene Ther. 2010;17(3):370-379. [103] LIN L, SHEN Q, LENG H, et al. Synergistic inhibition of endochondral bone formation by silencing Hif1α and Runx2 in trauma-induced heterotopic ossification. Mol Ther. 2011;19(8):1426-1432. [104] TU B, LIU S, YU B, et al. Mir-203 inhibits the traumatic heterotopic ossification by targeting Runx2. Cell Death Dis. 2016;7(10):e2436. [105] 张秀梅,崔亚洲,周小艳,等.Micrornas参与韧带成纤维细胞成骨分化相关分子表达的调节[J].中国生物化学与分子生物学报,2011,27(6):540-547. [106] 张燕,严冰浩,徐辰,等.Mir-92b-3p促进人脐带间充质干细胞的成骨分化[J].基础医学与临床,2016,36(5):598-603. [107] YIN J, ZHUANG G, ZHU Y, et al. Mir-615-3p inhibits the osteogenic differentiation of human lumbar ligamentum flavum cells via suppression of osteogenic regulators GDF5 and FOXO1. Cell Biol Int. 2017;41(7):779-786. [108] XU C, ZHANG H, GU W, et al. The microrna-10a/ID3/RUNX2 axis modulates the development of ossification of posterior longitudinal ligament. Sci Rep. 2018; 8(1):9225. [109] LOGAN NJ, CAMMAN M, WILLIAMS G, et al. Demethylation of ITGAV accelerates osteogenic differentiation in a blast-induced heterotopic ossification in vitro cell culture model. Bone. 2018;117:149-160. [110] ZHANG J, WANG L, CAO H, et al. Neurotrophin-3 acts on the endothelial-mesenchymal transition of heterotopic ossification in rats. J Cell Mol Med. 2019; 23(4):2595-2609. [111] LI D, TIAN Y, YIN C, et al. Silencing of lncrna AK045490 promotes osteoblast differentiation and bone formation via β-Catenin/TCF1/Runx2 signaling axis. Int J Mol Sci. 2019;20(24):6229. [112] KIM JM, YANG YS, PARK KH, et al. A RUNX2 stabilization pathway mediates physiologic and pathologic bone formation. Nat Commun. 2020;11(1):2289. [113] ROBINSON TE, COX SC, GROVER LM. Heterotopic ossification following traumatic blast injury. Aikawa E, Hutcheson JD. cardiovascular calcification and bone mineralization. Cham: Springer International Publishing. 2020:297-315. [114] 田建,范存义,芮永军,等.环氧化酶2、骨形态形成蛋白2和血管内皮生长因子在异位骨化组织中的表达[J].中国组织工程研究,2012,16(42):7814-7818. [115] 董利薇, 董国栋, 曹建业,等.环氧化酶2、血管内皮生长因子mrna在异位骨化组织中的表达[J].临床合理用药杂志,2018,11(1):152-153. [116] SUNG HH, CHUNG MT, ALLEN RM, et al. Evaluation of salivary cytokines for diagnosis of both trauma-induced and genetic heterotopic ossification. Front Endocrinol (Lausanne). 2017;8:74. [117] HWANG C, MARINI S, HUBER AK, et al. Mesenchymal VEGFA induces aberrant differentiation in heterotopic ossification. Bone Res. 2019;7(1):1-17. [118] 李国松,赫明堂,马立峰,等.大鼠跟腱异位骨化模型中低氧诱导因子1α及血管内皮生长因子的表达及作用[J].中国现代药物应用,2015,9(14):278-279. [119] ZHAO Y, LIU P, CHEN Q, et al. Development process of traumatic heterotopic ossification of the temporomandibular joint in mice. J Craniomaxillofac Surg. 2019;47(7):1155-1161. [120] HU JJ, YIN Z, SHEN WL, et al. Pharmacological regulation of in situ tissue stem cells differentiation for soft tissue calcification treatment. Stem Cells. 2016;34(4):1083-1096. [121] HUANG Y, WANG X, LIN H. The hypoxic microenvironment: a driving force for heterotopic ossification progression. Cell Commun Signal. 2020;18(1):20. [122] PETERS N, BALTIN CT, BARHAM M, et al. An unusual finding: Heterotopic ossification located in the subcutis of the iliac region-A case report in the context of current literature. Transl Res Anat. 2021. doi:10.1016/J.TRIA.2021. 100137. [123] HU X, SUN Z, LI F, et al. Burn-induced heterotopic ossification from incidence to therapy: key signaling pathways underlying ectopic bone formation. Cell Mol Biol Lett. 2021;26(1):34. [124] AGARWAL S, LODER S, BROWNLEY C, et al. Inhibition of Hif1α prevents both trauma-induced and genetic heterotopic ossification. Proc Natl Acad Sci U S A. 2016;113(3):E338-E347. [125] BAN HS, UTO Y, NAKAMURA H. Hypoxia-inducible factor (HIF) inhibitors: a patent survey (2016-2020). Expert Opin Ther Pat. 2021;31(5):387-397. [126] ZHANG D, HUANG J, SUN X, et al. Targeting local lymphatics to ameliorate heterotopic ossification via FGFR3-BMPR1a pathway. Nat Commun. 2021;12(1): 4391. [127] KAWAI M, HERRMANN D, FUCHS A, et al. Fgfr1 conditional-knockout in neural crest cells induces heterotopic chondrogenesis and osteogenesis in mouse frontal bones. Med Mol Morphol. 2019;52(3):156-163. [128] MAEDA S, KAWAMURA I, TOMINAGA H, et al. Histopathological features of ossification of the posterior longitudinal ligament. Springer. 2020. [129] DE VASCONCELLOS JF, JACKSON WM, DIMTCHEV A, et al. A microrna signature for impaired wound-healing and ectopic bone formation in humans. J Bone Joint Surg Am. 2020;102(21):1891-1899. [130] SUZUKI H, ITO Y, SHINOHARA M, et al. Gene targeting of the transcription factor Mohawk in rats causes heterotopic ossification of Achilles tendon via failed tenogenesis. Proc Natl Acad Sci U S A. 2016;113(28):7840-7845. [131] LIAO J, HU N, ZHOU N, et al. Sox9 potentiates BMP2-induced chondrogenic differentiation and inhibits BMP2-induced osteogenic differentiation. PLoS One. 2014;9(2):e89025. |
| [1] | Han Haihui, Ran Lei, Meng Xiaohui, Xin Pengfei, Xiang Zheng, Bian Yanqin, Shi Qi, Xiao Lianbo. Targeting fibroblast growth factor receptor 1 signaling to improve bone destruction in rheumatoid arthritis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(9): 1905-1912. |
| [2] | Yin Lu, Jiang Chuanfeng, Chen Junjie, Yi Ming, Wang Zihe, Shi Houyin, Wang Guoyou, Shen Huarui. Effect of Complanatoside A on the apoptosis of articular chondrocytes [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1541-1547. |
| [3] | De Ji, Suo Langda, Wei Yuchen, Wang Bin, Awangcuoji, Renqingcuomu, Cui Jiuzeng, Zhang Lei, Ba Gui. Comprehensive analysis of genes related to endometrial receptivity and alternative splicing events in northwest Tibetan cashmere goats [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1429-1436. |
| [4] | Chen Yuning, Jiang Ying, Liao Xiangyu, Chen Qiongjun, Xiong Liang, Liu Yue, Liu Tong. Buqi Huoxue Compounds intervene with the expression of related factors and autophagy related proteins in a rat model of cerebral ischemia/reperfusion [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(6): 1152-1158. |
| [5] | Lang Mecuo, Zhang Yilin, Wang Li. MiR-338-3p affects proliferation and apoptosis of alveolar bone osteoblasts by targeting receptor activator of nuclear factor-kappaB ligand [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(5): 899-907. |
| [6] | Xiang Pan, Che Yanjun, Luo Zongping. Compressive stress induces degeneration of cartilaginous endplate cells through the SOST/Wnt/beta-catenin pathway [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(5): 951-957. |
| [7] | Han Haihui, Meng Xiaohu, Xu Bo, Ran Le, Shi Qi, Xiao Lianbo. Effect of fibroblast growth factor receptor 1 inhibitor on bone destruction in rats with collagen-induced arthritis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(5): 968-977. |
| [8] | Liu Haowen, Qiao Weiping, Meng Zhicheng, Li Kaijie, Han Xuan, Shi Pengbo. Regulation of osteogenic effects by bone morphogenetic protein/Wnt signaling pathway: revealing molecular mechanisms of bone formation and remodeling [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(3): 563-571. |
| [9] | Wang Lian, Xie Na, Zhao Peiling, Chen Hao, Li Duyou, Wang Yuping. Effects of cannabidiol on hepatic stellate cell activation and hepatic fibrosis induced by transforming growth factor beta1 [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(23): 4965-4974. |
| [10] | Yu Lei, Zhang Wei, Qin Yi, Ge Gaoran, Bai Jiaxiang, Geng Dechun. Repair of femoral condyle defects using mesoporous bioactive glass grafted with bone morphogenetic protein 2 osteogenic peptide inspired by mussel [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(22): 4629-4638. |
| [11] | Yu Peng, Meng Dongfang, Li Huiying, Liu Hongfei, He Zike. Pinoresinol diglucoside activates the Wnt/beta-catenin signaling pathway to protect osteoblasts [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(2): 339-346. |
| [12] | Peng Yong, Hu Jiangping, Zhu Huan. Effects of low-load blood flow restriction exercise and high-intensity resistance exercise on the thigh microcirculation function of athletic young men [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(2): 393-401. |
| [13] | Hu Huali, Deng Fahua, Liu Yuancheng, Wang Siqi, Zhang Jingxin, Lu Tingting, Huang Hai, Wei Sixi. Effects of long non-coding RNA KIAA0125 on proliferation and apoptosis of acute myeloid leukemia U937 cells [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(19): 3983-3991. |
| [14] | Chen Jiahui, Dai Xiaoqi, Xu Yangang, Li Yuanchao, Huang Mei, Zhan Yifei, Du Yuxuan, Li Liuqiang, Guo Yaochuan, Bian Jun, Lai Dehui. Isolation, culture and differentiation of human urine-derived stem cells into smooth muscle cells [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(19): 4076-4082. |
| [15] | Israrguli · Maimeti, Jia Sen, Liu Jia. Bone morphogenetic protein 2-loaded hydrogel induces osteogenic differentiation of dental pulp stem cells [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(16): 3301-3310. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||