Chinese Journal of Tissue Engineering Research ›› 2024, Vol. 28 ›› Issue (4): 542-549.doi: 10.12307/2024.275
Previous Articles Next Articles
Comparison of protocols for constructing animal models of early traumatic knee osteoarthritis
Liu Yuhan1, Fan Yujiang1, 2, Wang Qiguang2
- 1Province-Ministry Collaborative Innovation Center for Regenerative Medicine and Medical Bioresource Development and Application, Guangxi Medical University, Nanning 530021, Guangxi Zhuang Autonomous Region, China; 2Center for Biomaterials, Sichuan University, Chengdu 610064, Sichuan Province, China
-
Received:2023-02-25Accepted:2023-04-04Online:2024-02-08Published:2023-07-14 -
Contact:Fan Yujiang, Professor, Doctoral supervisor, Province-Ministry Collaborative Innovation Center for Regenerative Medicine and Medical Bioresource Development and Application, Guangxi Medical University, Nanning 530021, Guangxi Zhuang Autonomous Region, China; Center for Biomaterials, Sichuan University, Chengdu 610064, Sichuan Province, China -
About author:Liu Yuhan, Province-Ministry Collaborative Innovation Center for Regenerative Medicine and Medical Bioresource Development and Application, Guangxi Medical University, Nanning 530021, Guangxi Zhuang Autonomous Region, China -
Supported by:the National Natural Science Foundation of China, No. 32071353 (to WQG)
CLC Number:
Cite this article
Liu Yuhan, Fan Yujiang, Wang Qiguang. Comparison of protocols for constructing animal models of early traumatic knee osteoarthritis[J]. Chinese Journal of Tissue Engineering Research, 2024, 28(4): 542-549.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
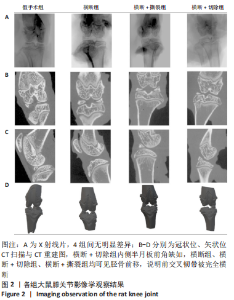
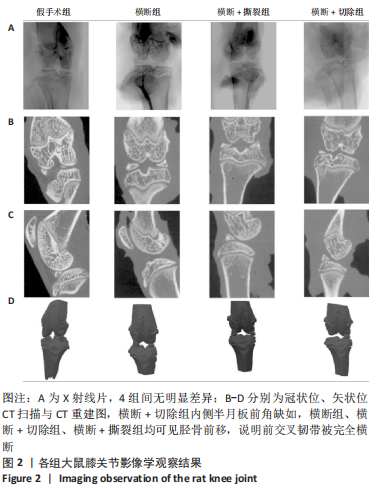
2.1 实验动物数量分析 48只大鼠全部进入结果分析,无脱失。 2.2 各组大鼠膝关节影像学检查结果 目前常见的影像学评分如Fairbank评分及K&L评分,均为主观性评分,他们所包含的个别标准很难定义[18]。尽管目前K&L评分仍被认为是影像学评价骨关节炎进展程度的金标准,但其仍缺乏准确性,特别是在骨关节炎早期[18],其对于骨关节炎的分级主要考虑以下4个指标:关节间隙、骨赘存在与否、软骨下硬化和骨畸形[19]。研究者普遍认为,站立位膝关节X射线片比仰卧位X射线片能更准确地反映关节软骨的损失,关节软骨损失是骨关节炎的一个标志[20-22]。但如果检测动物为大鼠,由于其并非直立行走,关节间隙无法因重力作用而出现狭窄,导致其关节间隙如图2A所示的衡量指标不具有特异性,此次实验4组大鼠膝关节下X射线片无明显差异。 CT扫描结果如图2B-D所示,横断+切除组内侧半月板前角缺如,横断组、横断+切除组、横断+撕裂组均可见胫骨前移,说明前交叉韧带被完全横断,图中未见其他骨组织损伤,说明以上造模方案均无副损伤,并达到手术预期损伤效果。"

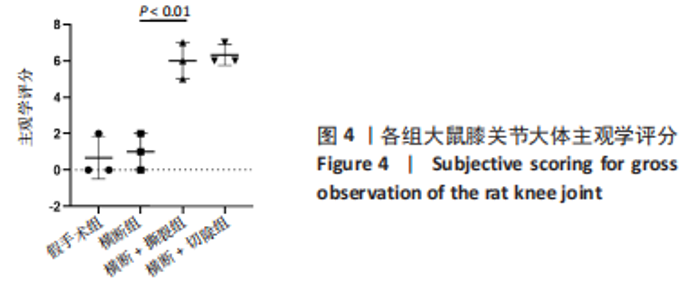
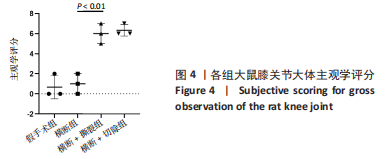
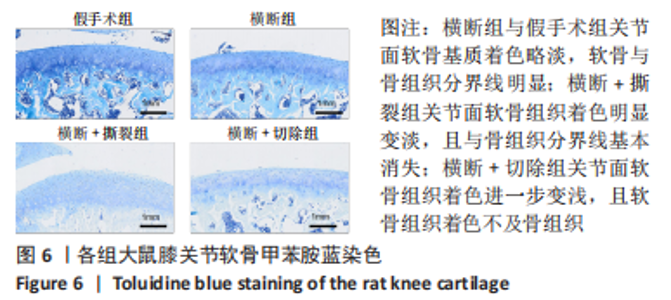
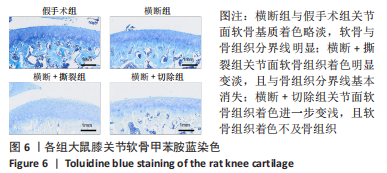
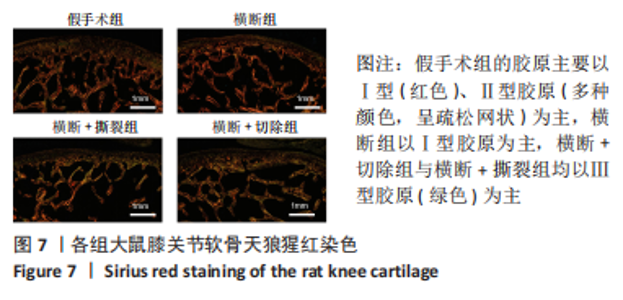
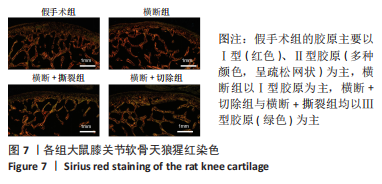
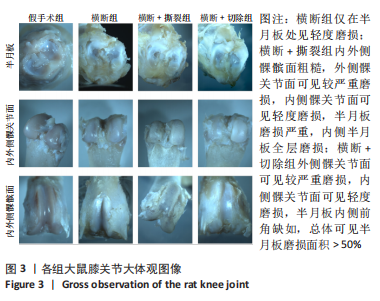
2.3 各组大鼠膝关节大体观察结果 大体观察选择了3个关节软骨磨损的易发区域:内外侧髁髌面、内外侧髁以及半月板,并观察是否出现骨赘,如图3所示。假手术组内外侧髁髌骨面、关节面及半月板光滑,无磨损迹象;横断组内外侧髁髌骨面、关节面光滑,仅在半月板处见轻度磨损;横断+撕裂组内外侧髁髌面粗糙,外侧髁关节面可见较严重磨损,内侧髁关节面可见轻度磨损,单侧损伤面积> 25%,半月板磨损严重,内侧半月板全层磨损;横断+切除组内外侧髁髌面可见串珠样改变,外侧髁关节面可见较严重磨损,内侧髁关节面可见轻度磨损,单侧损伤面积> 25%,半月板内侧前角缺如为手术所致,总体可见半月板磨损面积> 50%。横断组与假手术组大体观察并无明显差异,可能无法达到大部分实验需求;横断+撕裂组、横断+切除组造模方案与假手术组均存在较大差异,且两者所达到的效果相近,均可达到骨关节炎造模软骨损伤要求。"
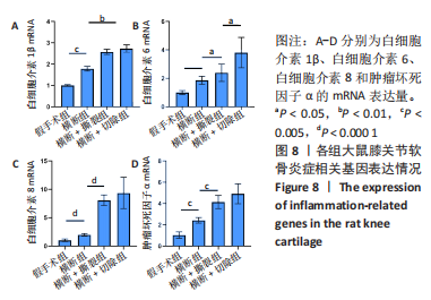
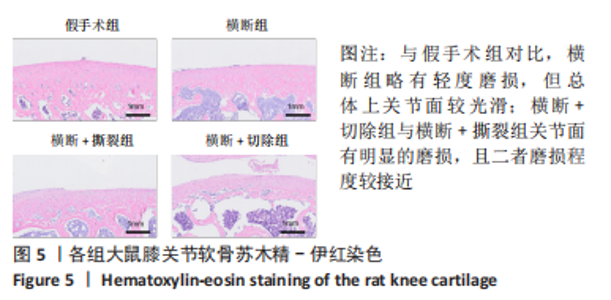
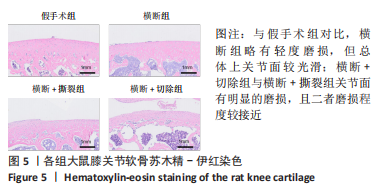
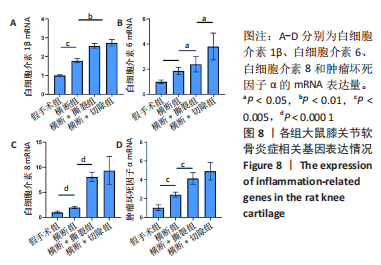
综上所述,可以从病理学切片的数据分析判断单纯的前交叉韧带横断术后膝关节组织学有一定变化,骨关节炎程度并不明显,而两种前交叉韧带横断联合内侧半月板失稳的手术方案可达到相近的骨关节炎效果。 2.5 各组大鼠膝关节PCR定量分析结果 从基因层面可知,相关的炎症因子在骨关节炎的发生发展中起着重要作用,其对关节软骨的退变以及骨关节炎的发展有重要影响,因此实验着重对相关炎性基因的表达进行了PCR表征,如图8所示。在骨关节炎发展中较为重要的炎症因子(白细胞介素1β、白细胞介素6、白细胞介素8和肿瘤坏死因子α)表达呈现出了较为相近的趋势,横断组白细胞介素1β、白细胞介素6、白细胞介素8及肿瘤坏死因子α的mRNA表达量高于假手术组(P < 0.005,P < 0.000 1),横断+撕裂组白细胞介素1β、白细胞介素6、白细胞介素8及肿瘤坏死因子α的mRNA表达量高于横断组(P < 0.05,P < 0.01,P < 0.005,P < 0.000 1),横断+切除组白细胞介素6的mRNA表达量高于横断+撕裂组(P < 0.05)。"
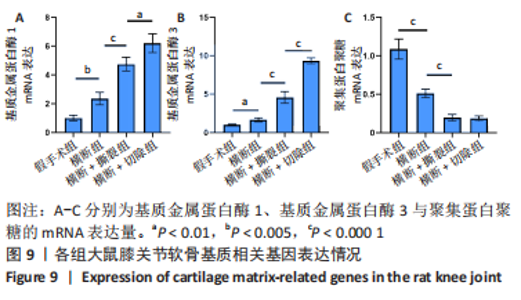
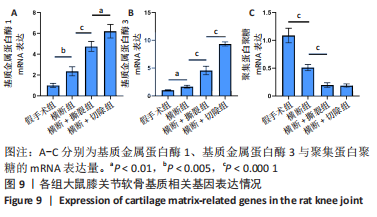
此外,各组大鼠膝关节软骨相关的基质以及基质降解酶的基因表达,如图9所示。与假手术组相比,横断组基质金属蛋白酶1、基质金属蛋白酶3的mRNA表达量升高(P < 0.01,P < 0.005),聚集蛋白聚糖的mRNA表达量降低(P < 0.000 1);与横断组相比,横断+撕裂组基质金属蛋白酶1、基质金属蛋白酶3的mRNA表达量升高(P < 0.000 1),聚集蛋白聚糖的mRNA表达量降低(P < 0.000 1);与横断+撕裂组相比,横断+切除组基质金属蛋白酶1、基质金属蛋白酶3的mRNA表达量升高(P < 0.01,P < 0.000 1),聚集蛋白聚糖的mRNA表达量无明显变化(P > 0.05)。"
| [1] CARBONE A, RODEO S. Review of current understanding of post-traumatic osteoarthritis resulting from sports injuries. J Orthop Res. 2017;35(3):397-405. [2] SRIKANTH VK, FRYER JL, ZHAI G, et al. A meta-analysis of sex differences prevalence, incidence and severity of osteoarthritis. Osteoarthritis Cartilage. 2005;13(9):769-781. [3] DE BOER TN, VAN SPIL WE, HUISMAN AM, et al. Serum adipokines in osteoarthritis; comparison with controls and relationship with local parameters of synovial inflammation and cartilage damage. Osteoarthritis Cartilage. 2012;20(8):846-853. [4] BROWN TD, JOHNSTON RC, SALTZMAN CL, et al. Posttraumatic osteoarthritis: a first estimate of incidence, prevalence, and burden of disease. J Orthop Trauma. 2006;20(10):739-744. [5] THOMAS AC, HUBBARD-TURNER T, WIKSTROM EA, et al. Epidemiology of Posttraumatic Osteoarthritis. J Athl Train. 2017;52(6):491-496. [6] FOY CG, PENNINX BW, SHUMAKER SA, et al. Long-term exercise therapy resolves ethnic differences in baseline health status in older adults with knee osteoarthritis. J Am Geriatr Soc. 2005;53(9):1469-1475. [7] MESSIER SP, MIHALKO SL, LEGAULT C, et al. Effects of intensive diet and exercise on knee joint loads, inflammation, and clinical outcomes among overweight and obese adults with knee osteoarthritis: the IDEA randomized clinical trial. JAMA. 2013;310(12):1263-1273. [8] TARUC-UY RL, LYNCH SA. Diagnosis and treatment of osteoarthritis. Prim Care. 2013;40(4):821-536. [9] MENENDEZ MI, HETTLICH B, WEI L, et al. Feasibility of Na18F PET/CT and MRI for Noninvasive In Vivo Quantification of Knee Pathophysiological Bone Metabolism in a Canine Model of Post-traumatic Osteoarthritis. Mol Imaging. 2017;16:1536012117714575. [10] RIORDAN EA, LITTLE C, HUNTER D. Pathogenesis of post-traumatic OA with a view to intervention. Best Pract Res Clin Rheumatol. 2014; 28(1):17-30. [11] LAMPROPOULOU-ADAMIDOU K, LELOVAS P, KARADIMAS EV, et al. Useful animal models for the research of osteoarthritis. Eur J Orthop Surg Traumatol. 2014;24(3):263-271. [12] TEEPLE E, JAY GD, ELSAID KA, et al. Animal models of osteoarthritis: challenges of model selection and analysis. AAPS J. 2013;15(2):438-446. [13] VINCENT TL, WILLIAMS RO, MACIEWICZ R, et al. Arthritis Research UK animal models working group. Mapping pathogenesis of arthritis through small animal models. Rheumatology (Oxford). 2012;51(11): 1931-1941. [14] MILLER RE, MALFAIT AM. Osteoarthritis pain: What are we learning from animal models? Best Pract Res Clin Rheumatol. 2017;31(5):676-687. [15] LIEBERTHAL J, SAMBAMURTHY N, SCANZELLO CR. Inflammation in joint injury and post-traumatic osteoarthritis. Osteoarthritis Cartilage. 2015;23(11):1825-1834. [16] LIM CY, IN J. Randomization in clinical studies. Korean J Anesthesiol. 2019;72(3):221-232. [17] CHAMBERS MG, BAYLISS MT, MASON RM. Chondrocyte cytokine and growth factor expression in murine osteoarthritis. Osteoarthritis Cartilage. 1997;5(5):301-308. [18] BRANDT KD, FIFE RS, BRAUNSTEIN EM, et al. Radiographic grading of the severity of knee osteoarthritis: relation of the Kellgren and Lawrence grade to a grade based on joint space narrowing, and correlation with arthroscopic evidence of articular cartilage degeneration. Arthritis Rheum. 1991;34(11):1381-1386. [19] FELSON DT, NIU J, GUERMAZI A, et al. Defining radiographic incidence and progression of knee osteoarthritis: suggested modifications of the Kellgren and Lawrence scale. Ann Rheum Dis. 2011;70(11):1884-1886. [20] AHLBÄCK S. Osteoarthrosis of the knee. A radiographic investigation. Acta Radiol Diagn (Stockh). 1968:Suppl 277:7-72. [21] LEACH RE, GREGG T, SIBER FJ. Weight-bearing radiography in osteoarthritis of the knee. Radiology. 1970;97(2):265-268. [22] ALTMAN RD, FRIES JF, BLOCH DA, et al. Radiographic assessment of progression in osteoarthritis. Arthritis Rheum. 1987;30(11):1214-1225. [23] XIA B, DI CHEN, ZHANG J, et al. Osteoarthritis pathogenesis: a review of molecular mechanisms. Calcif Tissue Int. 2014;95(6):495-505. [24] GOLDRING SR, GOLDRING MB. Changes in the osteochondral unit during osteoarthritis: structure, function and cartilage-bone crosstalk. Nat Rev Rheumatol. 2016;12(11):632-644. [25] GOLDRING MB, GOLDRING SR. Articular cartilage and subchondral bone in the pathogenesis of osteoarthritis. Ann N Y Acad Sci. 2010;1192: 230-237. [26] ZHEN G, CAO X. Targeting TGFβ signaling in subchondral bone and articular cartilage homeostasis. Trends Pharmacol Sci. 2014;35(5):227-236. [27] LI G, YIN J, GAO J, et al. Subchondral bone in osteoarthritis: insight into risk factors and microstructural changes. Arthritis Res Ther. 2013; 15(6):223. [28] FUNCK-BRENTANO T, COHEN-SOLAL M. Subchondral bone and osteoarthritis. Curr Opin Rheumatol. 2015;27(4):420-426. [29] LOESER RF, GOLDRING SR, SCANZELLO CR, et al. Osteoarthritis: a disease of the joint as an organ. Arthritis Rheum. 2012;64(6):1697-1707. [30] MARTEL-PELLETIER J, BARR AJ, CICUTTINI FM, et al. Osteoarthritis. Nat Rev Dis Primers. 2016;2:16072. [31] SWINGLER TE, NIU L, SMITH P, et al. The function of microRNAs in cartilage and osteoarthritis. Clin Exp Rheumatol. 2019;37 Suppl 120(5):40-47. [32] ILAS DC, CHURCHMAN SM, MCGONAGLE D, et al. Targeting subchondral bone mesenchymal stem cell activities for intrinsic joint repair in osteoarthritis. Future Sci OA. 2017;3(4):FSO228. [33] PRIMORAC D, MOLNAR V, ROD E, et al. Knee Osteoarthritis: A Review of Pathogenesis and State-Of-The-Art Non-Operative Therapeutic Considerations. Genes (Basel). 2020;11(8):854. [34] PRATTA MA, SU JL, LEESNITZER MA, et al. Development and characterization of a highly specific and sensitive sandwich ELISA for detection of aggrecanase-generated aggrecan fragments. Osteoarthritis Cartilage. 2006;14(7):702-713. [35] LOESER RF. Molecular mechanisms of cartilage destruction: mechanics, inflammatory mediators, and aging collide. Arthritis Rheum. 2006; 54(5):1357-1360. [36] HARKEY MS, LUC BA, GOLIGHTLY YM, et al. Osteoarthritis-related biomarkers following anterior cruciate ligament injury and reconstruction: a systematic review. Osteoarthritis Cartilage. 2015; 23(1):1-12. [37] MOBASHERI A, HENROTIN Y. Biomarkers of (osteo)arthritis. Biomarkers. 2015;20(8):513-518. [38] BOEHME KA, ROLAUFFS B. Onset and Progression of Human Osteoarthritis-Can Growth Factors, Inflammatory Cytokines, or Differential miRNA Expression Concomitantly Induce Proliferation, ECM Degradation, and Inflammation in Articular Cartilage? Int J Mol Sci. 201;19(8):2282. [39] GOLDRING MB, GOLDRING SR. Osteoarthritis. J Cell Physiol. 2007; 213(3):626-634. [40] ENGLUND M, GUERMAZI A, LOHMANDER LS. The meniscus in knee osteoarthritis. Rheum Dis Clin North Am. 2009;35(3):579-590. [41] ENGLUND M, GUERMAZI A, ROEMER FW, et al. Meniscal tear in knees without surgery and the development of radiographic osteoarthritis among middle-aged and elderly persons: The Multicenter Osteoarthritis Study. Arthritis Rheum. 2009;60(3):831-839. [42] ANDERSON DD, MARSH JL, BROWN TD. The pathomechanical etiology of post-traumatic osteoarthritis following intraarticular fractures. Iowa Orthop J. 2011;31:1-20. [43] SWÄRD P, FROBELL R, ENGLUND M, et al. Cartilage and bone markers and inflammatory cytokines are increased in synovial fluid in the acute phase of knee injury (hemarthrosis)--a cross-sectional analysis. Osteoarthritis Cartilage. 2012;20(11):1302-1308. [44] HAN PF, WEI L, DUAN ZQ, et al. Contribution of IL-1β, 6 and TNF-α to the form of post-traumatic osteoarthritis induced by “idealized” anterior cruciate ligament reconstruction in a porcine model. Int Immunopharmacol. 2018;65:212-220. [45] BIGONI M, SACERDOTE P, TURATI M, et al. Acute and late changes in intraarticular cytokine levels following anterior cruciate ligament injury. J Orthop Res. 2013;31(2):315-321. [46] AIGNER T, COOK JL, GERWIN N, et al. Histopathology atlas of animal model systems - overview of guiding principles. Osteoarthritis Cartilage. 2010;18 Suppl 3:S2-6. [47] COHEN-SOLAL M, HAY E, FUNCK-BRENTANO T. Animal models in OA: a means to explore bone. Osteoporos Int. 2012;23 Suppl 8:S853-856. [48] LITTLE CB, ZAKI S. What constitutes an “animal model of osteoarthritis”--the need for consensus? Osteoarthritis Cartilage. 2012;20(4):261-267. [49] 廖建钊,夏天.细胞外基质在骨关节炎发生、发展中的作用及 临床研究价值[J].中国组织工程研究,2022,26(12):1937-1943. |
| [1] | Zhang Kefan, Shi Hui. Research status and application prospect of cytokine therapy for osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(6): 961-967. |
| [2] | Zhang Zeyi, Yang Yimin, Li Wenyan, Zhang Meizhen. Effect of foot progression angle on lower extremity kinetics of knee osteoarthritis patients of different ages: a systematic review and meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(6): 968-975. |
| [3] | Shen Feiyan, Yao Jixiang, Su Shanshan, Zhao Zhongmin, Tang Weidong. Knockdown of circRNA WD repeat containing protein 1 inhibits proliferation and induces apoptosis of chondrocytes in knee osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(4): 499-504. |
| [4] | Maisituremu·Heilili, Zhang Wanxia, Nijiati·Nuermuhanmode, Maimaitituxun·Tuerdi. Effect of intraarticular injection of different concentrations of ozone on condylar histology of rats with early temporomandibular joint osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(4): 505-509. |
| [5] | Qiao Hujun, Wang Guoxiang. Evaluation of rat osteoarthritis chondrocyte models induced by interleukin-1beta [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(4): 516-521. |
| [6] | Zhang Yaru, Chen Yanjun, Zhang Xiaodong, Chen Shenghua, Huang Wenhua. Effect of ferroptosis mediated by glutathione peroxidase 4 in the occurrence and progression of synovitis in knee osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(4): 550-555. |
| [7] | Liu Luxing, Di Mingyuan, Yang Qiang. Signaling pathways in the mechanism underlying active ingredients of Chinese medicine in the treatment of osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(4): 609-614. |
| [8] | Yan Binghan, Li Zhichao, Su Hui, Xue Haipeng, Xu Zhanwang, Tan Guoqing. Mechanisms of traditional Chinese medicine monomers in the treatment of osteoarthritis by targeting autophagy [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(4): 627-632. |
| [9] | Chen Junyan, Meng Qingqi, Li Siming. Cartilage targeting function in the drug delivery system by intra-articular injection for the treatment of osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(3): 458-463. |
| [10] | Liu Baofang, Xu Bin, Chen Lei. Pueraria decoction in the treatment of osteoarthritis: network pharmacology analysis and animal model validation [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(2): 193-199. |
| [11] | Meng Zhicheng, Qiao Weiping, Zhao Yang, Liu Hongfei, Li Kaijie, Ma Bo. Effects of immune cells and related cytokines in the pathogenesis and treatment of osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(2): 280-287. |
| [12] | Wang Xianfeng, Wang Kun, Sun Han, Sun Xiaoliang, Yan Litao. Mechanism underlying exosomal lncRNA H19 derived from umbilical cord mesenchymal stem cells promotes cartilage injury repair [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(1): 20-25. |
| [13] | Shen Feiyan, Yao Jixiang, Su Shanshan, Zhao Zhongmin, Tang Weidong. Knockdown of circRNA WD repeat containing protein 1 inhibits proliferation and induces apoptosis of chondrocytes in knee osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(在线): 1-6. |
| [14] | Li Xiaomin, Tian Xiangdong, Tan Yetong, Zhu Guangyu, Wang Rongtian, Wang Jian, Xue Zhipeng, Ma Sheng, Hu Yuanyi, Huang Ye, Ding Tiansong. Changes of lower limb force line and knee function after high tibial osteotomy in osteoporotic medial ventricular knee osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(9): 1325-1329. |
| [15] | Huang Linke, Wei Linhua, Jiang Jie, Liu Qian, Chen Weiwei. Effects of estrogen combined with treadmill exercise on bone mass and articular cartilage in ovariectomized mice [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(8): 1166-1171. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||