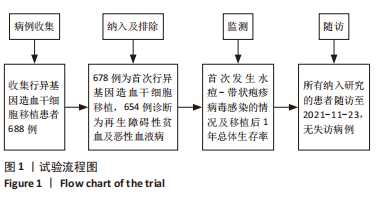
Chinese Journal of Tissue Engineering Research ›› 2023, Vol. 27 ›› Issue (33): 5292-5297.doi: 10.12307/2023.728
Previous Articles Next Articles
Analysis of risk factors for varicella-zoster virus infection after allogeneic hematopoietic stem cell transplantation
Qin Jing, Zhang Suping, Li Li, Zhang Ran, Peng Yingnan, Gao Siyu, Fan Jinpeng, Bian Zhilei, Wan Dingming
- Hematopoietic Stem Cell Transplantation Center, Department of Hematology, First Affiliated Hospital of Zhengzhou University, Zhengzhou 450052, Henan Province, China
-
Received:2022-10-09Accepted:2022-11-23Online:2023-11-28Published:2023-03-30 -
Contact:Wan Dingming, MD, Professor, Chief physician, Master’s supervisor, Hematopoietic Stem Cell Transplantation Center, Department of Hematology, First Affiliated Hospital of Zhengzhou University, Zhengzhou 450052, Henan Province, China -
About author:Qin Jing, Master candidate, Hematopoietic Stem Cell Transplantation Center, Department of Hematology, First Affiliated Hospital of Zhengzhou University, Zhengzhou 450052, Henan Province, China Zhang Suping, Attending physician, Hematopoietic Stem Cell Transplantation Center, Department of Hematology, First Affiliated Hospital of Zhengzhou University, Zhengzhou 450052, Henan Province, China -
Supported by:the National Natural Science Foundation of China (General Program), No. 82170211 (to BZL); the Major Scientific Research Project of Henan Provincial Universities, No. 20A320021 (to ZR)
CLC Number:
Cite this article
Qin Jing, Zhang Suping, Li Li, Zhang Ran, Peng Yingnan, Gao Siyu, Fan Jinpeng, Bian Zhilei, Wan Dingming. Analysis of risk factors for varicella-zoster virus infection after allogeneic hematopoietic stem cell transplantation[J]. Chinese Journal of Tissue Engineering Research, 2023, 27(33): 5292-5297.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks

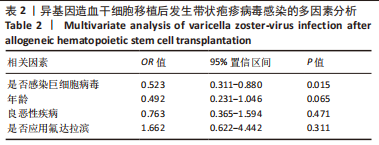
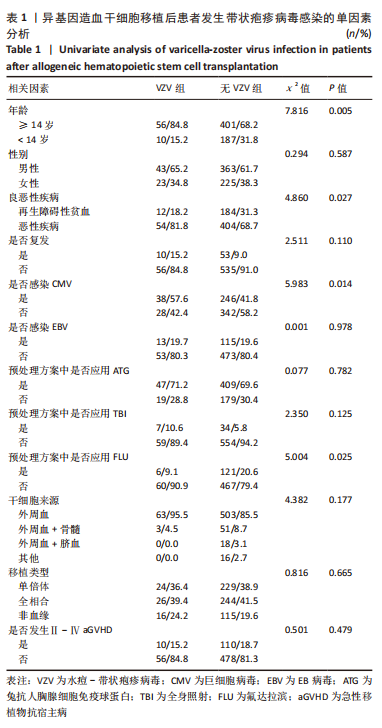
2.3 VZV感染发生率及影响因素分析 654例患者中有66例发生带状疱疹病毒感染,发病率为10.1%,其中男43例,女23例;发病中位年龄为21.5岁(4-62岁)。66例患者中,年龄 < 14岁有10例;合并巨细胞病毒血症38例;预处理方案中应用氟达拉滨6例;再生障碍性贫血12例,急性髓系白血病25例,急性淋巴细胞白血病20例,骨髓增生异常综合征6例,非霍奇金淋巴瘤3例。异基因造血干细胞移植后VZV感染发生率与年龄、预处理方案中是否应用氟达拉滨及基础疾病的良恶性有关(P < 0.05),也与移植后是否感染巨细胞病毒有关(P < 0.05)。与复发、移植方式、干细胞来源及预处理方案中是否应用兔抗人胸腺细胞免疫球蛋白等无关,结果见表1。在多因素分析中,VZV感染的发生与是否合并巨细胞病毒感染有关,结果见表2。"

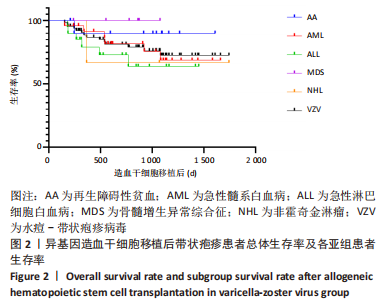
2.4 发病时间 发病的中位时间为149 d(19-1 179 d),在66例VZV患者中,1年内有51例(77.3%)发生VZV感染,2年内有62例(93.9%)发生VZV感染,3年内有65例(98.5%)发生VZV感染,最长发病时间为1 179 d。 2.5 临床表现 多数患者发病前可有局部皮肤的持续性疼痛,后出现成簇水疱,继而出现粟粒大小斑丘疹,常沿一侧周围神经分布,后发展为晶莹透亮的水疱。66例中13例发生于头颈或颜面部,8例为全身泛发性疱疹,41例发生于胸腰腹及会阴处,4例发生于四肢。有7例患者发生带状疱疹性脑炎,17例患者发生带状疱疹后神经痛,多数患者后遗神经痛随时间延长逐渐减轻,1例患者右耳失聪。 2.6 治疗情况 ①局部治疗:应用更昔洛韦眼用凝胶或重组人干扰素α-2b凝胶局部外用涂抹,水疱较大者碘伏消毒后予以抽吸疱液;②抗病毒治疗:静脉输注阿糖腺苷、喷昔洛韦或膦甲酸钠,或口服阿昔洛韦治疗,合并巨细胞病毒感染者可应用更昔洛韦治疗;③支持治疗:66例中有40例予以静脉输注丙种球蛋白400 mg/(kg?d)提高免疫力;④对症治 疗:有明显神经痛者给予止痛药物及甲钴胺等营养神经治疗,合并感染者给予相应的抗感染治疗。 2.7 造血重建及植入情况 66例患者均获造血重建。①粒细胞植入标准:连续3 d中性粒细胞> 0.5×109 L-1;②血小板植入标准:脱离血小板输注的情况下,连续7 d血小板> 20×109 L-1,中性粒细胞植入中位时间为13 d(9-25 d),血小板植入中位时间为13.5 d(6-32 d)。患者血型转为供者型,患者染色体及嵌合均为供者型。 2.8 预后 所有患者治疗后均出现水疱干瘪结痂,病情逐渐好转至痊愈,原疱疹部位遗留色素沉着斑,部分患者遗留疱疹后神经痛,治疗的中位时间为11 d(3-38 d)。有1例患者因发生带状疱疹感染而失聪。在66例患者中,有14例死亡,其中8例死于原发病复发,4例死于真菌感染,1例死于肝脏及肠道Ⅳ度移植物抗宿主病,1例死于脓毒血症。如图2所示,VZV组患者1年生存率为87%,2年生存率为81%,3年生存率为73%。在恶性疾病中,骨髓增生异常综合征患者预后最佳,急性淋巴细胞白血病预后最差,生存率因原发病不同而各有不同。"
| [1] 王昱,黄晓军.造血干细胞移植在血液疾病中的应用进展[J].中华血液学杂志,2019,40(8):704-708. [2] SAHIN U, TOPRAK SK, ATILLA PA, et al. An overview of infectious complications after allogeneic hematopoietic stem cell transplantation. J Infect Chemother. 2016;22(8):505-514. [3] HELDMAN MR, AAGAARD KM, HILL JA. Assessing and restoring adaptive immunity to HSV, VZV, and HHV-6 in solid organ and hematopoietic cell transplant recipients. Clin Microbiol Infect. 2022; 28(10):1345-1350. [4] 魏伟,明英姿.浅谈实体器官移植后水痘-带状疱疹病毒感染[J].器官移植,2018,9(5):399-401. [5] DÜVER F, WEIßBRICH B, EYRICH M, et al. Viral reactivations following hematopoietic stem cell transplantation in pediatric patients - A single center 11-year analysis. PLoS One. 2020;15(2):e0228451. [6] 黄胜萍,廖烈兰,方润平.带状疱疹并发症的诊治与预防的研究进展[J].内科,2022,17(3):301-304. [7] DADWAL SS. Herpes Virus Infections Other than Cytomegalovirus in the Recipients of Hematopoietic Stem Cell Transplantation. Infect Dis Clin North Am. 2019;33(2):467-484. [8] LJUNGMAN P, LÖNNQVIST B, GAHRTON G, et al. Clinical and subclinical reactivations of varicella-zoster virus in immunocompromised patients. J Infect Dis. 1986;153(5):840-847. [9] CAUDA R, GROSSI CE, WHITLEY RJ, et al. Analysis of immune function in herpes zoster patients: demonstration and characterization of suppressor cells. J Immunol. 1987;138(4):1229-1233. [10] BOCCARD M, CONRAD A, MOUTON W, et al. A Simple-to-Perform ifn-γ mRNA Gene Expression Assay on Whole Blood Accurately Appraises Varicella Zoster Virus-Specific Cell-Mediated Immunity After Allogeneic Hematopoietic Stem Cell Transplantation. Front Immunol. 2022;13:919806. [11] BAUMRIN E, CHENG MP, KANJILAL S, et al. Severe Herpes Zoster Requiring Intravenous Antiviral Treatment in Allogeneic Hematopoietic Cell Transplantation Recipients on Standard Acyclovir Prophylaxis. Biol Blood Marrow Transplant. 2019;25(8):1642-1647. [12] KOC Y, MILLER KB, SCHENKEIN DP, et al. Varicella zoster virus infections following allogeneic bone marrow transplantation: frequency, risk factors, and clinical outcome. Biol Blood Marrow Transplant. 2000; 6(1):44-49. [13] BUUS-GEHRIG C, BOCHENNEK K, HENNIES MT, et al. Systemic viral infection in children receiving chemotherapy for acute leukemia. Pediatr Blood Cancer. 2020;67(12):e28673. [14] ERARD V, GUTHRIE KA, VARLEY C, et al. One-year acyclovir prophylaxis for preventing varicella-zoster virus disease after hematopoietic cell transplantation: no evidence of rebound varicella-zoster virus disease after drug discontinuation. Blood. 2007;110(8):3071-3077. [15] SEO HM, KIM YS, BANG CH, et al. Antiviral prophylaxis for preventing herpes zoster in hematopoietic stem cell transplant recipients: A systematic review and meta-analysis. Antiviral Res. 2017;140:106-115. [16] UMEZAWA Y, KAKIHANA K, OSHIKAWA G, et al. Clinical features and risk factors for developing varicella zoster virus dissemination following hematopoietic stem cell transplantation. Transpl Infect Dis. 2014;16(2):195-202. [17] BAUMRIN E, IZAGUIRRE NE, BAUSK B, et al. Safety and reactogenicity of the recombinant zoster vaccine after allogeneic hematopoietic cell transplantation. Blood Adv. 2021;5(6):1585-1593. [18] XUE E, XIE H, LEISENRING WM, et al. High Incidence of Herpes Zoster After Cord Blood Hematopoietic Cell Transplant Despite Longer Duration of Antiviral Prophylaxis. Clin Infect Dis. 2021;72(8):1350-1357. [19] 沈悌,赵永强.血液病诊断及疗效标准[M].4版.北京:科学出版社,2018. [20] 中华医学会血液学分会干细胞应用学组.中国异基因造血干细胞移植治疗血液系统疾病专家共识(Ⅲ)——急性移植物抗宿主病(2020年版)[J].中华血液学杂志,2020,41(7):529-536. [21] SCHOEMANS HM, LEE SJ, FERRARA JL, et al. EBMT-NIH-CIBMTR Task. Force position statement on standardized terminology & guidance for graft-versus-host disease assessment. Bone Marrow Transplant. 2018; 53(11):1401-1415. [22] CHUN JY, KIM K, LEE MK, et al. Immunogenicity and safety of a live herpes zoster vaccine in hematopoietic stem cell transplant recipients. BMC Infect Dis. 2021;21(1):117. [23] STOREK J, DAWSON MA, STORER B, et al. Immune reconstitution after allogeneic marrow transplantation compared with blood stem cell transplantation. Blood. 2001;97(11):3380-3389. [24] 郝新健,周健,房佰俊,等.异基因造血干细胞移植后带状疱疹的临床分析[J].中华实验和临床感染病杂志(电子版),2012,6(3):221-224. [25] BEYAR-KATZ O, BITTERMAN R, ZUCKERMAN T, et al. Anti-herpesvirus prophylaxis, pre-emptive treatment or no treatment in adults undergoing allogeneic transplant for haematological disease: systematic review and meta-analysis. Clin Microbiol Infect. 2020;26(2):189-198. [26] HAN SB, KIM SK, LEE JW, et al. Varicella zoster virus infection after allogeneic hematopoietic cell transplantation in children using a relatively short duration of acyclovir prophylaxis: A retrospective study. Medicine (Baltimore). 2017;96(14):e6546. [27] ASANO-MORI Y, KANDA Y, OSHIMA K, et al. Long-term ultra-low-dose acyclovir against varicella–zoster virus reactivation after allogeneic hematopoietic stem cell transplantation. Am J Hematol 2008;83:472-476. [28] MASCARENHAS K, TEH JB, PENG K, et al. Efficacy of low-dose zoster prophylaxis in patients undergoing allogeneic hematopoietic cell transplantation. Bone Marrow Transplant. 2020;55(8):1662-1664. [29] BLENNOW O, FJAERTOFT G, WINIARSKI J, et al. Varicella-zoster reactivation after allogeneic stem cell transplantation without routine prophylaxis--the incidence remains high. Biol Blood Marrow Transplant. 2014;20(10):1646-1649. [30] LIZZI J, HILL T, JAKUBOWSKI J. Varicella Zoster Virus Encephalitis. Clin Pract Cases Emerg Med. 2019;3(4):380-382. [31] OH JH, TUMMALA S, HUSNAIN MG. Disseminated herpes zoster with acute encephalitis in an immunocompetent elderly man. BMJ Case Rep. 2020;13(6):e232928. [32] GRATAMA JW, WEILAND HT, HEKKER AC, et al. Herpes virus immunity and acute graft-versus-host disease. Transplant Proc. 1987;19(1 Pt 3): 2680-2682. [33] ARVIN A, ABENDROTH A. VZV: immunobiology and host response//ARVIN A, CAMPADELLI-FIUME G, MOCARSKI E, et al. editors. Human Herpesviruses: Biology, Therapy, and Immunoprophylaxis. Cambridge: Cambridge University Press. 2007:Chapter 39. [34] KENNEDY PGE, GERSHON AA. Clinical Features of Varicella-Zoster Virus Infection. Viruses. 2018;10(11):609. [35] LEWIS DJ, SCHLICHTE MJ, DAO H JR. Atypical disseminated herpes zoster: management guidelines in immunocompromised patients. Cutis. 2017;100(5):321,324,330. [36] 宋微,马良娟.移植与带状疱疹相关性新进展[J].中国麻风皮肤病杂志,2021,37(4):260-262. [37] KANG M, ASLAM S. Varicella zoster virus encephalitis in solid organ transplant recipients: Case series and review of literature. Transpl Infect Dis. 2019;21(2):e13038. [38] NABI S, KAHLON P, GOGGINS M, et al. VZV encephalitis following successful treatment of CMV infection in a patient with kidney transplant. BMJ Case Rep. 2014;2014:bcr2014206655. [39] 王卓,何岳林,廖建云等.异基因造血干细胞移植术后患儿带状疱疹病毒感染的临床研究[J].中华实用儿科临床杂志,2019,34(12): 930-933. [40] DOKI N, MIYAWAKI S, TANAKA M, et al. Visceral varicella zoster virus infection after allogeneic stem cell transplantation.Transpl Infect Dis. 2013;15(3):314-318. |
| [1] | You Zhengqiu, Zhang Zhongzu, Wang Qunbo. Early symptomatic intervertebral disc pseudocysts after discectomy detected on MRI [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(9): 1403-1409. |
| [2] | Zhao Wei, Feng Wei, Yang Tieyi, Ren Wei, Wang Yuxin, Lyu Huicheng, Chang Zhiqiang, Feng Xiaodong, Wang Ziheng, Guo Shibing. Antibiotic bone cement intramedullary nail prepared using 3D printed mold for the treatment of long bone infection in lower limbs [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(7): 1023-1030. |
| [3] | Zhang Wei, Huang Zhichao, Zhao Ruifeng, Liang Huan, Ma Yufeng, Shen Yanguang, Zhong Honggang, Chen Zhaojun, Zhang Jichuan, Chen Weiheng. Efficacy of gutta-percha splint on a rabbit fracture model [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(7): 1055-1061. |
| [4] | Lu Zhaohua, Sun Tianze, Zhang Jing, Zhang Wentao, Yang Ming, Li Zhonghai. Kyphoplasty via different approaches for osteoporotic vertebral compression fractures [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(36): 5834-5839. |
| [5] | Zhai Hongjie, Han Guanda, Li Lei, Dong Xiaohui, Jiang Zhiquan, Lou Feiyun. 3D printed polyetheretherketone material for skull defect repair [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(3): 380-384. |
| [6] | Zhou Jie, Pei Xibo, Wan Qianbing. Advances and biological application of asymmetric dressings [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(3): 434-440. |
| [7] | Wang Kaiyu, Hu Peng, Wei Zairong, Huang Guangtao, Zhou Jian, He Guijia, Nie Kaiyu. Use of expanders and implants in breast reconstruction complicated with infection [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(3): 461-469. |
| [8] | Han Tao, Hao Jianqiang, Li Wenbo, Shi Jie, Gao Qiuming. Advantages and problems of antibiotic-loaded bone cements for bone and joint infections [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(3): 470-477. |
| [9] | Ma Tianyong, Wang Dewei. Finite element analysis of middle clavicle fracture with ortho-bridge system intramedullary fixation [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(29): 4599-4606. |
| [10] | Liu Guanjuan, Song Na, Huo Hua, Luo Shanshan, Cheng Yuting, Xiong Yue, Hong Wei, Liao Jian. Zoledronic acid inhibits lipopolysaccharide-induced osteoclast differentiation by regulating NLRP3 signaling pathway [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(29): 4677-4683. |
| [11] | Chen Jinlun, Deng Peng, Ye Pengcheng, Cao Houran, Zeng Huiliang, Feng Wenjun, Zeng Jianchun, Zeng Yirong. Dynamic monitoring of plasma human alpha-defensin 1-3 and peripheral blood inflammatory markers after primary total knee arthroplasty [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(27): 4304-4311. |
| [12] | Gao Yue, Fu Ziwei, Wu Yanbo, Pan Shinong, Lu Zhao. Clinical and imaging features of slipped capital femoral epiphysis [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(27): 4421-4428. |
| [13] | Liu Zhilun, Guan Zhiyu, Jiang Taiping, Li Chengxi, Liu Zhaoming. Mechanism of stone balm on the healing of infected refractory wounds in rats [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(26): 4126-4131. |
| [14] | Wang Shan, Wang Cheng, Shi Yehong. Clinical application of vascular cryoprotectants [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(24): 3920-3925. |
| [15] | Ma Maoxiao, Cui Guofeng, Zhang Xue, Zhang Hong, Liu Youwen, Yue Chen. Complications after spinal fusion in patients with metabolic syndrome: a systematic review and meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(22): 3602-3608. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||