Chinese Journal of Tissue Engineering Research ›› 2023, Vol. 27 ›› Issue (7): 1023-1030.doi: 10.12307/2023.026
Previous Articles Next Articles
Antibiotic bone cement intramedullary nail prepared using 3D printed mold for the treatment of long bone infection in lower limbs
Zhao Wei, Feng Wei, Yang Tieyi, Ren Wei, Wang Yuxin, Lyu Huicheng, Chang Zhiqiang, Feng Xiaodong, Wang Ziheng, Guo Shibing
- Department of Orthopedic, Second Affiliated Hospital of Inner Mongolia Medical University, Orthopedic Institute of Inner Mongolia Autonomous Region, Hohhot 010030, Inner Mongolia Autonomous Region, China
-
Received:2021-08-26Accepted:2022-01-15Online:2023-03-08Published:2022-07-18 -
Contact:Guo Shibing, Chief physician, Professor, Department of Orthopedic, Second Affiliated Hospital of Inner Mongolia Medical University, Orthopedic Institute of Inner Mongolia Autonomous Region, Hohhot 010030, Inner Mongolia Autonomous Region, China -
About author:Zhao Wei, Master, Associate chief physician, Department of Orthopedic, Second Affiliated Hospital of Inner Mongolia Medical University, Orthopedic Institute of Inner Mongolia Autonomous Region, Hohhot 010030, Inner Mongolia Autonomous Region, China
CLC Number:
Cite this article
Zhao Wei, Feng Wei, Yang Tieyi, Ren Wei, Wang Yuxin, Lyu Huicheng, Chang Zhiqiang, Feng Xiaodong, Wang Ziheng, Guo Shibing. Antibiotic bone cement intramedullary nail prepared using 3D printed mold for the treatment of long bone infection in lower limbs[J]. Chinese Journal of Tissue Engineering Research, 2023, 27(7): 1023-1030.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
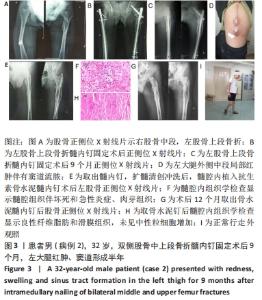
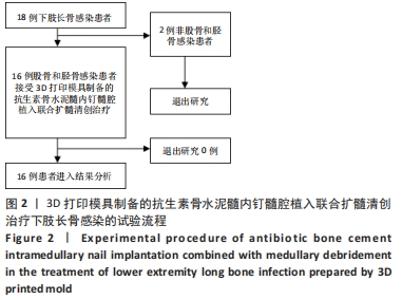
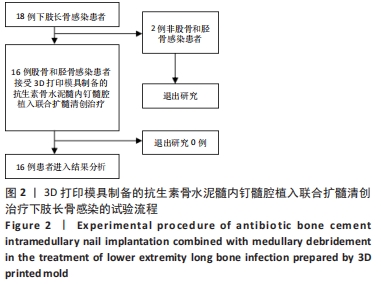
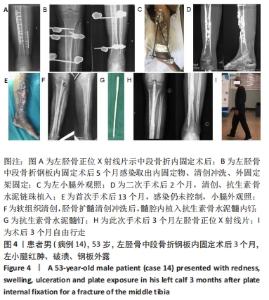
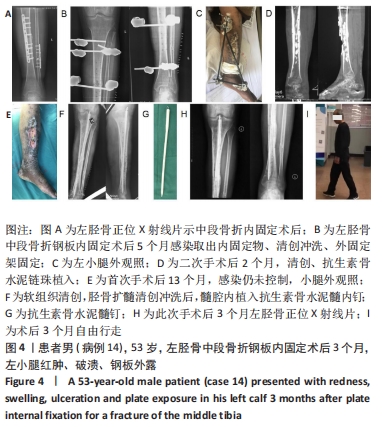
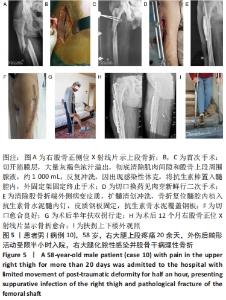
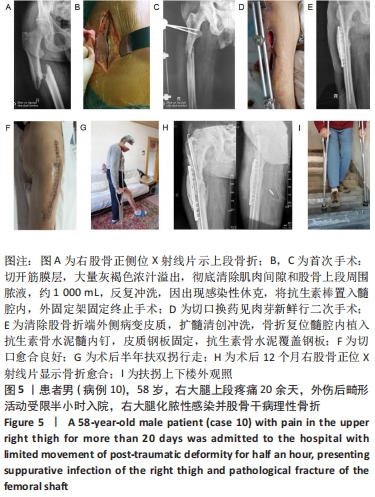
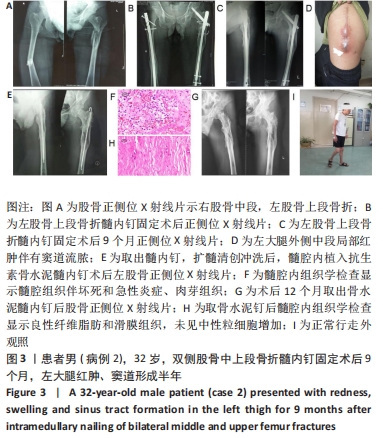
2.2 一般结果 14例股骨和胫骨骨折患者内固定术后骨感染以及2例股骨慢性骨髓炎患者均顺利完成手术,手术时间40-120 min;术中失血量50-400 mL。1例股骨陈旧骨折外固定架固定后2个月拆除,6个月骨折愈合;1例股骨新鲜骨折术后骨牵引,10个月骨折畸形愈合。16例患者住院时间7-53个月,平均(26.3±13.1)个月;医疗费用0.6-4.94万元,平均(3.86±1.63)万元。术后均获得随访,随访时间12-24个月,平均(15.9±4.7)个月。抗生素骨水泥髓内钉植入时间6-24个月(至随访),6例患者已取出。 2.3 髓内感染细菌培养情况及治疗 术前切口感染或窦道处细菌培养阳性10例,细菌培养阴性6例,见表2。13例患者入院后根据经验治疗给予头孢类或青霉素药物,之后根据术前细菌培养及药敏结果改为头孢类抗生素或左氧氟沙星治疗。4例患者术前、术后经多次培养结果为阴性,但红细胞沉降率及C-反应蛋白或降钙素原升高,病理结果为急慢性炎症者均应用头孢类和左氧氟沙星。2例培养结果阴性者术前术后均未静脉滴注抗生素。 2.4 感染及炎性指标变化情况 5例患者术前白细胞、血沉、C-反应蛋白均高于正常值,4例患者术前降钙素原高于正常值,均在术后2-6周恢复正常至水平。另有8例患者术前仅血沉和/或C-反应蛋白高于正常值,术后2-4周后异常指标逐渐恢复正常。 2.5 髓内感染控制情况 10例骨折髓内钉固定术后感染患者采用局部清创取内固定,并扩髓清创、冲洗、抗生素骨水泥髓内钉植入治疗,9例感染得到控制,其中3例已去除抗生素骨水泥髓内钉,至随访(12-24个月)时感染无复发,3例骨折均愈合,见图3。"
| [1] METSEMAKERS WJ, EMANUEL N, COHEN O, et al. A doxycycline-loaded polymer-lipid encapsulation matrix coating for the prevention of implant-related osteomyelitis due to doxycycline-resistant methicillin-resistant Staphylococcus aureus. J Control Release. 2015; 209:47-56. [2] YOUNG S, LIE SA, HALLAN G, et al. Risk factors for infection after 46,113 intramedullary nail operations in low- and middle-income countries. World J Surg. 2013;37(2):349-355. [3] DOSHI P, GOPALAN H, SPRAGUE S, et al. Incidence of infection following internal fixation of open and closed tibia fractures in India (INFINITI): a multi-centre observational cohort study. BMC Musculoskelet Disord. 2017;18(1):156. [4] MAKRIDIS KG, TOSOUNIDIS T, GIANNOUDIS PV. Management of infection after intramedullary nailing of long bone fractures: treatment protocols and outcomes. Open Orthop J. 2013;7:219-226. [5] HAKE ME, OH JK, KIM JW, et al. Difficulties and challenges to diagnose and treat post-traumatic long bone osteomyelitis. Eur J Orthop Surg Traumatol. 2015;25(1):1-3. [6] SANCINETO CF, BARLA JD. Treatment of long bone osteomyelitis with a mechanically stable intramedullar antibiotic dispenser: nineteen consecutive cases with a minimum of 12 months follow-up. J Trauma. 2008;65(6):1416-1420. [7] SANDERS J, MAUFFREY C. Long bone osteomyelitis in adults: fundamental concepts and current techniques. Orthopedics. 2013; 36(5):368-375. [8] LIDGREN L, TÖRHOLM C. Intramedullary reaming in chronic diaphyseal osteomyelitis: a preliminary report. Clin Orthop Relat Res. 1980;(151): 215-221. [9] ZALAVRAS CG, SINGH A, PATZAKIS MJ. Novel technique for medullary canal débridement in tibia and femur osteomyelitis. Clin Orthop Relat Res. 2007;461:31-34. [10] TOSOUNIDIS TH, CALORI GM, GIANNOUDIS PV. The use of Reamer-irrigator-aspirator in the management of long bone osteomyelitis: an update. Eur J Trauma Emerg Surg. 2016;42(4):417-423. [11] KANAKARIS N, GUDIPATI S, TOSOUNIDIS T, et al. The treatment of intramedullary osteomyelitis of the femur and tibia using the Reamer-Irrigator-Aspirator system and antibiotic cement rods. Bone Joint J. 2014;96-B(6):783-788. [12] ZIMMERLI W, SENDI P. Orthopaedic biofilm infections. APMIS. 2017; 125(4):353-364. [13] ARCIOLA CR, CAMPOCCIA D, MONTANARO L. Implant infections: adhesion, biofilm formation and immune evasion. Nat Rev Microbiol. 2018;16(7):397-409. [14] 郑强,潘志军,李杭,等.抗生素骨水泥棒治疗交锁钉术后髓内感染[J].中华创伤杂志,2007,23(4):265-267. [15] KLEMM K, HENRY SL, SELIGSON D. The treatment of infection after interlocking nailing. Techniques Orthop.1988;3(3):54-61. [16] PALEY D, HERZENBERG JE. Intramedullary infections treated with antibiotic cement rods: preliminary results in nine cases. J Orthop Trauma. 2002;16(10):723-729. [17] CONWAY J, MANSOUR J, KOTZE K, et al. Antibiotic cement-coated rods: an effective treatment for infected long bones and prosthetic joint nonunions. Bone Joint J. 2014;96-B(10):1349-1354. [18] QIANG Z, JUN PZ, JIE XJ, et al. Use of antibiotic cement rod to treat intramedullary infection after nailing: preliminary study in 19 patients. Arch Orthop Trauma Surg. 2007;127(10):945-951. [19] BHADRA AK, ROBERTS CS. Indications for antibiotic cement nails. J Orthop Trauma. 2009;23(5 Suppl):S26-30. [20] SELHI HS, MAHINDRA P, YAMIN M, et al. Outcome in patients with an infected nonunion of the long bones treated with a reinforced antibiotic bone cement rod. J Orthop Trauma. 2012;26(3):184-188. [21] WASKO MK, BORENS O. Antibiotic cement nail for the treatment of posttraumatic intramedullary infections of the tibia: midterm results in 10 cases. Injury. 2013;44(8):1057-1060. [22] WASKO MK, KAMINSKI R. Custom-Made Antibiotic Cement Nails in Orthopaedic Trauma: Review of Outcomes, New Approaches, and Perspectives. Biomed Res Int. 2015;2015:387186. [23] HAKE ME, YOUNG H, HAK DJ, et al. Local antibiotic therapy strategies in orthopaedic trauma: Practical tips and tricks and review of the literature. Injury. 2015;46(8):1447-1456. [24] REILLY RM, ROBERTSON T, O’TOOLE RV, et al. Are antibiotic nails effective in the treatment of infected tibial fractures? Injury. 2016; 47(12):2809-2815. [25] CHO JW, KIM J, CHO WT, et al. Antibiotic coated hinged threaded rods in the treatment of infected nonunions and intramedullary long bone infections. Injury. 2018;49(10):1912-1921. [26] 薛德挺,李杭,潘志军,等.下肢长骨骨折髓内钉固定术后早期和迟发骨感染的处理[J].中华骨科杂志,2018,38(9):556-562. [27] FINELLI CA, DOS REIS FB, FERNANDES HA, et al. Intramedullary reaming modality for management of postoperative long bone infection: a prospective randomized controlled trial in 44 patients. Patient Saf Surg. 2019;13:39. [28] 王上增.抗生素骨水泥间置器治疗髓内固定术后骨髓炎合并骨不连[J].中国修复重建外科杂志,2011,25(8):972-975. [29] THONSE R, CONWAY J. Antibiotic cement-coated interlocking nail for the treatment of infected nonunions and segmental bone defects. J Orthop Trauma. 2007;21(4):258-268. [30] THONSE R, CONWAY JD. Antibiotic cement-coated nails for the treatment of infected nonunions and segmental bone defects. J Bone Joint Surg Am. 2008;90 Suppl 4:163-174. [31] NOH JH, KOH SJ, LEE KH. Treatment of Proximal Femur Osteomyelitis Occurred after Proximal Femoral Nail Antirotation Fixation, with Antibiotic Cement-coated Tibia Intramedullary Nail: A Case Report. Hip Pelvis. 2018;30(1):45-52. [32] SHYAM AK, SANCHETI PK, PATEL SK, et al. Use of antibiotic cement-impregnated intramedullary nail in treatment of infected non-union of long bones. Indian J Orthop. 2009;43(4):396-402. [33] 张彦龙,冯晨晨,田书伟,等.扩髓清创联合抗生素骨水泥髓腔植入治疗髓内钉术后感染[J].中华骨科杂志,2018,38(9):523-529. [34] CIERNY G 3RD, MADER JT, PENNINCK JJ. A clinical staging system for adult osteomyelitis. Clin Orthop Relat Res. 2003;(414):7-24. [35] KIM JW, CUELLAR DO, HAO J, et al. Custom-made antibiotic cement nails: a comparative study of different fabrication techniques. Injury. 2014;45(8):1179-1184. [36] PENNER MJ, MASRI BA, DUNCAN CP. Elution characteristics of vancomycin and tobramycin combined in acrylic bone-cement. J Arthroplasty. 1996;11(8):939-944. [37] JIANG N, WU HT, LIN QR, et al. Direct healthcare costs and utilization for inpatiets with extremity post-traumatic osteomyelitis:a retrospective single-center observational study in Southern China. Helsinkin, Finland: 37th Annual Meeting of the European Bone and Joint Infection Society, 2018. |
| [1] | Li Xiaomin, Tian Xiangdong, Tan Yetong, Zhu Guangyu, Wang Rongtian, Wang Jian, Xue Zhipeng, Ma Sheng, Hu Yuanyi, Huang Ye, Ding Tiansong. Changes of lower limb force line and knee function after high tibial osteotomy in osteoporotic medial ventricular knee osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(9): 1325-1329. |
| [2] | Wu Taoguang, Nie Shaobo, Chen Hua, Zhu Zhengguo, Qi Lin, Tang Peifu. Biomechanical characteristics of a new multi-dimensional cross locking plate in the treatment of subtrochanteric nonunion [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(9): 1330-1334. |
| [3] | Zheng Bo, Zhang Xiuli, Zhou Hao, He Zebi, Zhou Jin, Zhou Weiyun, Li Peng. Arthroscopy-assisted locking hollow screw fixation and open reduction plate internal fixation in the treatment of Schatzker II-III tibial plateau fractures: early CT evaluation [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(9): 1410-1416. |
| [4] | Yu Jiaan, Liu Xinwei, Lian Hongyu, Liu Kexin, Li Zitao. Medial open-wedge tibial osteotomy versus lateral closed-wedge tibial osteotomy for unicompartmental knee osteoarthritis: a meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(4): 632-639. |
| [5] | Xu Xinzhong, Wu Zhonghan, Yu Shuisheng, Zhao Yao, Xu Chungui, Zhang Xin, Zheng Meige, Jing Juehua. Biomechanical analysis of different ways of inserting Steinmann Pins into the femoral head [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(9): 1313-1317. |
| [6] | Wei Guoqiang, Li Yunfeng, Wang Yi, Niu Xiaofen, Che Lifang, Wang Haiyan, Li Zhijun, Shi Guopeng, Bai Ling, Mo Kai, Zhang Chenchen, Xu Yangyang, Li Xiaohe. Biomechanical analysis of non-uniform material femur under different loads [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(9): 1318-1322. |
| [7] | Xu Kuishuai, Zhang Liang, Chen Jinli, Ren Zhongkai, Zhao Xia, Li Tianyu, Yu Tengbo. Effect of force line changes on lower limb joints after medial open wedge high tibial osteotomy [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(6): 821-826. |
| [8] | Li Jie, Zhang Haitao, Chen Jinlun, Ye Pengcheng, Zhang Hua, Zhou Bengen, Zhao Changqing, Sun Youqiang, Chen Jianfa, Xiang Xiaobing, Zeng Yirong. Anterior cruciate ligament rupture and patellofemoral joint stability before sagittal and axial measurement using MRI [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(6): 969-972. |
| [9] | Liu Dongcheng, Zhao Jijun, Zhou Zihong, Wu Zhaofeng, Yu Yinghao, Chen Yuhao, Feng Dehong. Comparison of different reference methods for force line correction in open wedge high tibial osteotomy [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(6): 827-831. |
| [10] | Li Shuo, Su Peng, Zhang Li, Wu Qiulong, Hu Xiangyu, Lai Yuliang. Positive effect of supracondylar femoral osteotomy on the correction of knee varus based on three-dimensional reconstruction and finite element analysis [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(6): 858-863. |
| [11] | Feng Jianbo, Li Chencheng, Liu Jinyue, Wang Xiaomin, Peng Jiachen. Implantation of Kirschner wire with Staphylococcus aureus biofilm establishes a traumatic osteomyelitis model in rats [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(5): 700-705. |
| [12] | Zhang Qiang, Wu Zongde, Liu Liang, Wei Guohua, Peng Liang. Finite element analysis of medial and lateral locking plates for fixation of externally rotated spiral fractures of the lower tibia [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(36): 5750-5754. |
| [13] | Li Weixiang, Xu Bin, Jiang Shaowei. A new evaluation index for patellofemoral instability: tibial nodule torsion angle [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(36): 5781-5786. |
| [14] | He Guowen, Hu Baijun, Gao Dawei, Chen Liang. Treatment of Schatzker type V and VI tibial plateau fractures with 3D printing preoperative planning combined with double reverse traction device [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(36): 5764-5769. |
| [15] | Pan Jianke, Zhao Di, Jin Xiao, Yang Weiyi, Luo Minghui, Liu Jun, Han Yanhong, Cao Houran. Implant-related errors and complications in medial open-wedge high tibial osteotomy [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(36): 5849-5856. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||