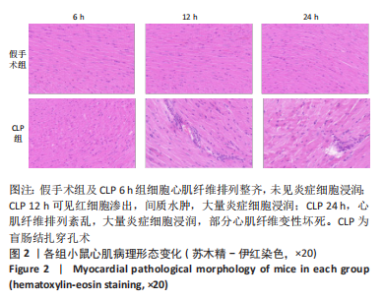
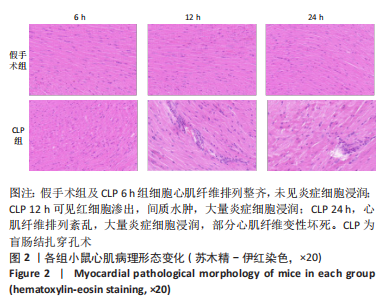
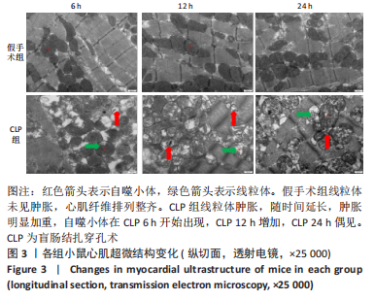
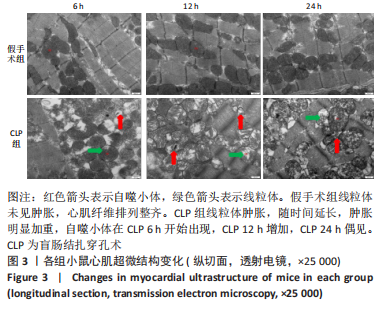
Chinese Journal of Tissue Engineering Research ›› 2023, Vol. 27 ›› Issue (23): 3667-3673.doi: 10.12307/2023.472
Previous Articles Next Articles
The role of NLRC4 inflammasome-mediated autophagy in a mouse model of sepsis-induced myocardial dysfunction
Jiang Dajun, Jiang Wanwei, Zhou Ying, Tian Yong, Yang Guohui
- Affiliated Hospital, Guizhou Medical University, Guiyang 550004, Guizhou Province, China
-
Received:2022-06-16Accepted:2022-07-21Online:2023-08-18Published:2023-01-16 -
Contact:Yang Guohui, Professor, Chief physician, Affiliated Hospital, Guizhou Medical University, Guiyang 550004, Guizhou Province, China -
About author:Jiang Dajun, Master candidate, School of Clinical Medicine, Guizhou Medical University, Guiyang 550004, Guizhou Province, China -
Supported by:the National Natural Culture Foundation of China for the Affiliated Hospital of Guizhou Medical University, No. gyfynsfc-2021-54 (to YGH); Guiyang Science and Technology Planning Project, No. [2019]9-1-9 (to YGH)
CLC Number:
Cite this article
Jiang Dajun, Jiang Wanwei, Zhou Ying, Tian Yong, Yang Guohui. The role of NLRC4 inflammasome-mediated autophagy in a mouse model of sepsis-induced myocardial dysfunction[J]. Chinese Journal of Tissue Engineering Research, 2023, 27(23): 3667-3673.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
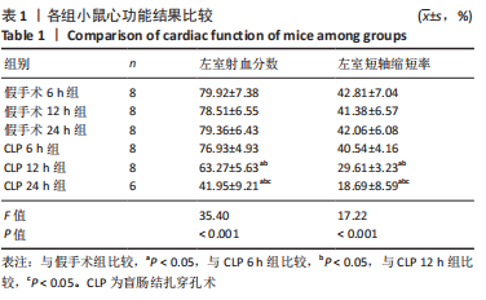
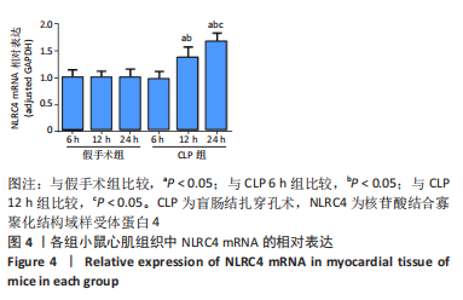
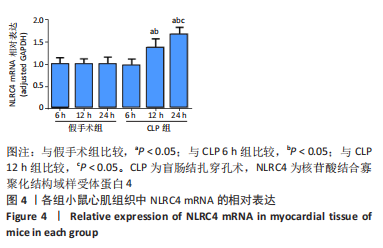
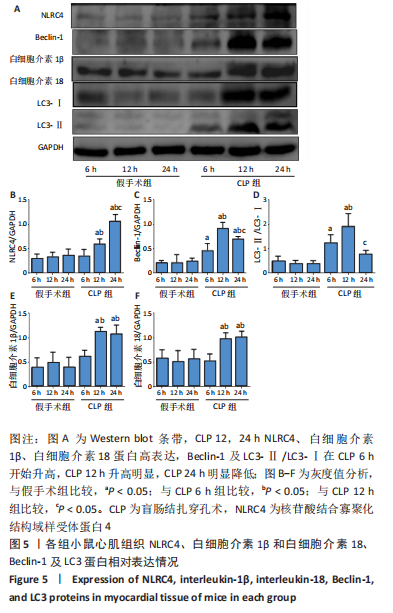
2.1 实验动物数量及一般情况分析 假手术组24只全存活,反应灵敏,正常进食。CLP 6 h组8只小鼠全存活,精神稍萎靡,活动减少,呼吸稍快;CLP 12 h组8只小鼠全存活,精神萎靡,活动明显减少,对刺激反应减弱,不进食,腹泻;CLP 24 h组死亡2只,其中1只在做B超时死亡,嗜睡、皮温冰凉、呼吸短促、寒战、毛发竖立、腹泻,运动迟缓、对刺激反应性迟钝。 2.2 小鼠B超心功能比较 CLP组与假手术组左室射血分数、左室短轴缩短率差异有显著性意义(P < 0.05);其中与假手术组比较,CLP 6 h组左室射血分数、左室短轴缩短率无显著差异(P > 0.05);与假手术组及CLP 6 h组比较,CLP 12 h组、CLP 24 h组小鼠左室射血分数、左室短轴缩短率均显著降低(P < 0.05);与CLP 12 h组比较,CLP 24 h组左室射血分数、左室短轴缩短率均显著降低(P < 0.05),见表1。"
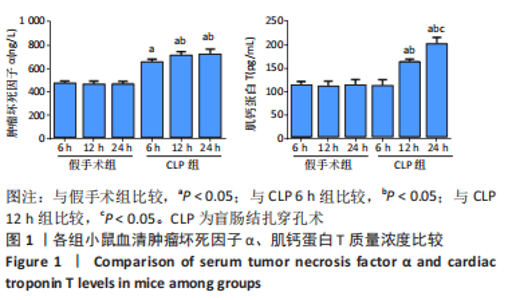
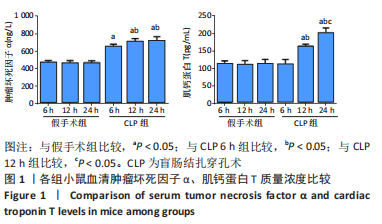
2.3 各组小鼠血清TNF-α、肌钙蛋白T 质量浓度比较 与假手术组比,CLP 6 h组血清TNF-α质量浓度显著升高(P < 0.05);与CLP 6 h组比较,CLP 12,24 h组小鼠血清TNF-α质量浓度均显著升高(P < 0.05);与CLP 12 h比较,CLP 24 h小鼠血清TNF-α质量浓度无明显变化(P > 0.05);与假手术组比较,CLP组血清肌钙蛋白T质量浓度升高,差异有显著性意义(P < 0.05),其中CLP 6 h组与假手术组无显著差异;与CLP 6 h组比较,CLP 12,24 h组血清肌钙蛋白T质量浓度显著升高(P < 0.05);与CLP 12 h比较,CLP 24 h血清肌钙蛋白T水平升高,差异有显著性意义(P < 0.05),见图1。"
| [1] SINGER M, DEUTSCHMAN CS, SEYMOUR CW, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016;315(8):801. [2] 严静, 沈延飞. 脓毒症的诊断及治疗进展[J]. 浙江医学,2021,43(14): 1479-1482,1488. [3] MORGAN RW, FITZGERALD JC, WEISS SL, et al. Sepsis-associated in-hospital cardiac arrest: Epidemiology, pathophysiology, and potential therapies. J Crit Care. 2017;40:128-135. [4] LIN H, WANG W, LEE M, et al. Current Status of Septic Cardiomyopathy: Basic Science and Clinical Progress. Front Pharmacol. 2020;11:210. [5] MARTIN L, DERWALL M, AL ZS, et al. The Septic Heart: Current Understanding of Molecular Mechanisms and Clinical Implications. Chest. 2019;155(2):427-437. [6] HOLLENBERG SM, SINGER M. Pathophysiology of sepsis-induced cardiomyopathy. Nat Rev Cardiol. 2021;18(6):424-434. [7] YAO RQ, REN C, ZHENG LY, et al. Advances in Immune Monitoring Approaches for Sepsis-Induced Immunosuppression. Front Immunol. 2022;13:891024. [8] BERGMANN CB, BECKMANN N, SALYER CE, et al. Lymphocyte Immunosuppression and Dysfunction Contributing to Persistent Inflammation, Immunosuppression, and Catabolism Syndrome (PICS). Shock. 2021;55(6):723-741. [9] MIRA JC, GENTILE LF, MATHIAS BJ, et al. Sepsis Pathophysiology, Chronic Critical Illness, and Persistent Inflammation-Immunosuppression and Catabolism Syndrome. Crit Care Med. 2017;45(2):253-262. [10] 杨贵芳,彭文,赵琴,等.心力衰竭免疫学机制及治疗的研究进展[J]. 中国循环杂志,2015,30(2):193-195. [11] PIQUEREAU J, GODIN R, DESCHENES S, et al. Protective role of PARK2/Parkin in sepsis-induced cardiac contractile and mitochondrial dysfunction. Autophagy. 2013;9(11):1837-1851. [12] WASYLUK W, NOWICKA-STAZKA P, ZWOLAK A. Heart Metabolism in Sepsis-Induced Cardiomyopathy-Unusual Metabolic Dysfunction of the Heart. Int J Environ Res Public Health. 2021;18(14):7598. [13] WEN X, XIE B, YUAN S, et al. The “Self-Sacrifice” of ImmuneCells in Sepsis. Front Immunol. 2022;13:833479. [14] BARDET J, LAVERDURE N, FUSARO M, et al. NLRC4 GOF Mutations, a Challenging Diagnosis from Neonatal Age to Adulthood. J Clin Med. 2021;10(19):4369. [15] ZHANG X, CUI Y, DING X, et al. Analysis of mRNAlncRNA and mRNAlncRNA-pathway coexpression networks based on WGCNA in developing pediatric sepsis. Bioengineered. 2021;12(1):1457-1470. [16] RITTIRSCH D, HUBER-LANG MS, FLIERL MA, et al. Immunodesign of experimental sepsis by cecal ligation and puncture. Nat Protoc. 2009;4(1):31-36. [17] ANTONUCCI E, FIACCADORI E, DONADELLO K, et al. Myocardial depression in sepsis: from pathogenesis to clinical manifestations and treatment. J Crit Care. 2014;29(4):500-511. [18] L’HEUREUX M, STERNBERG M, BRATH L, et al. Sepsis-Induced Cardiomyopathy: a Comprehensive Review. Curr Cardiol Rep. 2020; 22(5):35. [19] BELTRAN-GARCIA J, OSCA-VERDEGAL R, ROMA-MATEO C, et al. Epigenetic biomarkers for human sepsis and septic shock: insights from immunosuppression. Epigenomics. 2020;12(7):617-646. [20] HALADE GV, LEE DH. Inflammation and resolution signaling in cardiac repair and heart failure. EBio Medicine. 2022;79:103992. [21] LEE H, LIM JM, LEE J, et al. Positive Role of Delta Neutrophil Index (DNI) as a Prodiagnostic Marker in Cecal Ligation and Puncture (CLP)-Induced Sepsis Murine Model. Medicina (Kaunas). 2022;58(3):369. [22] SUNDARAM B, KANNEGANTI TD. Advances in Understanding Activation and Function of the NLRC4 Inflammasome. Int J Mol Sci. 2021;22(3):1048. [23] KANNEGANTI TD. Intracellular innate immune receptors: Life inside the cell. Immunol Rev. 2020;297(1):5-12. [24] DINARELLO CA, SIMON A, VAN DER MEER JW. Treating inflammation by blocking interleukin-1 in a broad spectrum of diseases. Nat Rev Drug Discov. 2012;11(8):633-652. [25] AINSCOUGH JS, GERBERICK GF, KIMBER I, et al. Interleukin-1beta Processing Is Dependent on a Calcium-mediated Interaction with Calmodulin. J Biol Chem. 2015;290(52):31151-31161. [26] VECCHIE A, BONAVENTURA A, TOLDO S, et al. IL-18 and infections: Is there a role for targeted therapies? J Cell Physiol. 2021;236(3):1638-1657. [27] OKUHARA Y, YOKOE S, IWASAKU T, et al. Interleukin-18 gene deletion protects against sepsis-induced cardiac dysfunction by inhibiting PP2A activity. Int J Cardiol. 2017;243:396-403. [28] LIU G, LIU Y, TAO J, et al. [Differential gene expression and bioinformatics analysis in sepsis secondary to pneumonia]. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2022;34(2):138-144. [29] WANG SS, YAN CS, LUO JM. NLRC4 gene silencing-dependent blockade of NOD-like receptor pathway inhibits inflammation, reduces proliferation and increases apoptosis of dendritic cells in mice with septic shock. Aging (Albany NY). 2021;13(1):1440-1457. [30] VANDEN BT, DEMON D, BOGAERT P, et al. Simultaneous targeting of IL-1 and IL-18 is required for protection against inflammatory and septic shock. Am J Respir Crit Care Med. 2014;189(3):282-291. [31] SMEDING L, PLOTZ FB, GROENEVELD AB, et al. Structural changes of the heart during severe sepsis or septic shock. Shock. 2012;37(5):449-456. [32] CHEN P, AN Q, HUANG Y, et al. Prevention of endotoxin-induced cardiomyopathy using sodium tanshinone IIA sulfonate: Involvement of augmented autophagy and NLRP3 inflammasome suppression. Eur J Pharmacol. 2021;909:174438. [33] HUANG Z, ZHANG H, FU X, et al. Autophagy-driven neutrophil extracellular traps: The dawn of sepsis. Pathol Res Pract. 2022;234:153896. [34] HSIAO HW, TSAI KL, WANG LF, et al. The decline of autophagy contributes to proximal tubular dysfunction during sepsis. Shock. 2012; 37(3):289-296. [35] HOTCHKISS RS, TINSLEY KW, SWANSON PE, et al. Depletion of dendritic cells, but not macrophages, in patients with sepsis. J Immunol. 2002; 168(5):2493-2550. [36] SCHMID D, PYPAERT M, MUNZ C. Antigen-loading compartments for major histocompatibility complex class II molecules continuously receive input from autophagosomes. Immunity. 2007;26(1):79-92. [37] HOHLSTEIN P, GUSSEN H, BARTNECK M, et al. Prognostic Relevance of Altered Lymphocyte Subpopulations in Critical Illness and Sepsis. J Clin Med. 2019;8(3):353. [38] WATANABE E, MUENZER JT, HAWKINS WG, et al. Sepsis induces extensive autophagic vacuolization in hepatocytes: a clinical and laboratory-based study. Lab Invest. 2009;89(5):549-561. [39] JABIR MS, RITCHIE ND, LI D, et al. Caspase-1 cleavage of the TLR adaptor TRIF inhibits autophagy and beta-interferon production during Pseudomonas aeruginosa infection. Cell Host Microbe. 2014;15(2): 214-227. [40] PU Q, GAN C, LI R, et al. Atg7 Deficiency Intensifies Inflammasome Activation and Pyroptosis in Pseudomonas Sepsis. J Immunol. 2017; 198(8):3205-3213. |
| [1] | Nie Chenchen, Su Kaiqi, Gao Jing, Fan Yongfu, Ruan Xiaodi, Yuan Jie, Duan Zhaoyuan, Feng Xiaodong. The regulatory role of circular RNAs in cerebral ischemia-reperfusion injury [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(8): 1286-1291. |
| [2] | Zhang Qijian, Xu Ximing. Acquisition and application of ectodermal mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(6): 928-934. |
| [3] | Wu Yujie, Wan Xiaofang, Wei Mianxing, Peng Shiyuan, Xu Xiaomei. Correlation between autophagy and the Hippo-YAP protein pathway in periodental ligament cells on the pressure side of a mouse model of orthodontic tooth movement [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(5): 683-689. |
| [4] | Li Long, Li Guangdi, Shi Hao, Deng Keqi. Circular RNA as a competing endogenous RNA is involved in the regulation of osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(5): 751-757. |
| [5] | Li Zhichao, Tan Guoqing, Su Hui, Xu Zhanwang, Xue Haipeng. Regulatory role of non-coding RNAs as potential therapeutic targets in spinal cord injury [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(5): 758-764. |
| [6] | Chen Chao, Wang Xuenan, Zhan Enyu, Lyu Zhengpin, Zhang Fan. Mechanism underlying the mutual regulation between circadian rhythm and autophagy [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(29): 4696-4703. |
| [7] | Chen Cai, Zeng Ping, Liu Jinfu. Long non-coding RNAs regulate osteoarthritis by mediating chondrocyte-related mechanisms [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(29): 4729-4735. |
| [8] | Liu Jihuan, Wang Peng, Yuan Shunling, Jian Ye, Huang Shaoze, Yao Sisi, Liu Wenfeng. Mechanism by which aerobic endurance exercise regulates cardiomyocyte autophagy in rats [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(23): 3714-3720. |
| [9] | Yang Huixia, Jie Yuzhen, Bai Zhigang, Jiao Yun, Yang Yong, Ma Tianlong, Ma Shengchao, Jiang Yideng. Role of LncRNA MALAT1 in myocardial autophagy reduction in aging rats after ischemic postconditioning [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(20): 3173-3179. |
| [10] | Wang Huanhuan, Wang Qing, Tang Peng, Zhang Rui, Min Hongwei. Effects of extracorporeal shock wave on the proliferation and autophagy of chondrocytes from osteoarthritis rats [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(2): 252-257. |
| [11] | Zhao Yan, Xia Qiuqiu, Xiang Shaojie, Mao Qiming, Kong Weijun, Du Qian, Liao Wenbo, Xin Zhijun. Exosomal miRNA in the repair mechanism of degenerative diseases of the spine and joints [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(19): 3040-3051. |
| [12] | Wang Yaxin, Lin Yican, Guo Yinuo, Zhang Bo, Ma Yuying, He Xiaoping. Methods, technologies and future of Chinese medicine modulation of cellular autophagy in the treatment of spinal cord injury [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(13): 2116-2123. |
| [13] | Zhang Min, Bai Shulin, Li Shenghong, Fan Zhibo, Xie Yijia, Xu Xiaomei. Effects of high mobility group box 1 and ERK1/2 pathway on autophagy of human periodontal ligament cells under tensile stress [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(10): 1560-1566. |
| [14] | Cui Xing, Sun Xiaoqi, Zheng Wei, Ma Dexin. Huangqin Decoction regulates autophagy to intervene with intestinal acute graft-versus-host disease in mice [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1057-1062. |
| [15] | Fan Jilin, Zhu Tingting, Tian Xiaoling, Liu Sijia, Su Jing, Zhang Shiliang. Effect and mechanism of non-coding RNA regulating autophagy in myocardial ischemia-reperfusion injury [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(35): 5716-5723. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||