Chinese Journal of Tissue Engineering Research ›› 2022, Vol. 26 ›› Issue (24): 3921-3927.doi: 10.12307/2022.577
Previous Articles Next Articles
Human umbilical cord mesenchymal stem cells in endometrial injury repair
Liu Xingyu, Hu Xiaofang, Xu Guangli, Wang Rui, Li Peiyao, Wang Mengyuan, Peng Li, Zhu Xiangying
- Department of Reproductive Medicine, First Affiliated Hospital of Henan University of Chinese Medicine, Zhengzhou 450000, Henan Province, China
-
Received:2021-06-30Accepted:2021-07-02Online:2022-08-28Published:2022-01-24 -
Contact:Xu Guangli, MD, Associate chief physician, Department of Reproductive Medicine, First Affiliated Hospital of Henan University of Chinese Medicine, Zhengzhou 450000, Henan Province, China Hu Xiaofang, Professor, Master’s supervisor, Chief physician, Department of Reproductive Medicine, First Affiliated Hospital of Henan University of Chinese Medicine, Zhengzhou 450000, Henan Province, China -
About author:Liu Xingyu, Master candidate, Department of Reproductive Medicine, First Affiliated Hospital of Henan University of Chinese Medicine, Zhengzhou 450000, Henan Province, China -
Supported by:Medical Science and Technology Research Project of Henan Province, No. LHGJ20170001 (to XGL)
CLC Number:
Cite this article
Liu Xingyu, Hu Xiaofang, Xu Guangli, Wang Rui, Li Peiyao, Wang Mengyuan, Peng Li, Zhu Xiangying. Human umbilical cord mesenchymal stem cells in endometrial injury repair[J]. Chinese Journal of Tissue Engineering Research, 2022, 26(24): 3921-3927.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
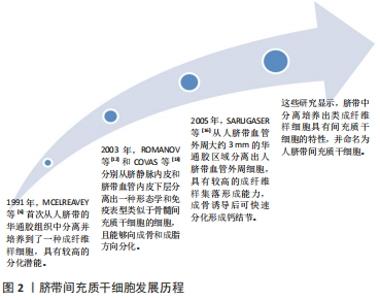
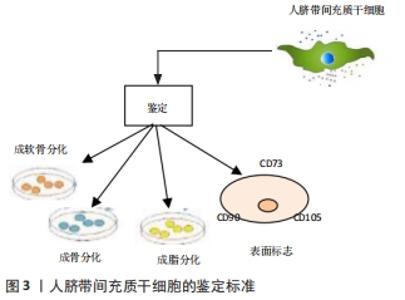
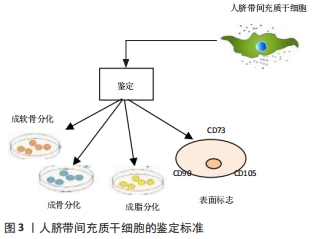
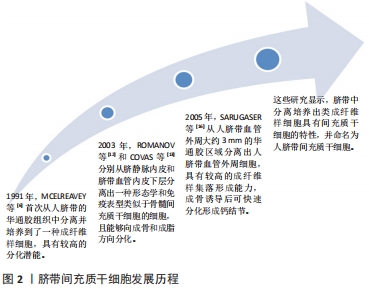
2.1.1 人脐带间充质干细胞的发现及应用价值 脐带为胎儿与胎盘连接的纽带,外层为羊膜,内有2条管腔较小、管壁较厚的脐动脉和1条管腔较大、管壁较薄的脐静脉,脐血管周围有起保护作用的胚胎结缔组织称为华通胶。1991年,MCELREAVEY等[6]首次从人脐带的华通胶组织中分离并培养到了一种成纤维样细胞,具有较高的分化潜能。2003年,ROMANOV等[12]和COVAS等[13]分别从脐静脉内皮和脐带血管内皮下层分离出一种形态学和免疫表型类似于骨髓间充质干细胞的细胞,且能够向成骨和成脂方向分化。随后,多位研究者从脐带华通胶组织中分离培养出类成纤维样细胞[14-15]。2005年,SARUGASER等[16]从人脐带血管外周大约3 mm的华通胶区域分离出人脐带血管外周细胞,具有较高的成纤维样集落形成能力,成骨诱导后可快速分化形成钙结节。上述这些研究显示,脐带中分离培养出类成纤维样细胞具有间充质干细胞的特性,并命名为人脐带间充质干细胞,脐带间充质干细胞发展历程,见图2。"

2.1.2 人脐带间充质干细胞的多向分化潜能 人脐带间充质干细胞的多向分化潜能使得其具有促进组织修复、调节免疫反应的特性,间充质干细胞具有黏附、克隆和表达细胞表面独特表型的能力,具有高度的可扩展性,能够向不同的细胞系进行分化,作为一种原始干细胞,可以通过定向诱导分化形成神经元样细胞、胰岛样细胞、上皮细胞、血管内皮细胞等具有特定功能的成体细胞,参与受损组织的修复,从而恢复机体的正常功能,达到治疗疾病的效果[22-25],因此间充质干细胞被认为是某些临床疾病的潜在治疗方案,迅速成为干细胞再生医学领域的一个很有前途的研究对象。CHEN等[26]诱导人脐带间充质干细胞分化为神经干细胞,诱导后细胞表现为神经元细胞分化,神经元特异性烯醇化酶和神经源性分化蛋白1阳性细胞分别达到87.3%和72.6%(在评估神经分化时,因为缺少神经特异性细胞内标记,因此常依赖于在神经谱系细胞的发育和生理过程中起重要作用的细胞表面标记)。LI等[27]对人脐带间充质干细胞进行诱导分化培养实验,在4代人脐带间充质干细胞中加入含有B27、碱性成纤维细胞生长因子和神经生长因子的无血清诱导分化培养基,成功在体外分化生成神经元样细胞。MAJIDI等[28]将人脐带间充质干细胞与小鼠睾丸细胞进行共培养从而定向诱导其分化为生殖样细胞,揭示了特定的组织微环境对于人脐带间充质干细胞定向诱导分化的潜能。金诚[29]将人脐带间充质干细胞以睾丸支持细胞作为共培养细胞,加入视黄酸可以诱导分化为视网膜色素上皮细胞,方法简便可行。SHI等[30]研究表明1 μmol/L的17β-雌二醇是促进人脐带华通胶间充质干细胞向子宫内膜上皮细胞样细胞分化的良好诱导剂,而8-Br-cAMP联合雌激素和生长因子可诱导人脐带华通胶间充质干细胞向子宫内膜间质细胞样细胞分化,这些发现为治疗子宫内膜损伤和其他子宫内膜疾病提供了一种有前途的方法。研究还发现人脐带间充质干细胞可分化为心肌细胞、胰岛样细胞,对治疗心肌梗死和糖尿病等多发性疾病具有重大的临床意义[31-32],见表1。"

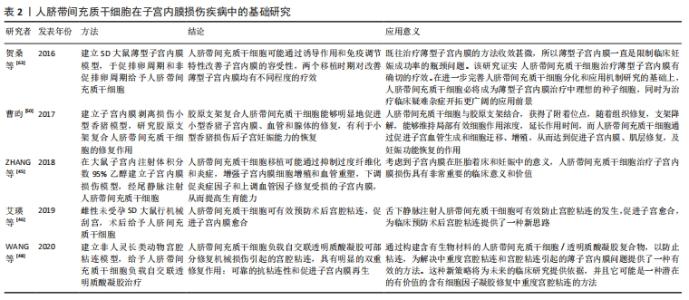
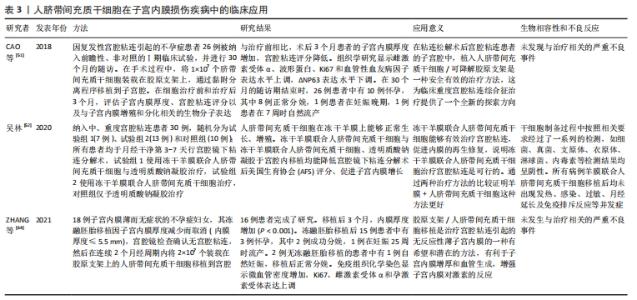
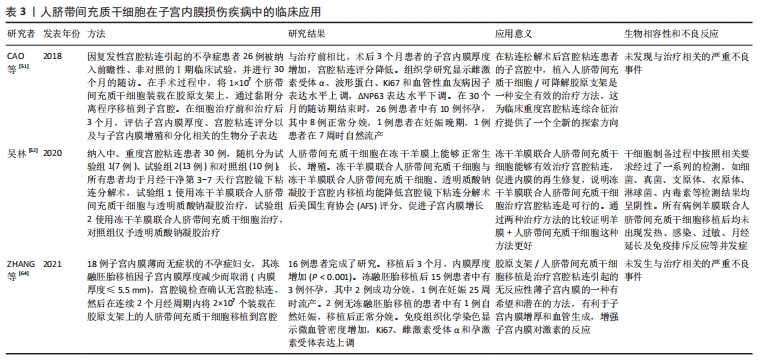
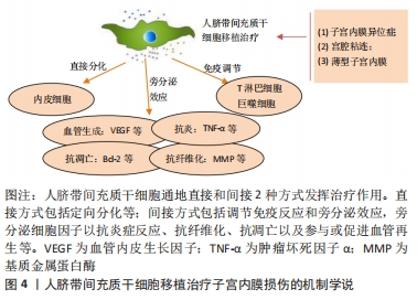
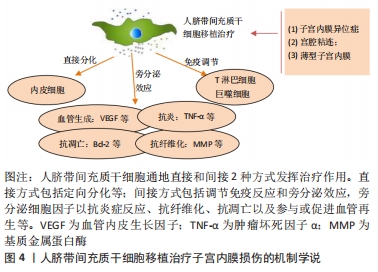
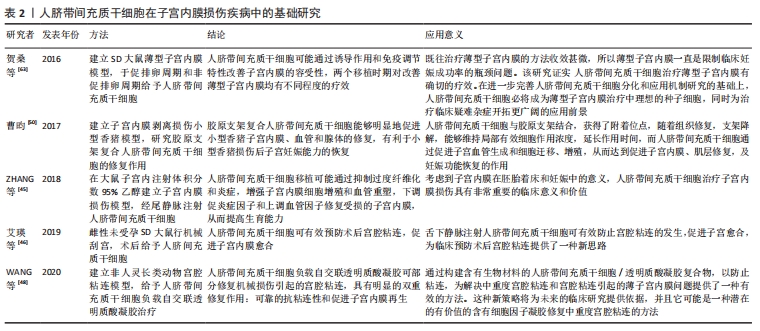
2.1.3 人脐带间充质干细胞的免疫调节能力 除了较强的自我更新和分化能力外,人脐带间充质干细胞还具有较强的免疫调节能力,在对细胞治疗的研究中,通过将年轻小鼠的血液移植到老年小鼠,发现血液中的年轻干细胞可以帮助老年小鼠恢复肌肉和神经元的功能[33]。许多研究表明间充质干细胞具有免疫调节作用,从细胞中分泌许多生物分子和介质,如细胞因子,在免疫过程中起着重要作用[34],此外,间充质干细胞还抑制促炎细胞因子的分泌,增加受损细胞的存活,可以调节树突状细胞、巨噬细胞和单核细胞的细胞因子分泌,分泌的细胞因子可以诱导巨噬细胞向抗炎细胞的转变,由于缺乏细胞表面HLA-DR和共同刺激分子,如CD40,CD80和CD86,间充质干细胞具有较低的免疫原性,因此,间充质干细胞可以影响细胞的免疫功能,发挥免疫抑制作用,这些特性可以赋予发挥间充质干细胞移植治疗疾病的优势[35]。 2.1.4 人脐带间充质干细胞的旁分泌功能 近年来,外泌体被认为是一种新的旁分泌因子,由包括间充质干细胞在内的各种细胞通过出芽方式释放,对各种细胞功能具有重要意义。外泌体是一种膜囊泡,直径40-150 nm,被磷脂双层包围[36]。它们是细胞间通信的重要介质,保护其携带的生物活性物质免受高温、各种pH值环境、反复冻融和其他不利条件的影响。近年来,越来越多研究观察到间充质干细胞可以通过外泌体的细胞外囊泡,进行细胞间信号传递并发挥生物学功能,外泌体可通过一定的机制调节免疫,其有望代替间充质干细胞成为临床上治疗免疫相关疾病的新工具,并且通过体外的外泌体功能测定筛选合适的外泌体进行治疗[37-38]。 研究发现,同一浓度不同间充质干细胞来源外泌体对脐血单个核细胞的增殖抑制能力不同,其中人脐带间充质干细胞来源外泌体抑制脐血单个核细胞的增殖能力最强,分泌细胞因子的不同可能是作用差异的重要原因[39]。郭礼妍等[40]研究人脐带间充质干细胞来源外泌体对Treg和Th17细胞的调节作用,发现外泌体能影响外周血单个核细胞的T细胞亚群分布,且具有增加Treg细胞和降低Th17细胞的作用,提示人脐带间充质干细胞来源外泌体具有免疫调节功能。 2.2 人脐带间充质干细胞在子宫内膜损伤疾病中的应用 2.2.1 子宫内膜异位症 是一种慢性妇科疾病,其特征是子宫内膜组织生长在子宫腔以外的部位。异位子宫内膜组织见于卵巢、子宫骶骨韧带、阔韧带以及盆腔腹膜。子宫内膜异位症的患病率为6%-10%,是一种多因素疾病[41-42]。近年来越来越多的研究证明,凋亡通过清除异位子宫内膜细胞并维持月经周期中细胞内环境的稳定中起着重要作用[43]。脐带间充质干细胞可以通过旁分泌作用分泌一些细胞因子,包括抗凋亡分子、免疫调节分子、血管生成分子、趋化作用分子等,进而调控细胞的生物学活性,参与组织修复等过程。通过研究发现,PTEN基因表达的改变与多种子宫内膜相关病变之间存在一定的关系,并认为是子宫内膜组织中的“管家基因”。王春梅等[44]研究结果表明人脐带间充质干细胞可以通过上调PTEN基因表达,抑制异位子宫内膜细胞增殖,并促进其凋亡。在动物模型中,ZHANG等[45]在大鼠子宫内注射体积分数为95%乙醇建立子宫内膜损伤模型,经尾静脉注射人脐带间充质干细胞,分为1,2,3次给药,结果显示移植后第8天子宫内膜形态和胚胎着床率明显改善。此外,人脐带间充质干细胞可以减轻纤维化,降低纤维化标志物的表达,子宫内膜中细胞增殖标志物Ki-67有阳性表达,子宫内膜间质标志物波形蛋白和上皮标志物细胞角蛋白19表达明显增加,血管标志物CD31、血管内皮生长因子A和基质金属蛋白9的表达上调;第2次和第3次给予人脐带间充质干细胞后,促炎因子肿瘤坏死因子α及白细胞介素2水平均显著下调。结果表明,人脐带间充质干细胞移植可能通过抑制过度纤维化和炎症,增强子宫内膜细胞增殖和血管重塑,下调促炎症因子和上调血管因子修复受损的子宫内膜,从而提高生育能力。 2.2.2 Asherman综合征 宫腔粘连也被称为Asherman综合征,由Asherman于1948年首次报道,是由于刮宫时损伤宫颈管黏膜或子宫内膜基底层、肌层,局部创面形成而致粘连。子宫内膜层损伤常伴有瘢痕形成,导致子宫腔部分或完全阻塞,以致不孕或流产。虽然许多方法已被用来治疗严重宫腔粘连,但是因复发率较高和子宫内膜变薄,使其治疗效果有限。艾瑛等[46]研究表明,脐带间充质干细胞可有效预防术后宫腔粘连,促进子宫内膜愈合。江春燕[47]研究表明中药左归丸、脐带间充质干细胞对受损子宫内膜均有一定程度的修复作用,可以增加子宫内膜厚度、腺体个数及抑制子宫内膜纤维化的趋势,其作用机制与激活信号通路PI3K/AKT有关,经宫腔原位注射的脐带间充质干细胞在经过治疗28 d后仍有部分存在于子宫内膜中。WANG等[48]研究表明人脐带间充质干细胞负载自交联透明质酸凝胶可部分修复机械损伤引起的宫腔粘连,具有明显的双重修复作用:可靠的抗粘连性和促进子宫内膜再生。LIU等[49]研究表明,与单纯胶原支架和单纯人脐带间充质干细胞相比,胶原支架联合人脐带间充质干细胞可显著增加子宫内膜腺体数量,减少纤维化面积,可能是治疗宫腔粘连的一种新方法。曹昀[50]采用子宫内膜剥离损伤的小型香猪模型,研究胶原支架复合人脐带间充质干细胞的修复作用,结果显示,胶原支架复合人脐带间充质干细胞能够明显促进小型香猪子宫内膜、血管和腺体的修复,有利于小型香猪损伤后子宫妊娠能力的恢复。研究过程中获得“中国第一株临床使用级脐带间充质干细胞”,进行了临床小样本研究注册方案(NCT02313415)并初步探究了Asherman综合征的分子病理特征[51],经胶原支架/人脐带间充质干细胞治疗后,宫腔粘连减少,宫腔粘连评分降低,子宫内膜厚度显著增加,月经量较治疗之前增加,月经期延长;子宫内膜活检组织的免疫组织化学检查结果显示,治疗后受试者子宫内膜组织雌激素受体α,波形蛋白,Ki67表达量增加,血管性血友病因子密度升高,结果表明,胶原支架/人脐带间充质干细胞能够明显促进重度宫腔粘连综合征患者子宫内膜再生性修复,促进血管再生,恢复内膜功能。吴林[52]研究发现冻干羊膜联合人脐带间充质干细胞治疗宫腔粘连也是可行的,这一全新的治疗方式为重度官腔粘连综合征的临床治疗带来了新的治疗选择。 2.2.3 薄型子宫内膜 子宫内膜厚度可用于预测子宫内膜容受性[53]。子宫内膜厚度临床定义为子宫肌层与子宫内膜之间的距离,可通过超声测量[54]。正常情况下,在不同的时期子宫内膜的厚度也是不一样的,月经期子宫内膜厚度1-4 mm,增生中期 4-8 mm,卵泡晚期8-14 mm,分泌期7-14 mm[55]。子宫上皮和间质细胞增殖缓慢可导致子宫内膜变薄[56]。据报道,Asherman综合征、既往宫内手术、盆腔放疗等多种病理状况是子宫内膜变薄的原因[57]。理论上,薄的子宫内膜可以通过子宫内膜间质和上皮细胞的再生得到改善,从而改善着床[58]。一些报道表明,厚度超过17 mm的子宫内膜与提高妊娠率有关[59]。由薄子宫内膜引起的不孕症,目前尚没有较好的治疗方法。目前,干细胞作为再生医学的一种新的治疗方法,尤其是对于人类子宫内膜疾病如薄子宫内膜的再生有着广泛的兴趣[60]。已有证据表明,在子宫内膜区移植不同来源干细胞对子宫内膜有影响,如减少纤维化面积,增加腺体数量,刺激血管生成,增加子宫内膜厚度,形成更好的组织结构,保护妊娠,提高怀孕率[61-62]。国内贺桑等[63]研究表明人脐带间充质干细胞可能通过诱导作用和免疫调节特性改善子宫内膜的容受性,两个移植时期对改善薄型子宫内膜均有不同程度的疗效。一项临床研究注册方案(NCT03724617)研究表明胶原支架/人脐带间充质干细胞是治疗Asherman 综合征引起的无反应性薄子宫内膜的一种很有前途和潜力的方法[64]。 近年来,人脐带间充质干细胞在子宫内膜损伤疾病中的基础和临床研究已经取得了很多成果,具体见表2,3和图4。"
| [1] 勾亚婷,张文文,李长江,等.NF-κB信号通路在人羊膜间充质干细胞治疗宫腔粘连中的作用[J].第三军医大学学报,2020,42(11):1101-1108. [2] SONG A, WANG J, TONG Y, et al. BKCa channels regulate the immunomodulatory properties of WJ-MSCs by affecting the exosome protein profiles during the inflammatory response. Stem Cell Res Ther. 2020;11(1):440. [3] 汪子涵,刘义,鞠明艳,等.间充质干细胞治疗成骨不全:是直接分化为功能细胞还是旁分泌作用[J].中国组织工程研究,2018,22(33):5373-5378. [4] BABENKO VA, SILACHEV DN, DANILINA TI, et al. Age-Related Changes in Bone-Marrow Mesenchymal Stem Cells. Cells. 2021;10(6):1273. [5] ZHAN XS, EL-ASHRAM S, LUO DZ, et al. A comparative study of biological characteristics and transcriptome profiles of mesenchymal stem cells from different canine tissues. Int J Mol Sci. 2019;20(6):1485. [6] MCELREAVEY KD, IRVINE AI, ENNIS KT, et al. Isolation, culture and characterisation of fibroblast-like cells derived from the Wharton’s jelly portion of human umbilical cord. Biochem Soc Trans. 1991;19(1):29S. [7] BAI X, LIU J, CAO S, et al. Mechanisms of endometrial fibrosis and the potential application of stem cell therapy. Discov Med. 2019;27(150):267-279. [8] FAN D, WU S, YE S, et al. Umbilical cord mesenchyme stem cell local intramuscular injection for treatment of uterine niche: protocol for a prospective, randomized, double-blinded, placebo-controlled clinical trial. Medicine (Baltimore). 2017;96(44):e8480. [9] XIA L, MENG Q, XI J, et al. The synergistic effect of electroacupuncture and bone mesenchymal stem cell transplantation on repairing thin endometrial injury in rats. Stem Cell Res Ther. 2019;10(1):244. [10] SHAO X, AI G, WANG L, et al. Adipose-derived stem cells transplantation improves endometrial injury repair. Zygote. 2019;27(6):367-374. [11] SINGH N, SHEKHAR B, MOHANTY S, et al. Autologous bone marrow-derived stem cell therapy for asherman’s syndrome and endometrial atrophy: a 5-year follow-up study. J Hum Reprod Sci. 2020;13(1):31-37. [12] ROMANOV YA, SVINTSITSKAYA VA, SMIRNOV VN. Searching for alternative sources of postnatal human mesenchymal stem cells: candidate MSC-like cells from umbilical cord. Stem Cells. 2003;21(1):105-110. [13] COVAS DT, SIUFI JL, SILVA AR, et al. Isolation and culture of umbilical vein mesenchymal stem cells. Braz J Med Biol Res. 2003;36(9):1179-1183. [14] MITCHELL KE, WEISS ML, MITCHELL BM, et al. Matrix cells from Wharton’s jelly form neurons and glia. Stem Cells. 2003;21(1):50-60. [15] MA L, FENG XY, CUI BL, et al. Human umbilical cord Wharton’s Jelly-derived mesenchymal stem cells differentiation into nerve-like cells. Chin Med J (Engl). 2005;118(23):1987-1993. [16] SARUGASER R, LICKORISH D, BAKSH D, et al. Human umbilical cord perivascular (HUCPV) cells: a source of mesenchymal progenitors. Stem Cells. 2005;23(2):220-229. [17] BEERAVOLU N, MCKEE C, ALAMRI A, et al. Isolation and characterization of mesenchymal stromal cells from human umbilical cord and fetal placenta. J Vis Exp. 2017;(122):55224. [18] HU Y, ZHANG Y, NI CY, et al. Human umbilical cord mesenchymal stromal cells-derived extracellular vesicles exert potent bone protective effects by CLEC11A-mediated regulation of bone metabolism. Theranostics. 2020; 10(5):2293-2308. [19] IWATANI S, YOSHIDA M, YAMANA K, et al. Isolation and characterization of human umbilical cord-derived mesenchymal stem cells from preterm and term infants. J Vis Exp. 2019;(143):58806. [20] SHI Y, NAN C, YAN Z, et al. Synaptic plasticity of human umbilical cord mesenchymal stem cell differentiating into neuron-like cells in vitro induced by edaravone. Stem Cells Int. 2018;2018:5304279. [21] ABOU-ELNAGA A, EL-CHENNAWI F, IBRAHIM KAMEL S, et al. The potentiality of human umbilical cord isolated mesenchymal stem/stromal cells for cardiomyocyte generation. Stem Cells Cloning. 2020;13:91-101. [22] GUAN YT, XIE Y, LI DS, et al. Comparison of biological characteristics of mesenchymal stem cells derived from the human umbilical cord and decidua parietalis. Mol Med Rep. 2019;20(1):633-639. [23] PAN XH, HUANG X, RUAN GP, et al. Umbilical cord mesenchymal stem cells are able to undergo differentiation into functional islet-like cells in type 2 diabetic tree shrews. Mol Cell Probes. 2017;34:1-12. [24] SHI Q, GAO J, JIANG Y, et al. Differentiation of human umbilical cord Wharton’s jelly-derived mesenchymal stem cells into endometrial cells. Stem Cell Res Ther. 2017;8(1):246. [25] ZHANG S, ZHANG W, LI Y, et al. Human umbilical cord mesenchymal stem cell differentiation into odontoblast-like cells and endothelial cells: a potential cell source for dental pulp tissue engineering. Front Physiol. 2020;11:593. [26] CHEN S, ZHANG W, WANG JM, et al. Differentiation of isolated human umbilical cord mesenchymal stem cells into neural stem cells. Int J Ophthalmol. 2016;9(1):41-47. [27] LI Y, YANG J, FU G, et al. Human umbilical cord mesenchymal stem cells differentiate into neuron-like cells after induction with B27-supplemented serum-free medium. Nan Fang Yi Ke Da Xue Xue Bao. 2020;40(9):1340-1345. [28] MAJIDI F, BAMEHR H, SHALCHIAN Z, et al. Differentiation of human umbilical cord mesenchymal stem cell into germ-like cell under effect of co-culture with testicular cell tissue. Anat Histol Embryol. 2020;49(3):359-364. [29] 金诚.人脐带间充质干细胞向视网膜色素上皮细胞分化的研究[J].石家庄:河北医科大学,2014. [30] SHI Q, GAO J, JIANG Y, et al. Differentiation of human umbilical cord Wharton’s jelly-derived mesenchymal stem cells into endometrial cells. Stem Cell Res Ther. 2017;8(1):246. [31] YANG L, ZHU S, LI Y, et al. Overexpression of Pygo2 increases differentiation of human umbilical cord mesenchymal stem cells into cardiomyocyte-like cells. Curr Mol Med. 2020;20(4):318-324. [32] BELAME SHIVAKUMAR S, BHARTI D, BAREGUNDI SUBBARAO R, et al. Pancreatic endocrine-like cells differentiated from human umbilical cords Wharton’s jelly mesenchymal stem cells using small molecules. J Cell Physiol. 2019;234(4):3933-3947. [33] VILLEDA SA, PLAMBECK KE, MIDDELDORP J, et al. Young blood reverses age-related impairments in cognitive function and synaptic plasticity in mice. Nat Med. 2014;20(6):659-663. [34] MILLÁN-RIVERO JE, NADAL-NICOLÁS FM, GARCÍA-BERNAL D, et al. Human Wharton’s jelly mesenchymal stem cells protect axotomized rat retinal ganglion cells via secretion of anti-inflammatory and neurotrophic factors. Sci Rep. 2018;8(1):16299. [35] THAWEESAPPHITHAK S, TANTRAWATPAN C, KHEOLAMAI P, et al. Human serum enhances the proliferative capacity and immunomodulatory property of MSCs derived from human placenta and umbilical cord. Stem Cell Res Ther. 2019;10(1):79. [36] YAGHOUBI Y, MOVASSAGHPOUR A, ZAMANI M, et al. Human umbilical cord mesenchymal stem cells derived-exosomes in diseases treatment. Life Sci. 2019;233:116733. [37] YIN S, JI C, WU P, et al. Human umbilical cord mesenchymal stem cells and exosomes: bioactive ways of tissue injury repair. Am J Transl Res. 2019;11(3): 1230-1240. [38] MAO F, WU Y, TANG X, et al. Exosomes derived from human umbilical cord mesenchymal stem cells relieve inflammatory bowel disease in mice. Biomed Res Int. 2017;2017:5356760. [39] 梁亚会,张青宜,郭子宽,等.人脐带间充质干细胞来源的外泌体免疫调节功能异质性研究[J].军事医学,2017,41(6):434-439. [40] 郭礼妍,赖沛龙,耿素霞.等.人脐带间充质干细胞来源外泌体对Treg和TH17细胞的调节作用[J].中国实验血液学杂志,2019,27(1):221-226. [41] SASSON IE, TAYLOR HS. Stem cells and the pathogenesis of endometriosis. Ann N Y Acad Sci. 2008;1127:106-115. [42] CECCARONI M, ROVIGLIONE G, ROSENBERG P, et al. Pericardial, pleural and diaphragmatic endometriosis in association with pelvic peritoneal and bowel endometriosis: a case report and review of the literature. Wideochir Inne Tech Maloinwazyjne. 2012;7(2):122-131. [43] 李清雪,陈景伟,杨剑.OTUD7B对子宫内膜异位症患者子宫内膜基质细胞增殖、侵袭与凋亡的影响[J].中国临床药理学杂志,2021,37(8): 1004-1007. [44] 王春梅,李洁,孙文萍,等.人脐带间充质干细胞对异位子宫内膜细胞增殖及凋亡的影响[J].中国组织工程研究,2015,19(41):6628-6632. [45] ZHANG L, LI Y, GUAN CY, et al. Therapeutic effect of human umbilical cord-derived mesenchymal stem cells on injured rat endometrium during its chronic phase. Stem Cell Res Ther. 2018;9(1):36. [46] 艾瑛,廖诗平,王玉芳,等.脐带间充质干细胞移植预防宫腔粘连的研究[J].现代妇产科进展,2019,28(5):342-345. [47] 江春燕.左归丸联合脐带间充质干细胞修复受损子宫内膜的研究[D].武汉:湖北中医药大学,2017. [48] WANG L, YU C, CHANG T, et al. In situ repair abilities of human umbilical cord-derived mesenchymal stem cells and autocrosslinked hyaluronic acid gel complex in rhesus monkeys with intrauterine adhesion. Sci Adv. 2020;6(21):eaba6357. [49] LIU Y, CAI J, LUO X, et al. Collagen scaffold with human umbilical cord mesenchymal stem cells remarkably improves intrauterine adhesions in a rat model. Gynecol Obstet Invest. 2020;85(3):267-276. [50] 曹昀.干细胞治疗宫腔粘连综合征的机制研究和临床转化[D].南京:南京大学,2017. [51] CAO Y, SUN H, ZHU H, et al. Allogeneic cell therapy using umbilical cord MSCs on collagen scaffolds for patients with recurrent uterine adhesion: a phase I clinical trial. Stem Cell Res Ther. 2018;9(1):192. [52] 吴林.冻干羊膜联合人脐带间充质干细胞治疗中重度宫腔粘连的研究[D].衡阳:南华大学,2020. [53] HEGER A, SATOR M, PIETROWSKI D. Endometrial receptivity and its predictive value for IVF/ICSI-outcome. Geburtshilfe Frauenheilkd. 2012; 72(8):710-715. [54] MAHAJAN N, SHARMA S. The endometrium in assisted reproductive technology: how thin is thin? J Hum Reprod Sci. 2016;9(1):3-8. [55] BAKOS O, LUNDKVIST O, BERGH T. Transvaginal sonographic evaluation of endometrial growth and texture in spontaneous ovulatory cycles-a descriptive study. Hum Reprod. 1993;8(6):799-806. [56] GROW DR, IROMLOO K. Oral contraceptives maintain a very thin endometrium before operative hysteroscopy. Fertil Steril. 2006;85(1):204-207. [57] LIU KE, HARTMAN M, HARTMAN A. Management of thin endometrium in assisted reproduction: a clinical practice guideline from the Canadian Fertility and Andrology Society. Reprod Biomed Online. 2019;39(1):49-62. [58] MIWA I, TAMURA H, TAKASAKI A, et al. Pathophysiologic features of ‘thin’ endometrium. Fertil Steril. 2009;91:998-1004. [59] AL-GHAMDI A, COSKUN S, AL-HASSAN S, et al. The correlation between endometrial thickness and outcome of in vitro fertilization and embryo transfer (IVF-ET) outcome. Reprod Biol Endocrinol. 2008;6:37. [60] AZIZI R, AGHEBATI-MALEKI L, NOURI M, et al. Stem cell therapy in Asherman syndrome and thin endometrium: stem cell-based therapy. Biomed Pharmacother. 2018;102:333-343. [61] YE MX, YU L, WANG SF, et al. Efficacy of gamma-irradiated adipose-derived stem cells for treatment of thin endometrium in rats. Nan Fang Yi Ke Da Xue Xue Bao. 2017;37(5):575-580. [62] MENG QY, XI J, XIA LJ, et al. Effect of combined administration of electroacupuncture and mesenchymal stem cells on expression of endometrium estrogen receptor and progesterone receptor in thin endometrium rats. Zhen Ci Yan Jiu. 2021;46(5):385-390. [63] 贺桑,卢永娟,黄向红,等.人脐带间充质干细胞移植对大鼠薄型子宫内膜的治疗作用[J].中国医学前沿杂志(电子版),2016,8(9):67-72. [64] ZHANG Y, SHI L, LIN X, et al. Unresponsive thin endometrium caused by Asherman syndrome treated with umbilical cord mesenchymal stem cells on collagen scaffolds: a pilot study. Stem Cell Res Ther. 2021;12(1):420. |
| [1] | Wang Jing, Xiong Shan, Cao Jin, Feng Linwei, Wang Xin. Role and mechanism of interleukin-3 in bone metabolism [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(8): 1260-1265. |
| [2] | Xiao Hao, Liu Jing, Zhou Jun. Research progress of pulsed electromagnetic field in the treatment of postmenopausal osteoporosis [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(8): 1266-1271. |
| [3] | Tian Chuan, Zhu Xiangqing, Yang Zailing, Yan Donghai, Li Ye, Wang Yanying, Yang Yukun, He Jie, Lü Guanke, Cai Xuemin, Shu Liping, He Zhixu, Pan Xinghua. Bone marrow mesenchymal stem cells regulate ovarian aging in macaques [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 985-991. |
| [4] | Hou Jingying, Guo Tianzhu, Yu Menglei, Long Huibao, Wu Hao. Hypoxia preconditioning targets and downregulates miR-195 and promotes bone marrow mesenchymal stem cell survival and pro-angiogenic potential by activating MALAT1 [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1005-1011. |
| [5] | Zhou Ying, Zhang Huan, Liao Song, Hu Fanqi, Yi Jing, Liu Yubin, Jin Jide. Immunomodulatory effects of deferoxamine and interferon gamma on human dental pulp stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1012-1019. |
| [6] | Liang Xuezhen, Yang Xi, Li Jiacheng, Luo Di, Xu Bo, Li Gang. Bushen Huoxue capsule regulates osteogenic and adipogenic differentiation of rat bone marrow mesenchymal stem cells via Hedgehog signaling pathway [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1020-1026. |
| [7] | Wang Jifang, Bao Zhen, Qiao Yahong. miR-206 regulates EVI1 gene expression and cell biological behavior in stem cells of small cell lung cancer [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1027-1031. |
| [8] | Liu Feng, Peng Yuhuan, Luo Liangping, Wu Benqing. Plant-derived basic fibroblast growth factor maintains the growth and differentiation of human embryonic stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1032-1037. |
| [9] | Wen Dandan, Li Qiang, Shen Caiqi, Ji Zhe, Jin Peisheng. Nocardia rubra cell wall skeleton for extemal use improves the viability of adipogenic mesenchymal stem cells and promotes diabetes wound repair [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1038-1044. |
| [10] | Zhu Bingbing, Deng Jianghua, Chen Jingjing, Mu Xiaoling. Interleukin-8 receptor enhances the migration and adhesion of umbilical cord mesenchymal stem cells to injured endothelium [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1045-1050. |
| [11] | Luo Xiaoling, Zhang Li, Yang Maohua, Xu Jie, Xu Xiaomei. Effect of naringenin on osteogenic differentiation of human periodontal ligament stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1051-1056. |
| [12] | Wang Xinmin, Liu Fei, Xu Jie, Bai Yuxi, Lü Jian. Core decompression combined with dental pulp stem cells in the treatment of steroid-associated femoral head necrosis in rabbits [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1074-1079. |
| [13] | Fang Xiaolei, Leng Jun, Zhang Chen, Liu Huimin, Guo Wen. Systematic evaluation of different therapeutic effects of mesenchymal stem cell transplantation in the treatment of ischemic stroke [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1085-1092. |
| [14] | Guo Jia, Ding Qionghua, Liu Ze, Lü Siyi, Zhou Quancheng, Gao Yuhua, Bai Chunyu. Biological characteristics and immunoregulation of exosomes derived from mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1093-1101. |
| [15] | Huang Chenwei, Fei Yankang, Zhu Mengmei, Li Penghao, Yu Bing. Important role of glutathione in stemness and regulation of stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1119-1124. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||