Chinese Journal of Tissue Engineering Research ›› 2022, Vol. 26 ›› Issue (11): 1772-1779.doi: 10.12307/2022.364
Previous Articles Next Articles
Active monomer composition of Epimedium influences the homeostasis of bone resorption and bone formation by regulating osteoporosis related signaling pathways
Li Shibin1, Xia Tian2, Zhang Xiaoyun1, Wang Weiwei1, Zhou Yi1, Lai Yu1
- 1Ruikang Hospital Affiliated to Guangxi University of Chinese Medicine, Nanning 530011, Guangxi Zhuang Autonomous Region, China; 2Guangxi University of Chinese Medicine, Nanning 530000, Guangxi Zhuang Autonomous Region, China
-
Received:2021-06-08Revised:2021-06-10Accepted:2021-07-16Online:2022-04-18Published:2021-12-13 -
Contact:Xia Tian, MD candidate, Associate professor, Guangxi University of Chinese Medicine, Nanning 530000, Guangxi Zhuang Autonomous Region, China -
About author:Li Shibin, Master candidate, Ruikang Hospital Affiliated to Guangxi University of Chinese Medicine, Nanning 530011, Guangxi Zhuang Autonomous Region, China -
Supported by:the National Natural Science Foundation of China, Nos. 81760796 and 81960803 (both to ZXY [project participant]); Guangxi Natural Science Foundation for the Youth, No. 2020GXNSFBA159053 (to ZXY); Young Teachers’ Basic Ability Improvement Project of Guangxi Universities, No. 2019KY0352 (to ZXY); Guangxi Innovation Projects for Graduate Education, No. YCBXJ2021019 (to XT) and YCSW2021219 (to XT [project participant])
CLC Number:
Cite this article
Li Shibin, Xia Tian, Zhang Xiaoyun, Wang Weiwei, Zhou Yi, Lai Yu. Active monomer composition of Epimedium influences the homeostasis of bone resorption and bone formation by regulating osteoporosis related signaling pathways[J]. Chinese Journal of Tissue Engineering Research, 2022, 26(11): 1772-1779.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
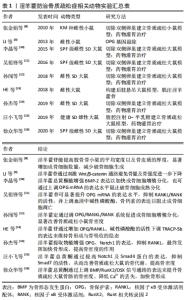
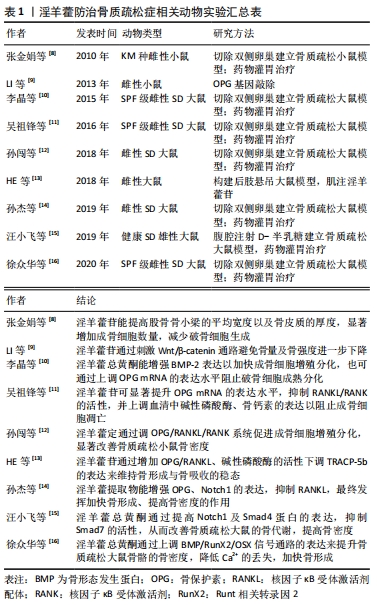
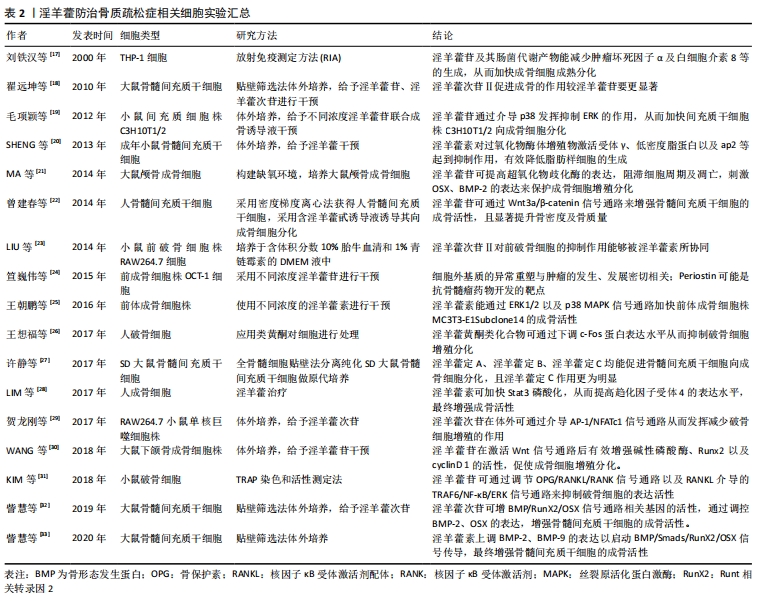
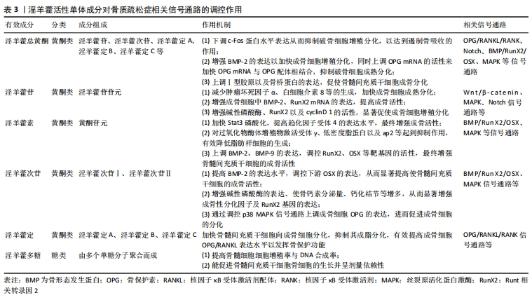
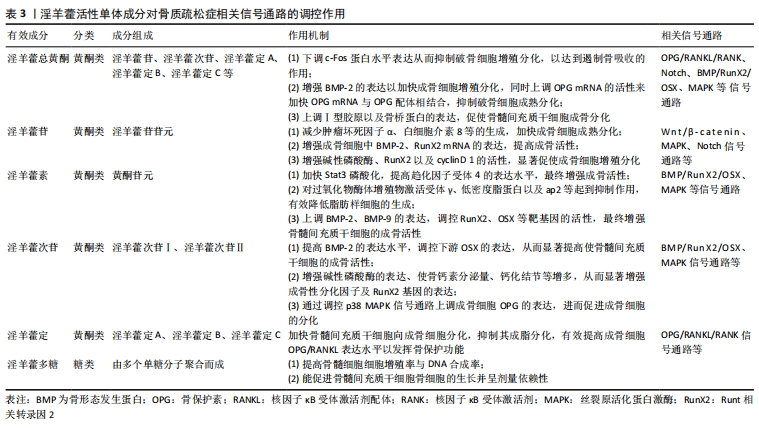
2.3.1 淫羊藿苷 淫羊藿苷是淫羊藿茎叶中一种具有广泛药理学活性的黄酮类化合物,已被众多研究证实其在人体骨骼系统中发挥着重要的作用,其能够增强成骨活性,有效抑制成骨细胞的凋亡。而成熟的成骨细胞能生成一种抑制破骨细胞吸收或破坏人体健康骨质作用的细胞因子,并促使破骨细胞凋亡,从而发挥抗骨质疏松的作用[34]。此外淫羊藿苷还能通过减少脂质形成、减少细胞中三酰甘油水平来抑制成脂转录因子以及提高子结合蛋白基因的表达来阻止成脂分化,最终增强成骨活性[35]。 有学者指出淫羊藿苷及其人肠内菌的代产物如淫羊藿苷元、宝藿苷Ⅰ等能够促进机体分泌一种有利于成骨细胞生长的因子来减少肿瘤坏死因子α及白细胞介素8等的生成,从而加快成骨细胞成熟分化[17]。同样,在去卵巢大鼠造模实验中发现淫羊藿苷能够提高股骨骨小梁的平均宽度以及骨皮质的厚度,同时显著增加成骨细胞数量,减少破骨细胞生成[8]。笪巍伟等[24]在研究中发现使用淫羊藿苷可有效增强成骨细胞中骨形态发生蛋白2、Runt相关转录因2 mRNA的表达,明显提高成骨活性。此外,也有学者发现淫羊藿苷可通过抑制活性氧及丙二醛来提高超氧化物歧化酶的表达,并阻滞细胞周期及凋亡,同时刺激成骨细胞特异性转录因子OSX、骨形态发生蛋白2基因的表达来保护成骨细胞增殖分化[21]。目前淫羊藿苷被认为是淫羊藿中最主要的活性成分,随着人们对其抗骨质疏松作用机制研究的深入,淫羊藿苷在细胞因子及信号通路方面促进成骨细胞的内在机制逐渐被揭示,这将对今后骨质疏松的防治有重要意义。 2.3.2 淫羊藿次苷 淫羊藿次苷包括淫羊藿次苷Ⅰ和淫羊藿次苷Ⅱ,是淫羊藿中另一主要活性成分,近年来众多研究证实了其抗氧化及加快细胞增殖分化的作用。目前发现淫羊藿次苷Ⅱ可增强诱导型一氧化氮合酶的表达,并加快一氧化氮的释放,从而有效提高骨髓间充质干细胞成骨分化的作用[36]。同时机体活性氧自由基长期积累时会对成骨细胞产生抑制作用,甚至诱导其凋亡,而淫羊藿次苷Ⅱ有较强的抗氧化作用,其在清除DPPH自由基、抑制脂质过氧化等方面效果显著[37]。 贺龙刚等[29]在实验中发现淫羊藿次苷Ⅰ及淫羊藿次苷Ⅱ在体外皆可通过介导AP-1/NFATc1信号通路从而发挥减少破骨细胞增殖的作用,且淫羊藿次苷Ⅱ效果更为明显。同样,訾慧等[32]在大鼠实验中证实了淫羊藿次苷Ⅰ及淫羊藿次苷Ⅱ在体外可增强BMP/RunX2/OSX信号通路相关基因的活性,并通过提高骨形态发生蛋白2的表达水平进一步调控下游成骨细胞特异性转录因子的转录表达,从而显著提高骨髓间充质干细胞的成骨活性。值得注意的是,淫羊藿次苷虽是由淫羊藿苷代谢所产生,但是有学者在研究中发现淫羊藿次苷Ⅱ可有效增强碱性磷酸酶的表达、使骨钙素分泌量、钙化结节等增多,从而显著增强成骨性分化因子及Runt相关转录因2的表达,其促进成骨的作用较淫羊藿苷要更显著[18]。经研究,目前认为淫羊藿次苷活性增强的原因可能与其脱去了7位碳上的葡萄糖残基有关,然而还需要进行更深层次的研究。 2.3.3 淫羊藿素 淫羊藿素属于黄酮类化合物,其生物活性同样广泛。近年来越来越多药理机制的研究围绕其骨保护及抗肿瘤作用展开。目前发现其通过降低肿瘤坏死因子受体相关因子6 (TNF receptor associated factor,TRAF6)的水平来下调核因子κB及活性氧信号通路的表达,发挥抑制NFATc1的活性以达到抗骨质疏松的目的[38]。此外,人们还发现淫羊藿素通过直接增强骨形态发生蛋白的活性来刺激骨髓间充质干细胞及人脂肪组织干细胞在体外向成骨分化。这些发现都为淫羊藿素能够防治骨质疏松及脆性骨折提供了强有力的证据[39]。 在大鼠造模实验中发现,预先服用淫羊藿素能够明显加快骨形成,促进骨质疏松大鼠骨松质、骨小梁结构的恢复[40]。LIM等[28]在实验中表明淫羊藿素可加快Stat3磷酸化,从而提高趋化因子受体4(CXC chemokine receptor 4,CXCR4)的表达水平,最终达到增强成骨活性的作用。同样,有实验证实了淫羊藿素不仅能够加快成骨分化,且有效阻止了成脂分化。在体外实验中,学者发现淫羊藿素在加快成骨细胞增殖分化的同时也在对过氧化物酶体增殖物激活受体γ、低密度脂蛋白以及ap2等起到抑制作用,有效降低脂肪样细胞的生成,从而发挥抗骨质疏松的作用[20]。而据最近的研究表明,淫羊藿次苷Ⅱ和淫羊藿素都有阻止破骨细胞生成的作用[23],有意思的是前者对前破骨细胞的抑制作用能够被淫羊藿素所协同,这从侧面证明淫羊藿中不同单体成分能协同作用以达到抗骨质疏松的目的。 2.3.4 淫羊藿定 淫羊藿定A、淫羊藿定B、淫羊藿定C同样作为淫羊藿中主要黄酮类化合物,已被证实不仅具有增加细胞基质钙的作用,也能加快体外培养的成骨细胞增殖分化[41]。在脊柱动物骨骼发育成形的过程中,OPG/RANKL/RANK系统始终发挥着维持动态平衡的作用。当骨保护素的表达水平高于核因子κB受体激活剂配体时,体内破骨细胞活性受到抑制,成骨细胞形成活跃,则骨量增加;反之骨量减少,最终造成骨质疏松,因此该系统对于调节骨代谢稳态,防治骨质疏松具有重要意义[42]。 孙闯等[12]在研究中发现淫羊藿定可提高骨组织中骨保护素mRNA的表达,从而显著改善骨质疏松大鼠股骨干骺端骨密度,说明淫羊藿定通过调控OPG/RANKL/RANK系统促进成骨细胞增殖分化,以达到抗骨质疏松的作用。此外越来越多研究发现淫羊藿定能促进骨髓间充质干细胞向成骨细胞分化。刘颖等[43]取正常小鼠骨髓间充质干细胞体外培养,分别加入不同剂量淫羊藿定A,应用ELISA技术观察淫羊藿定A对小鼠骨髓间充质干细胞的影响,结果发现淫羊藿定A可加快骨髓间充质干细胞向成骨细胞分化,抑制其成脂分化,有效提高成骨细胞OPG/RANKL表达水平以发挥骨保护功能。同样许静等[27]分离纯化SD大鼠骨髓间充质干细胞,加入淫羊藿黄酮类主要成分进行干预,他们发现淫羊藿定A、淫羊藿定B、淫羊藿定C均能促进骨髓间充质干细胞向成骨细胞分化,且淫羊藿定C作用更为明显。 2.4 淫羊藿调控分子信号通路抗骨质疏松症的作用机制 在骨形成与骨吸收的过程中有多条信号通路参与其中。"
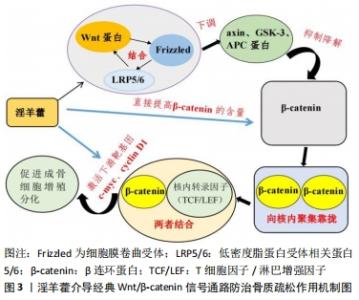
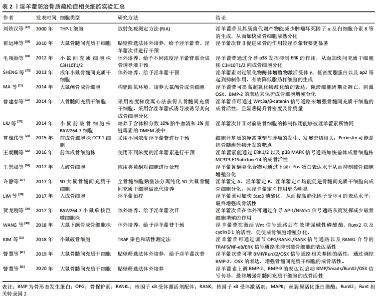
根据实验表明,目前发现淫羊藿主要通过干预Wnt/β-catenin、MAPK、OPG/RANKL/RANK以及BMP/RunX2/OSX、Notch等通路来促进骨形成、增加骨密度,维持骨代谢平衡以发挥抗骨质疏松的作用,见表3。 2.4.1 Wnt/β-catenin信号通路 Wnt/β-catenin信号通路目前被认为是Wnt信号通路传导途径中最经典的通路,其通过加快成骨细胞增殖分化、抑制成骨细胞凋亡等有效促进骨量增加,已被证实在抗骨质疏松方面发挥着重要的作用。 Wnt蛋白首先与细胞膜卷曲受体(Frizzled)以及低密度脂蛋白受体相关蛋白5/6相结合以激活Wnt/β-catenin信号通路,接下来下调axin、GSK-3及APC蛋白的表达以保护β-连环蛋白(β-catenin)免受降解,使其在细胞质内保持稳定水平,并最终与核内转录因子如T细胞因子/淋巴增强因子等相结合以达到加快下游c-myc、cyclin D1等靶基因表达的作用,从而促进细胞增殖分化并防治其凋亡。而随着人们对淫羊藿研究的不断深入,发现其众多药效成分均能干预Wnt/β-catenin信号通路,促进成骨细胞增殖分化从而有效维持骨代谢平衡,最终发挥抗骨质疏松的作用,见图3。"

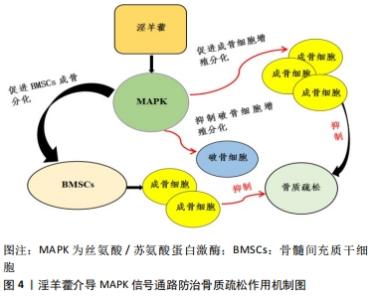
WANG等[30]发现淫羊藿苷在激活Wnt信号通路后有效增强了碱性磷酸酶、Runt相关转录因2以及cyclinD 1的活性,显著促使成骨细胞增殖分化。同样,LI等[9]在实验中发现,淫羊藿苷通过刺激Wnt/β-catenin 通路显著改善了小鼠颅骨的骨吸收情况,避免了骨量及骨强度进一步下降,从而维持小鼠体内的骨代谢以达到抗骨质疏松的目的。曾建春等[22]也证实了淫羊藿苷可通过Wnt3a/β-catenin信号通路来增强间充质干细胞的成骨活性,且显著提升骨密度及骨质量,最终起到抗骨质疏松的作用。由此可见淫羊藿通过Wnt/β-catenin信号通路在促进骨形成及维持骨代谢方面至关重要。 2.4.2 MAPK信号通路 MAPK是一种具有磷酸化功能的丝氨 酸/苏氨酸蛋白激酶,主要由细胞外信号调节激酶(extracellular Regulated Protein Kinases,ERK)、p38以及c-Jun氨基末端激酶(c-Jun N-terminal kinase JNK)3种激酶组成。细胞外信号调节激酶1/2信号可通过激活细胞生长因子的表达,在骨细胞形成的过程中发挥着关键作用。p38也被证实是成骨分化过程中的重要媒介,调控p38将有助于骨髓间充质干细胞成骨分化[44];而c-Jun氨基末端激酶对人骨膜细胞成骨活性的大小至关重要[45],总之MAPK信号通路在骨细胞的形成、发育以及凋亡过程中起着重要的作用。目前发现MAPK信号通路在淫羊藿苷的刺激下将促使成骨细胞增殖分化,抑制破骨细胞生成,同时促进骨髓间充质干细胞成骨分化,维持骨形成及骨吸收过程的稳态,并最终达到抗骨质疏松的作用[46],见图4。"
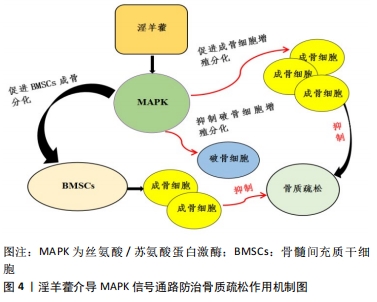
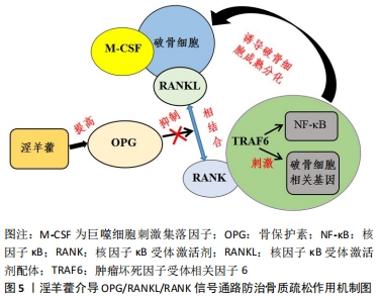
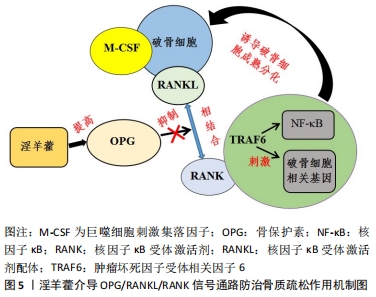
龚一昕等[47]在探究淫羊藿次苷Ⅱ抗骨质疏松作用机制的实验中发现,淫羊藿次苷Ⅱ通过介导p38 MAPK信号通路的表达从而促进成骨细胞骨保护素的活性,最终促使成骨细胞分化。王想福等[26]在实验中发现淫羊藿黄酮类化合物可通过下调p38 MAPK通路中c-Fos蛋白水平表达从而抑制破骨细胞增殖分化,以达到遏制骨吸收的作用。同样,王朝鹏[25]在其研究中发现当细胞外信号调节激酶1/2以及p38 MAPK信号通路被阻断后,淫羊藿素增强碱性磷酸酶以及骨桥蛋白活性的作用将明显减弱,这表明淫羊藿素能够通过细胞外信号调节激酶1/2以及p38 MAPK信号通路加快前体成骨细胞株 MC3T3-E1Subclone14 的成骨活性。毛项颖等[19]在研究中也证实了淫羊藿苷可通过介导MAPK信号通路上的p38,发挥抑制细胞外信号调节激酶的作用,并最终加快间充质干细胞株C3H10T1/2向成骨细胞分化。因此,以淫羊藿介导MAPK信号通路可能是治疗骨质疏松的靶点之一,但仍需要更多研究进一步证实。 2.4.3 OPG/RANKL/RANK信号通路 破骨细胞的存活主要与巨噬细胞刺激集落因子及核因子κB受体激活剂配体有关。目前发现当巨噬细胞刺激集落因子增加破骨前体细胞池后,核因子κB受体激活剂配体将与核因子κB受体激活剂发生结合,启动细胞内信号传导,并且与肿瘤坏死因子受体相关因子6发生协同作用,而肿瘤坏死因子受体相关因子6会进一步刺激核因子κB等信号传导途径诱导破骨细胞成熟分化。因此抑制核因子κB受体激活剂与核因子κB受体激活剂配体特异性结合是有效降低破骨细胞活性的方法之一。 在最近的实验中表明,淫羊藿苷可通过调节OPG/RANKL/RANK信号通路来减少破骨细胞的生成,亦可通过调节核因子κB受体激活剂配体介导的TRAF6/NF-κB/ERK信号通路来抑制破骨细胞的表达活性[31]。同时HE等[13]在研究中证实了淫羊藿苷能通过增强OPG/RANKL、碱性磷酸酶的活性下调TRACP-5b等骨吸收标志物的表达来减少骨吸收,促使成骨分化,以达到维持骨形成与骨吸收的稳定。吴祖锋等[11]发现淫羊藿苷可显著提升骨保护素蛋白的表达水平,从而有效抑制RANKL/RANK的活性,并上调血清中碱性磷酸酶、骨钙素的表达以阻止成骨细胞凋亡的进程。李晶等[10]也在实验中发现淫羊藿总黄酮不仅能增强骨形态发生蛋白2的表达以加快成骨细胞增殖分化,也可通过上调骨保护素蛋白的活性来加快骨保护素蛋白与骨保护素配体相结合,从而发挥抑制核因子κB受体激活剂配体、阻止破骨细胞成熟分化的作用,见图5。"
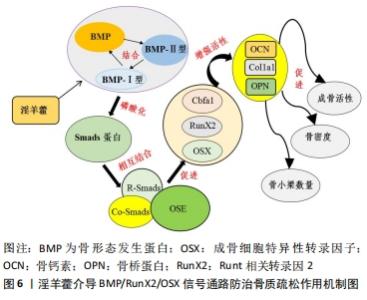
2.4.4 BMP/RunX2/OSX信号通路 目前发现由骨形态发生蛋白、Runt相关转录因2以及成骨细胞特异性转录因子组成的BMP/RunX2/OSX信号通路参与调控成骨细胞增殖分化,在骨形成过程中发挥着重要的作用[48]。骨形态发生蛋白作为转化生长因子超家族成员之一,其与异二聚体受体如BMP-Ⅰ型、Ⅱ型等发生特异性结合,促使Smads蛋白磷酸化,进一步加快R-Smads与Co-Smads蛋白在核内与OSE序列结合,从而调控Cbfa1/RunX2以及下游成骨细胞特异性转录因子基因的表达,上调骨钙素、骨桥蛋白、Col1a1等成骨细胞标志物的活性,促使膜内、软骨内骨化成骨,进而提高骨密度、骨小梁数量等以达到抗骨质疏松的作用,见图6。"
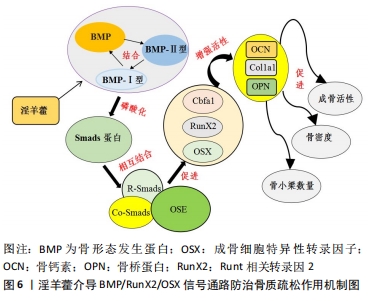

徐众华等[16]在其研究中发现淫羊藿总黄酮能通过上调BMP/RunX2/OSX信号通路的表达来提升绝经后骨质疏松模型大鼠骨骼的骨密度,且降低了Ca2+的丢失,加快骨形成,有效延缓骨质疏松的进一步发展。此外訾慧等[33]在实验中证实了淫羊藿素通过上调骨形态发生蛋白2、骨形态发生蛋白9的表达水平以启动BMP/Smads/RunX2/OSX信号传导,进而调控Runt相关转录因2、成骨细胞特异性转录因子等靶基因的活性,最终增强骨髓间充质干细胞的成骨活性。梁广胜等[49]在研究中使用淫羊藿总黄酮对大鼠进行干预,他发现大鼠体内Ⅰ型胶原以及骨桥蛋白的表达明显上调,且钙结节密度也较前明显增大,这说明淫羊藿总黄酮能通过BMP-2/RunX2/OSX信号通路促使骨髓间充质干细胞成骨分化。 2.4.5 Notch信号通路 Notch信号通路主要由Notch受体及其配体(CSL与DSL蛋白)、Notch调节因子及下游Hes、Hey等构成,在细胞增殖、分化以及凋亡等一系列发育过程中起着重要的调控作用。该通路的激活不需要胞内第二信使的参与,当Notch受体与其配体相结合后会在跨膜区胞外端经受3次切割裂解,生成NICD并使之与核内CSL蛋白特异性结合,并最终与DNA形成多蛋白-DNA复合体,激活成骨细胞与破骨细胞相关基因的表达[50]。 目前发现该信号通路在成骨细胞分化中扮演着双向调节作用[51],即Notch信号通路的转导活化在早期成骨分化中起促进成骨的作用,在后期则会抑制成骨细胞的增殖、分化,但淫羊藿通过Notch信号通路促进成骨分化这一观点已被普遍认可。邓宇等[52]发现淫羊藿苷不仅能上调Hesl、Runt相关转录因2的表达活性,且显著提高CBF1、Notch1以及Jagged-1蛋白的表达来促进骨髓间充质干细胞向成骨细胞分化。孙杰等[14]在实验中发现淫羊藿提取物能下调碱性磷酸酶的水平,并增强Notch1以及骨保护素的表达活性,对核因子κB受体激活剂配体起到抑制作用,最终发挥加快骨形成、提高骨密度的作用。同样汪小飞等[15]也证实了淫羊藿总黄酮通过提高Notch1及Smad4蛋白的表达水平,抑制Smad7蛋白的活性,从而改善老年骨质疏松大鼠的骨代谢,提高骨密度。总之Notch信号通路与骨代谢疾病密切相关,其在骨骼发育中发挥着重要作用。但目前国内外相关研究不多,期待未来的研究将致力于剖析这一复杂的调控机制,以揭示淫羊藿介导Notch信号通路发挥抗骨质疏松作用的确切机制。"

| [1] KANIS JA, MCCLOSKEY EV, HARVEY NC, et al. Intervention thresholds and the diagnosis of osteoporosis. J Bone Miner Res. 2015;30(10):1747-1753. [2] SI L, WINZENBERG TM, JIANG Q, et al. Projection of osteoporosis- related fractures and costs in China: 2010-2050. Osteoporos Int. 2015;26(7): 1929-1937. [3] BURNS RB, ROSEN H, BERRY S, et al. How would You manage this patient with osteoporosis? Grand rounds discussion from Beth Israel Deaconess Medical Center. Ann Intern Med. 2018;168(11):801-808. [4] VIDAL M, THIBODAUX RJ, NEIRA LFV, et al. Osteoporosis: A clinical and pharmacological update. Clin Rheumatol. 2019;38(2):385-395. [5] EASTELL R, SZULC P. Use of bone turnover markers in postmenopausal osteoporosis. Lancet Diabetes Endocrinol. 2017;5(11):908-923. [6] 李建国,谢兴文,李鼎鹏,等.中药淫羊藿治疗骨质疏松症的研究进展[J].中国骨质疏松杂志,2018,24(3):389-393. [7] AN J, YANG H, ZHANG Q, et al. Natural products for treatment of osteoporosis: The effects and mechanisms on promoting osteoblast-mediated bone formation. Life Sciences. 2016;147:46-58. [8] 张金娟,文娱,张贵林,等.淫羊藿苷对骨质疏松模型小鼠骨组织形态计量学指标的影响[J].贵州医药,2010,34(5):22-23. [9] LI XF, XU H, ZHAO YJ, et al. Icariin augments bone formation and reverses the phenotypes of osteoprotegerin-deficient mice through the activation of Wnt/β- Catenin-BMP signaling. Evid Based Complement Alternat Med. 2013;2013:652317. [10] 李晶,宋敏,罗晓,等.淫羊藿总黄酮对去势大鼠骨组织BMP-2和OPGmRNA表达的影响[J].中华中医药杂志,2015,30(7):2529-2531. [11] 吴祖锋,袁垒,吴风晴,等.淫羊藿苷对骨质疏松症模型大OPG/RANKL/RANK轴系统影响的实验研究[J].甘肃中医药大学学报,2016,33(3):4-7. [12] 孙闯,杨大威,姜明久,等.淫羊藿定对骨质疏松大鼠的治疗作用及机制研究[J].哈尔滨医科大学学报,2018,52(3):234-237. [13] HE JP, FENG X, WANG JF, et al. Icariin prevents bone loss by inhibiting bone resorption and stabilizing bone biological apatite in a hindlimb suspension rodent model. Acta Pharmacol Sin. 2018;39(11):1760-1767. [14] 孙杰,宋鑫,王健.淫羊藿提取物对骨质疏松骨折大鼠愈合过程Notch信号通路的影响[J].中国中医急症,2019,28(4):611-614. [15] 汪小飞,李晶晶.淫羊藿总黄酮对老年骨质疏松大鼠Notch和Smads通路蛋白表达的影响[J].中国中医骨伤科杂志,2019,27(2):1-5. [16] 徐众华,莫雨晴,周驰.基于BMP/Runx2/Osx信号通路研究淫羊藿总黄酮改善绝经后骨质疏松模型大鼠的作用机制[J].中国药房, 2020,31(19): 2333-2338. [17] 刘铁汉,王本祥,王毅,等.淫羊藿苷及其肠菌代谢产物对THP-1细胞分泌细胞因子的影响[J].药学学报,2000(4):245-248. [18] 翟远坤,葛宝丰,陈克明,等.淫羊藿苷与其代谢产物淫羊藿次苷Ⅱ对骨髓间充质干细胞成骨性分化影响的比较研究[J].中药材, 2010, 33(12):1896-1900. [19] 毛项颖,卞琴,沈自尹.淫羊藿苷介导MAPK信号通路促进间充质干细胞株C3H10T1/2成骨分化的体外研究[J].中西医结合学报, 2012, 10(11):1272-1278. [20] SHENG H, RUI XF, SHENG CJ, et al. A novel semisynthetic molecule icaritinstimulates osteogenic differentiation and inhibits adipogenesis of mesenchymalstem cells. Int J Med Sci. 2013;10(6):782-789. [21] MA HP, MA XN, GE BF, et al. Icariin attenuates hypoxia- induced oxidative stress and apoptosis in osteoblasts and preserves their osteogenic differentiation potential in vitro. Cell Proliferation. 2014;47(6):527-539. [22] 曾建春,曾意荣,樊粤光,等.淫羊藿甙诱导MSCs向成骨细胞分化过程中对Wnt信号通路的影响[J].广州中医药大学学报,2014,31(4): 607-611. [23] LIU YQ, YANG QX, CHENG MC, et al. Synergistic inhibitory effect of icariside Ⅱ with icaritin from herba Epimedii on pre-osteoclastic raw264.7 cell Growth. Phytomedicine. 2014;21(12):1633-1637. [24] 笪巍伟,赵永见,王拥军,等.淫羊藿苷对前成骨细胞株OCT1细胞BMP-2 mRNA、Runx-2 mRNA表达的影响[J].上海中医药杂志, 2015, 49(5):90-94. [25] 王朝鹏.淫羊蕾素对MC3T3-E1 Subclone14ER/MAPK信号通路的影响[D].广州:暨南大学,2016. [26] 王想福,孙凤歧,叶丙霖,等.类黄酮对人体外周血破骨细胞分化p38MAPK/c-Fos信号通路调控的研究[J].中国骨质疏松杂志,2017, 23(11):1488-1491,1529. [27] 许静,张晶晶,郭非非,等.淫羊藿黄酮类主要成分促进骨髓间充质干细胞向成骨细胞增殖分化作用及机制的影响[J].中国实验方剂学杂志, 2017,23(14):113-120. [28] LIM R, LI L, CHEW N, et al. The prenylflavonoid Icaritin enhances osteoblast proliferation and function by signal transducer and activator of transcription factor 3 (STAT-3) regulation of C-X-C chemokine receptor type 4 (CXCR4) expression. Bone. 2017;105:122-133. [29] 贺龙刚,高奥,邱煌沛,等.淫羊藿次苷Ⅰ及其代谢产物淫羊藿次苷Ⅱ通过AP-1/NFATc1信号通路调控破骨细胞生成[J].中华中医药杂志, 2017,32(3):1299-1302. [30] WANG Y, WANG R, ZHANG F. Icariin promotes the proliferation and differentiation of osteoblasts from the rat mandible by the Wnt /β-catenin signalling pathway. Mol Med Rep. 2018;18 (3):3445-3450. [31] 王宇,董婉茹,杨晓旭,等.淫羊藿活性单体抗骨质疏松药理研究进展[J].中药药理与临床,2016,32(3):197-201. [32] 訾慧,范颖,蒋宁,等.淫羊藿次苷Ⅰ及淫羊藿次苷Ⅱ通过BMP/Runx2/Osx信号通路促进大鼠骨髓间充质干细胞成骨分化的实验研究[J].中国骨质疏松杂志,2019,25(5):690-695. [33] 訾慧,郑洪新,蒋宁,等.淫羊藿素通过BMP/Runx2/Osx信号通路促进大鼠骨髓间充质干细胞成骨分化研究[J].中华中医药学刊, 2020,38(7): 212-215+270. [34] WANG Z, WANG D, YANG D, et al. The effect of icariin on bone metabolism and its potential clinical application. Osteoporos Int. 2018;29(3):535-544. [35] HUANG Z, CHENG C, WANG J, et al. Icariin regulates the osteoblast differentiation and cell proliferation of MC3T3-E1 cells through microRNA-153 by targeting Runt-related transcription factor 2. Exp Ther Med. 2018;15(6):5159-5166. [36] LIU M, XU H, MA Y, et al. Osteoblasts Proliferation and Differentiation Stimulating Activities of the Main Components of Epimedii folium. Pharmacogn Mag. 2017;13(49):90-94. [37] LIU W, MAO L, JI F, et al. Icariside II activates EGFR-Akt-Nrf2 signaling and protects osteoblasts from dexamethasone. Oncotarget. 2017;8(2): 2594-2603. [38] TAN EM, LI L, INDRAN IR, et al. TRAF6 Mediates Suppression of Osteoclastogenesis and Prevention of Ovariectomy-Induced Bone Loss by a Novel Prenylflavonoid. J Bone Miner Res. 2017;32(4):846-860. [39] LIM RZL, LI L, YONG EL, et al. STAT-3 regulation of CXCR4 is necessary for the prenylflavonoid icaritin to enhance mesenchymal stem cell proliferation,migration and osteogenic differentiation. Biochim Biophys Acta Gen Subj. 2018;1862(7):1680-1692. [40] KIM B, LEE KY, PARK B. Icariin abrogates osteoclast formationthrough the regulation of the RANKL-mediated TRAF6 /NF-κB / ERK signaling pathway in Raw264. 7 cells. Phytomedicine. 2018;51:181-190. [41] HUANG M, WANG Y, PENG R. Icariin alleviates glucocorticoid-induced osteoporosis through EphB4/ Ephrin-B2 axis. Evid Based Complement Alternat Med. 2020;2020:2982480. [42] XU S, ZHANG Y, LIU B, et al. Activation of mTORC1 in B Lymphocytes Promotes Osteoclast Formation via Regulation of β-Catenin and RANKL/OPG. J Bone Miner Res. 2016;31(7):1320-1333. [43] 刘颖,柴丽娟,黄菊阳,等.朝藿定A对原代骨髓间充质干细胞向成骨细胞分化的作用[J].中国兽医学报,2021,41(5):980-984+991. [44] TORRE E. Molecular signaling mechanisms behind polyphenol- induced bone anabolism. Phytochem Rev. 2017;16(6):1183-1226. [45] SONG N, WANG ZM, HE LJ, et al. Estradiol enhanced osteogenesis of rat bone marrow stromal cells is associated with the JNK pathway. Mol Med Rep. 2017;16(6):8589-8594. [46] WANG W, BAI J, ZHANG W, et al. Protective Effects of Punicalagin on Osteoporosis by Inhibiting Osteoclastogenesis and Inflammation via the NF-κB and MAPK Pathways. Front Pharmacol. 2020;11:696. [47] 龚一昕,刘尚全,袁媛,等.淫羊藿次苷II通过p38 MAPK调控成骨细胞护骨素表达的体外研究[J].安徽医科大学学报,2016,51(12):1790-1793,1794. [48] WEI Q, HOLLE A, LI J, et al. BMP-2 Signaling and Mechanotransduction Synergize to Drive Osteogenic Differentiation via YAP/TAZ. Adv Sci (Weinh). 2020;7(15):1902931. [49] 梁广胜,陈伟才,殷嫦嫦,等.淫羊藿总黄酮对大鼠骨髓间充质干细胞成骨分化过程BMP-2/RunX2/OSX通路的影响[J].中国中西医结合杂志, 2016,36(5):614-618. [50] YANG M, LIU H, WANG Y, et al. Hypoxia reduces the osteogenic differentiation of peripheral blood mesenchymal stem cells by upregulating Notch-1 expression. Connect Tissue Res. 2019;60(6):583-596. [51] ZANOTTI S, CANALIS E. Notch Signaling and the Skeleton. Endocr Rev. 2016; 37(3):223-253. [52] 邓宇,陈廖斌.淫羊藿苷通过激活Notch信号通路促进骨髓间充质干细胞向成骨细胞分化的实验研究[J].中医学报,2017,32(12):2393-2398. |
| [1] | Jiang Huanchang, Zhang Zhaofei, Liang De, Jiang Xiaobing, Yang Xiaodong, Liu Zhixiang. Comparison of advantages between unilateral multidirectional curved and straight vertebroplasty in the treatment of thoracolumbar osteoporotic vertebral compression fracture [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(9): 1407-1411. |
| [2] | Zhu Chan, Han Xuke, Yao Chengjiao, Zhou Qian, Zhang Qiang, Chen Qiu. Human salivary components and osteoporosis/osteopenia [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(9): 1439-1444. |
| [3] | Jin Tao, Liu Lin, Zhu Xiaoyan, Shi Yucong, Niu Jianxiong, Zhang Tongtong, Wu Shujin, Yang Qingshan. Osteoarthritis and mitochondrial abnormalities [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(9): 1452-1458. |
| [4] | Zhang Lichuang, Xu Hao, Ma Yinghui, Xiong Mengting, Han Haihui, Bao Jiamin, Zhai Weitao, Liang Qianqian. Mechanism and prospects of regulating lymphatic reflux function in the treatment of rheumatoid arthritis [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(9): 1459-1466. |
| [5] | Wang Jing, Xiong Shan, Cao Jin, Feng Linwei, Wang Xin. Role and mechanism of interleukin-3 in bone metabolism [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(8): 1260-1265. |
| [6] | Xiao Hao, Liu Jing, Zhou Jun. Research progress of pulsed electromagnetic field in the treatment of postmenopausal osteoporosis [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(8): 1266-1271. |
| [7] | Zhu Chan, Han Xuke, Yao Chengjiao, Zhang Qiang, Liu Jing, Shao Ming. Acupuncture for Parkinson’s disease: an insight into the action mechanism in animal experiments [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(8): 1272-1277. |
| [8] | Li Wei, Zhu Hanmin, Wang Xin, Gao Xue, Cui Jing, Liu Yuxin, Huang Shuming. Effect of Zuogui Wan on bone morphogenetic protein 2 signaling pathway in ovariectomized osteoporosis mice [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(8): 1173-1179. |
| [9] | Wang Baojuan, Zheng Shuguang, Zhang Qi, Li Tianyang. Miao medicine fumigation can delay extracellular matrix destruction in a rabbit model of knee osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(8): 1180-1186. |
| [10] | Guo Jia, Ding Qionghua, Liu Ze, Lü Siyi, Zhou Quancheng, Gao Yuhua, Bai Chunyu. Biological characteristics and immunoregulation of exosomes derived from mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1093-1101. |
| [11] | Wu Weiyue, Guo Xiaodong, Bao Chongyun. Application of engineered exosomes in bone repair and regeneration [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1102-1106. |
| [12] | Zhou Hongqin, Wu Dandan, Yang Kun, Liu Qi. Exosomes that deliver specific miRNAs can regulate osteogenesis and promote angiogenesis [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1107-1112. |
| [13] | Zhang Jinglin, Leng Min, Zhu Boheng, Wang Hong. Mechanism and application of stem cell-derived exosomes in promoting diabetic wound healing [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1113-1118. |
| [14] | Huang Chenwei, Fei Yankang, Zhu Mengmei, Li Penghao, Yu Bing. Important role of glutathione in stemness and regulation of stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1119-1124. |
| [15] | Hui Xiaoshan, Bai Jing, Zhou Siyuan, Wang Jie, Zhang Jinsheng, He Qingyong, Meng Peipei. Theoretical mechanism of traditional Chinese medicine theory on stem cell induced differentiation [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1125-1129. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||